Introduction
Prostate cancer is one of the leading causes of
mortality in men (1) and has a
highly variable natural history. Prostate cancer may be present as
an indolent and silent entity throughout a man’s life and then grow
rapidly following metastasis to the lymph nodes and bones with a
median life expectancy of 24–36 months (2). Therefore, prostate cancer is largely
asymptomatic until metastases are present and it is largely a
disease of the elderly. It is reasonable to propose that agents
which inhibit metastasis could have great therapeutic efficacy.
Quinazoline derivatives are known for multiple
effects, such as anti-malarial, anti-inflammatory (3), anti-bacterial, and antitumor
activities (4). In recent years,
we have designed and synthesized a series of quinazoline
derivatives as new anti-mitotic agents (5,6). Our
previous study showed that synthesized
6-pyrrolidinyl-4-quinazolinone derivative MJ-29 inhibited tubulin
polymerization through binding to β-tubulin at the
colchicine-binding site and acted as an anti-mitotic agent
(5). Furthermore, we demonstrated
that 6-fluoro-(3-fluorophenyl)-4-(3-methoxyanilino)quinazoline
(LJJ-10) exhibits anti-metastatic effects in human osteosarcoma U-2
OS cells through targeting the insulin-like growth factor-I
receptor (IGF-IR) (6). In the
present study, we investigated the effects of MJ-33 on invasion,
migration and adhesion in DU145 human prostate cancer cells. MJ-33
inhibited migration and invasion through downregulation of matrix
metalloproteinases (MMPs) and urokinase-type plasminogen activator
(u-PA) through the AP-1 and NF-κB signaling pathways.
Materials and methods
Chemicals and reagents
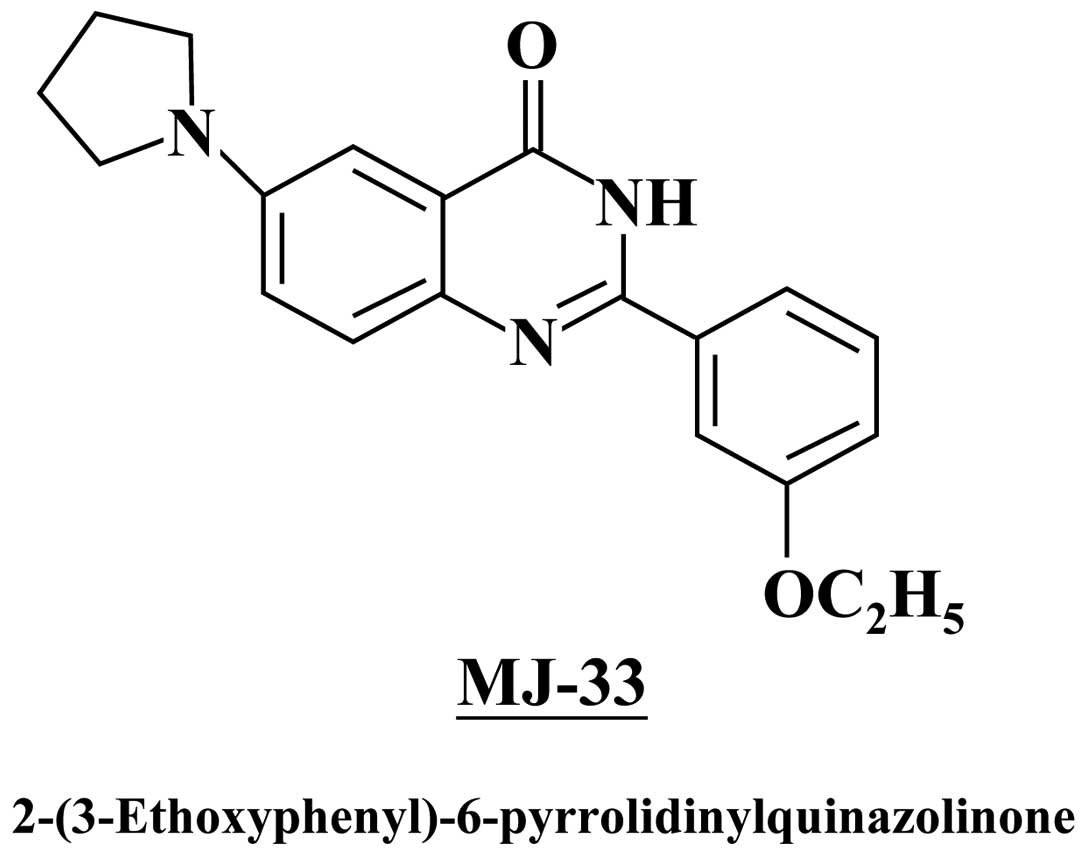
MJ-33 was designed and synthesized by Mann-Jen Hour
and Sheng-Chu Kuo (China Medical University, Taichung, Taiwan)
(Fig. 1). Dimethylsulfoxide
(DMSO), potassium phosphates, propidium iodide (PI) and triton
X-100 were obtained from Sigma-Aldrich (St. Louis, MO, USA).
RPMI-1640 medium, fetal bovine serum (FBS), L-glutamine,
penicillin-streptomycin, and trypsin-EDTA were obtained from
Gibco-BRL (Invitrogen, Grand Island, NY, USA). Antibodies against
phospho-AKT, phospho-JNK, phospho-ERK and phospho-p38 were
purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
Antibodies against AKT, JNK, ERK, p38, β-actin, MMP-2, MMP-9, u-PA,
NF-κB (p65), c-fos, c-Jun and all peroxidase-conjugated secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Antibody against β-actin was purchased from
Sigma-Aldrich.
Cell culture
The DU145, LNCaP and PC-3 human prostate cancer cell
lines were obtained from the Food Industry Research and Development
Institute (Hsinchu, Taiwan). All cells were individually plated
onto 75 cm2 tissue culture flasks with 90% RPMI-1640
medium. Cell medium with 2 mM L-glutamine was adjusted to contain
1.5 μg/ml sodium bicarbonate, supplemented with 10% FBS, 100
Units/ml penicillin and 100 μg/ml streptomycin. The cells were
grown at 37°C under a humidified 5% CO2 atmosphere.
Cell viability
Approximately 5x104 cells/well of DU145,
LNCaP and PC-3 cells were individually grown in 96-well plates for
24 h before different concentrations of MJ-33 were added (0, 50,
100, 250 and 500 nM). Cells were incubated at 37°C, 5%
CO2 and 95% air for 24 and 48 h. Following treatment,
the supernatant was discarded before a 100 μl solution of MTT (500
μg/ml) was added to each well for 4 h at 37°C. After incubation,
the violet formazan crystal produced from MTT was solubilized by
the addition of 100 μl of DMSO. The absorbance of the dissolved
formazan grained within the cells was measured at 570 nm by a
microplate reader as previously described (6).
Cell invasion assay
Twenty-four-well Transwell inserts with 8 μm
porosity polycarbonate filters (Millipore, Billerica, MA, USA) were
pre-conted with 30 μg Engelbreth-Holm-Swarm sarcoma tumor extract
(EHS Matrigel Basement Membrane Matrix) at room temperature for 1 h
and then formed a genuine reconstituted basement membrane. DU145
cells (1x104 cells/0.5 ml RPMI-1640) were placed onto
the upper compartment and incubated with MJ-33 (0, 50, 100 and 200
nM). The plates were then incubated at 37°C for 24 h in a
humidified atmosphere with 95% air and 5% CO2. The cells
were fixed with 4% formaldehyde in PBS and stained with 2% crystal
violet. Cells on the upper surface of the filter were removed by
wiping with a cotton swab, and cells that penetrated through the
matrigel to the lower surface of the filter were counted under a
light microscope at ×200. Each treatment was assayed in duplicate,
and three independent experiments were carried out as previously
described (7,8).
Cell migration assay
Approximately 5x104 DU145 cells/ml were
plated in 6-well plates for 24 h and then the cells in individual
wells were wounded by scratching with a pipette tip and incubated
with or without FBS free RPMI-1640 medium and treated with or
without MJ-33 (0, 50, 100 and 200 nM) for 24 h. The cells were
photographed under phase-contrast microscopy (x100) and calculated.
Each treatment was assayed in duplicate, and three independent
experiments were carried out as previously described (7,8).
Cell adhesion assay
The cell-matrix adhesion assay was used to determine
cell adhesion. DU145 cells were plated on a 24-well plate after
treatment with or without MJ-33 (0, 50, 100 and 200 nM) for 24 h.
Then the individual cells were removed to a plate coated with 150
μl of type I collagen (10 μg/ml) and cultured for 30 min.
Non-adherent cells were removed by PBS washing, and adherent cells
were fixed in ethanol. After staining with 0.1% crystal violet,
fixed cells were lysed in 0.2% Triton-100 and measured at 550 nm by
a microplate reader. Each treatment was assayed in duplicate, and
three independent experiments were done as previously described
(7,8).
Zymography assay
To determine the activity of MMP-2, MMP-9 and u-PA,
quantitative gelatin zymography was performed with standard
methods. Briefly, cells (1x107 cells/ml) were treated
with MJ-33 (0, 50, 100 and 200 nM) for 24 h. Cells were harvested
and separated by dilution in zymography sample buffer. Samples were
electrophoresed in an 8% SDS-polyacrylamide gel containing 1%
gelatin, and incubated in renaturing buffer (2.5% Triton X-100).
Electrophoresis was performed at 110 V for 3 h. The gel was
incubated with development buffer (50 mM Tris, pH 7.5, 200 mM NaCl,
5 mM CaCl2, 1 μM ZnCl2, 0.02% Brij-35) at
37°C for 18 h, and stained with 0.5% Coomassie blue G-250 for 3 h.
The gels were digitized using a scanning digitizing system and
analyzed using NIH image software. The u-PA activity was performed
by casein-plasminogen zymography. Briefly, 2% casein and 20 μg/ml
plasminogen were added to an 8% SDS-PAGE gel. Samples with a total
protein of approximately 30 μg were then loaded onto the gels. The
u-PA activity of cells treated with or without MJ-33 was measured
as described for the gelatin zymography assay (7,8).
Preparation of whole-cell lysate and
nuclear extract
Approximately 1x107 cells were treated
with MJ-33 (0, 50, 100 and 200 nM) for 6 or 24 h. The cells were
harvested and whole-cell lysed with iced-cold RIPA buffer (1%
NP-40, 50 mM Tris-base, 0.1% SDS, 0.5% deoxycholic acid, 150 mM
NaCl, pH 7.5), and then phenylmethanesulfonyl fluoride (10 mg/ml),
leupeptin (17 mg/ml), and sodium orthovanadate (10 mg/ml) were
added (9,10) and vortexed for 30 min on ice. The
samples were centrifuged at 12,000 g for 10 min. Nuclear extracts
were prepared from MJ-33-treated DU145 cells using the NE-PER
Nuclear and Cytoplasmic Extraction kit (Pierce, Rockford, IL, USA).
Each nuclear pellet was collected and was then re-suspended in
nuclear extract buffer (1.5 mM MgCl2, 10 mM HEPES, pH
7.9, 0.1 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM
phenylmethanesulfonyl fluoride, 25% glycerol, and 420 mM NaCl). The
nuclear suspension was incubated for 20 min on ice then centrifuged
at 14,000 g for 5 min. The supernatant (the soluble nuclear
fraction) was saved and the remaining pellet was solubilized by
sonication in PBS. The protein content in each sample was
determined by using Bio-Rad protein assay reagent using bovine
serum albumin as the standard. Nuclear extracts were prepared for
NF-κB, c-fos, and c-Jun western determination (11).
Western blotting
Cells were harvested and the total and nuclear
proteins were collected as described above. Protein abundance of
MMP-2, MMP-9 and u-PA, p-JNK, p-ERK, p-p38, p-AKT, JNK, ERK, p38,
AKT, NF-κB (p65), c-fos and c-Jun were determined by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western
blotting as previously described (5,12–15).
Electrophoretic Mobility Shift Assay
(EMSA)
Approximately 1x107 cells were treated
with MJ-33 (0, 100 and 200 nM) for 6 h. Nuclear extracts were
prepared from MJ-33-treated DU145 cells using the NE-PER Nuclear
and Cytoplasmic Extraction kit (Pierce). The protein concentrations
were determined and Biotin end-labeled oligonucleotide sequences
5′-Biotin-GATCCAGGGGACTTTCCCTAGC-3′ corresponded to the consensus
site of NF-κB and Biotin end-labeled oligonucleotide sequences
5′-Biotin-CGCTTGATGACTCAGCCGGAA-3′ corresponded to the consensus
site of AP-1. Nuclear extract proteins (5 μg) were used for EMSA
with a LightShift Chemiluminescent EMSA Kit according to the
manufacturer’s protocol. Biotin end-labeled duplex DNA was
incubated with a nuclear extract or purified factor and
electrophoresed on a 6% polyacrylamide native gel. For competition
experiments, a 100-fold excess of unlabeled double stranded
oligonucleotide was added to the reaction. The DNA was then rapidly
transferred to a positive nylon membrane, UV cross-linked, probed
with streptavidin-HRP conjugate and incubated with the substrate of
the ECL kit (16,17).
Statistical analysis
Student’s t-test was used to analyze differences
between treated and control groups. *p<0.05 was
considered to indicate a statistically significant difference.
Results
MJ-33 induces growth inhibition effects
on human prostate cancer cell lines
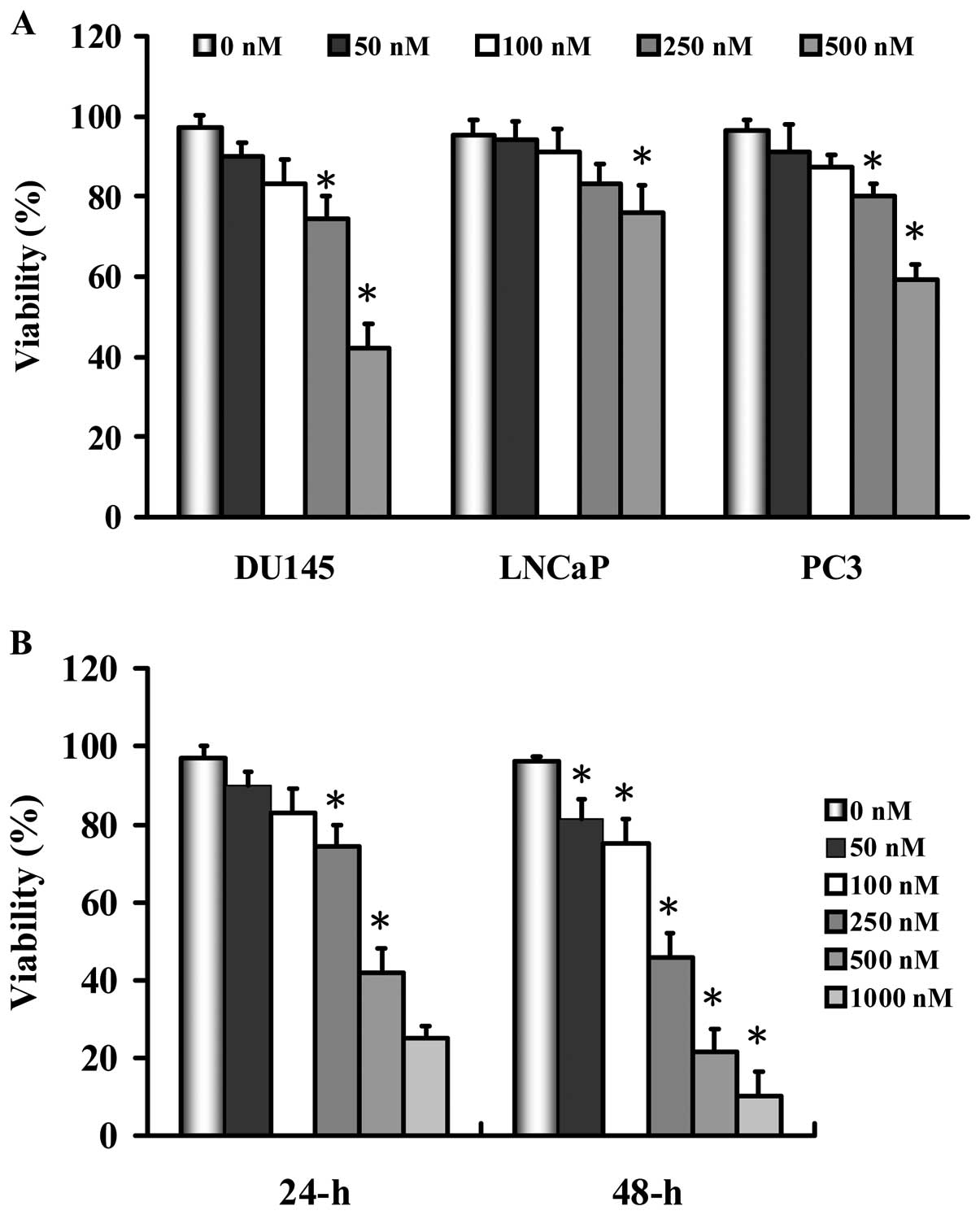
We determined the growth inhibition effects of MJ-33
on the human prostate cancer cell lines DU145, LNCaP and PC-3. As
shown in Fig. 2, MJ-33 inhibited
the cell growth of the three cell lines in a
concentration-dependent manner. DU145 cells were more sensitive by
MJ-33 than that of the other two cell lines. We therefore
investigated whether or not MJ-33 could induce a concentration- and
time-dependent growth inhibition effect on DU145 cells. As seen in
Fig. 2B, MJ-33 decreased the
percentage of viable DU145 cells in a concentration- and
time-dependent manner, but we selected less than 200 nM of MJ-33
for further works in this study.
MJ-33 inhibits invasion, migration and
adhesion of DU145 cells
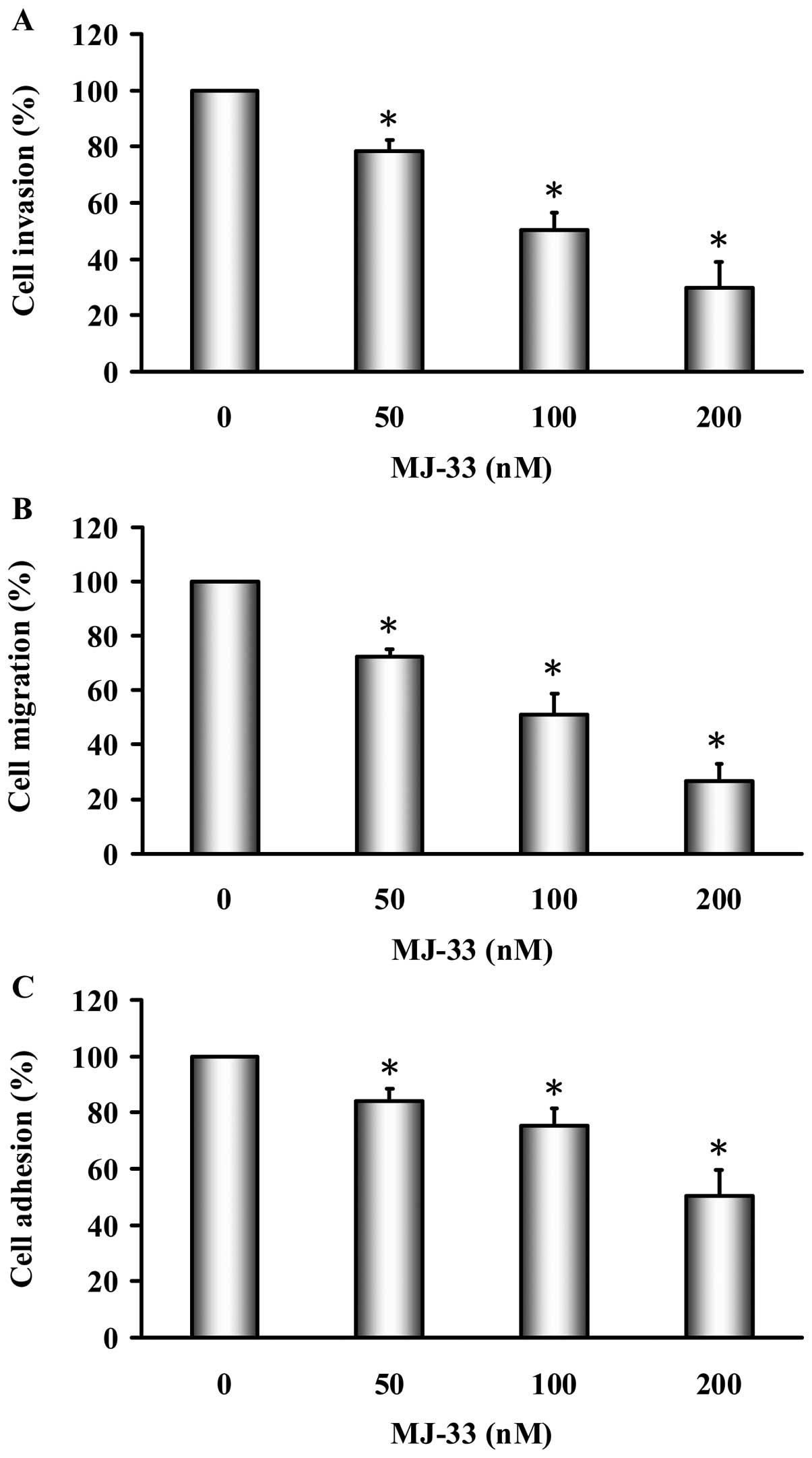
The effects of MJ-33 on cell invasion were examined
using Matrigel-coated Transwell assay in DU145 cells. As shown in
Fig. 3A, MJ-33 (50–200 nM)
significantly inhibited cell invasion in a concentration-dependent
manner; the percentage of inhibition ratio was 15–70%. The
inhibition of DU145 cell migration by MJ-33 was examined using the
wound-healing assay. As shown in Fig.
3B, MJ-33 (50–200 nM) significantly inhibited cell migration in
a concentration-dependent manner; the percentage of inhibition
ratio was 30–75%. The inhibition of DU145 cell adhesion by MJ-33
was examined by using cell adhesion assay. As shown in Fig. 3C, MJ-33 (50–200 nM) significantly
inhibited cell adhesion in a concentration-dependent manner; the
percentage of inhibition ratio was 10–45%. MJ-33 did not affect
cell viability at 50–200 nM of 24-h treatment. On the other hand,
the EC50 is 458.32±6.96 nM for 24 h in MJ-33-treated
DU145 cells. Our results demonstrated that MJ-33 inhibited the
effects of cell invasion, migration and adhesion in DU145 cells.
Also, the inhibitory effects of MJ-33 on invasion, migration and
adhesion are independent of cellular cytotoxicity.
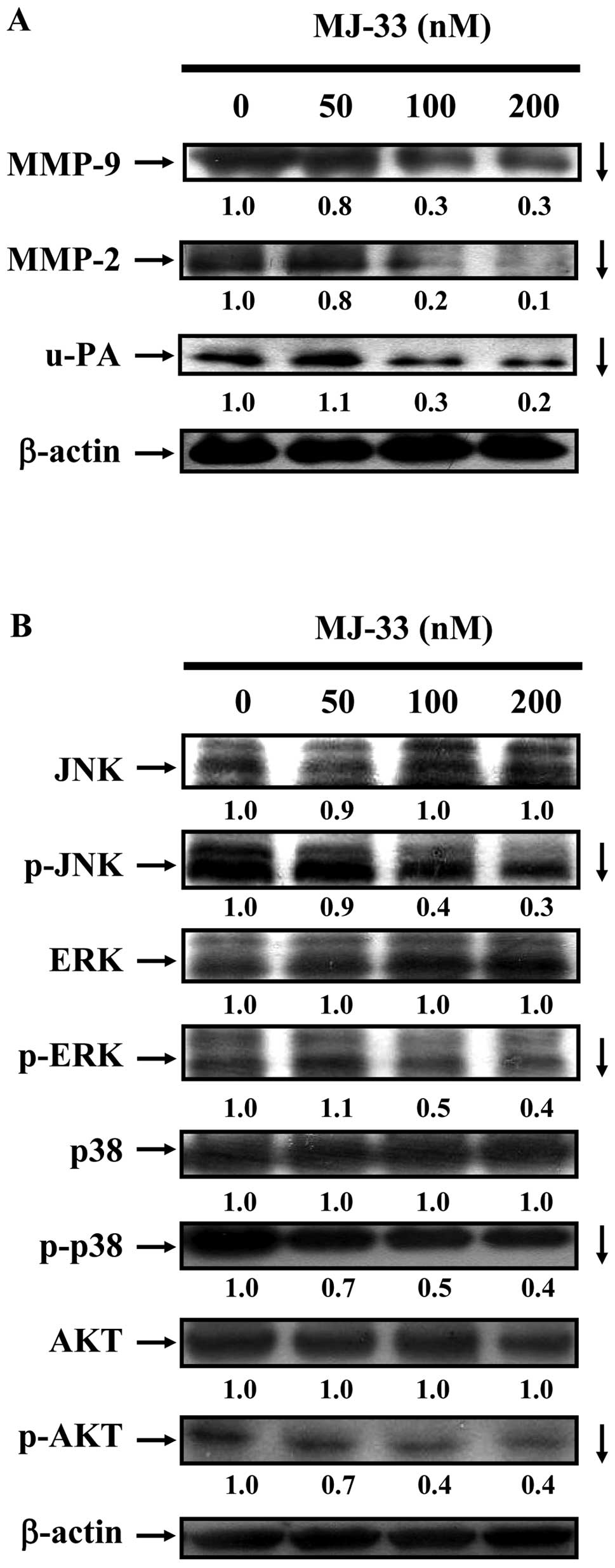
MJ-33 inhibits MMP-2, MMP-9 and u-PA
enzyme activities of DU145 cells
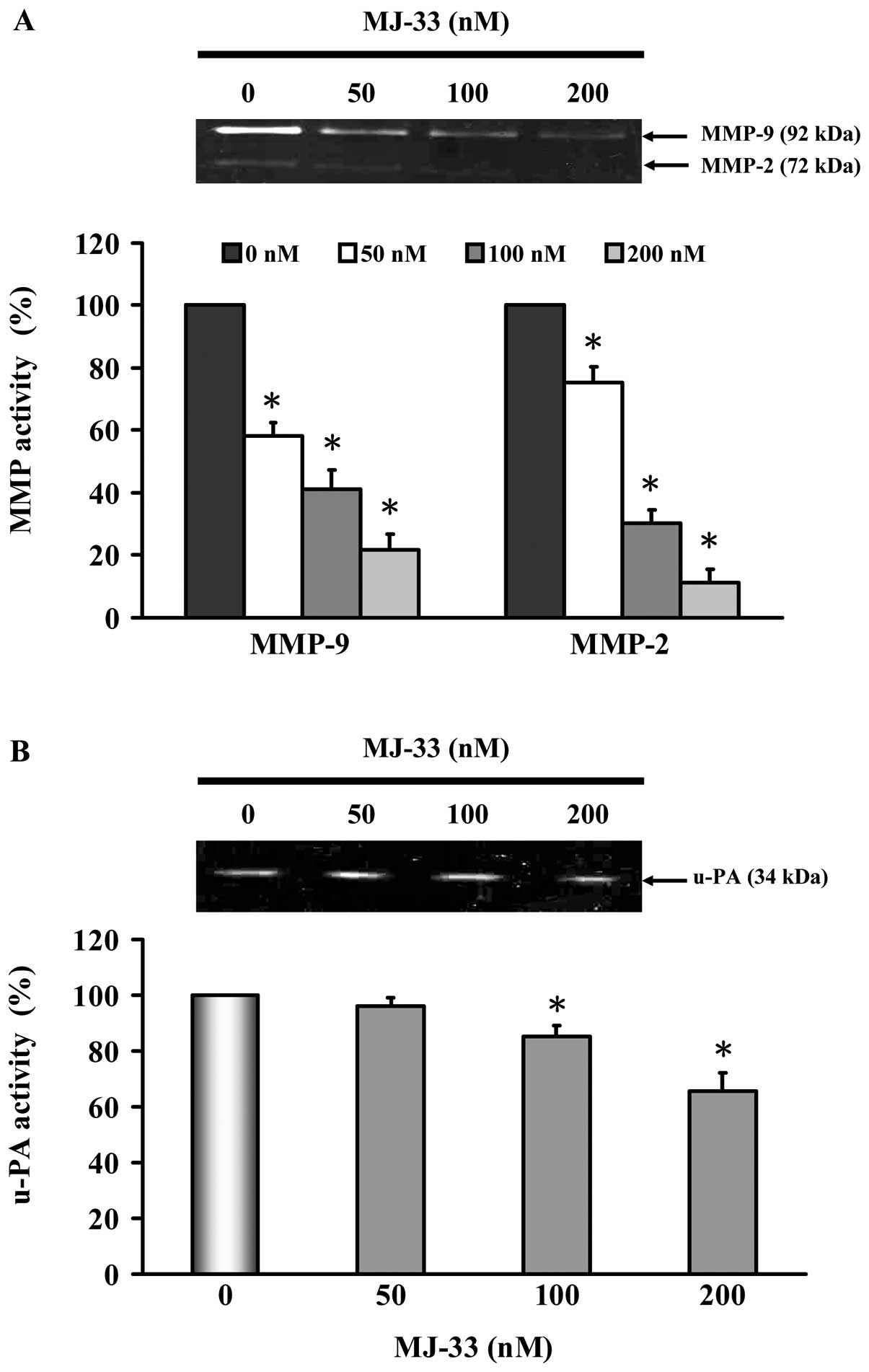
We investigated the mechanisms of cell invasive
phenotype by determining the involvement of MMP-2, MMP-9 and u-PA.
DU145 cells were treated with MJ-33 (0, 50, 100 and 200 nM) for 24
h. The MMP-2, MMP-9 and u-PA activities were determined by gelatin
or casein zymography. As shown in Fig.
4, we found that MJ-33 inhibited individual activity of MMP-2,
MMP-9 (Fig. 4A) and u-PA (Fig. 4B). Reductions in activity are
consistent with decreases in protein abundance of MMP-2, MMP-9 and
u-PA, as shown in Fig. 5A.
MJ-33 inhibits the MAPKs and AKT
signaling pathways in DU145 cells
We investigated the effects of MJ-33 on metastatic
protein levels in DU145 cells by western blotting. As shown in
Fig. 5A, we determined DU145 cells
after exposure to MJ-33 (0, 50, 100 and 200 nM) for 24 h. MJ-33
reduced the protein levels of MMP-2, MMP-9 and u-PA. To clarify the
possible upstream signaling pathways in MJ-33-treated DU145 cells,
we evaluated the related protein levels in the MAPK (JNK, p38 and
ERK) and AKT signaling pathways by western blotting. We determined
DU145 cells after exposure to MJ-33 (0, 50, 100 and 200 nM) for 6
h. We found that incubation of cells with MJ-33 reduced the protein
levels of p-JNK, p-ERK, p-p38 and p-AKT.
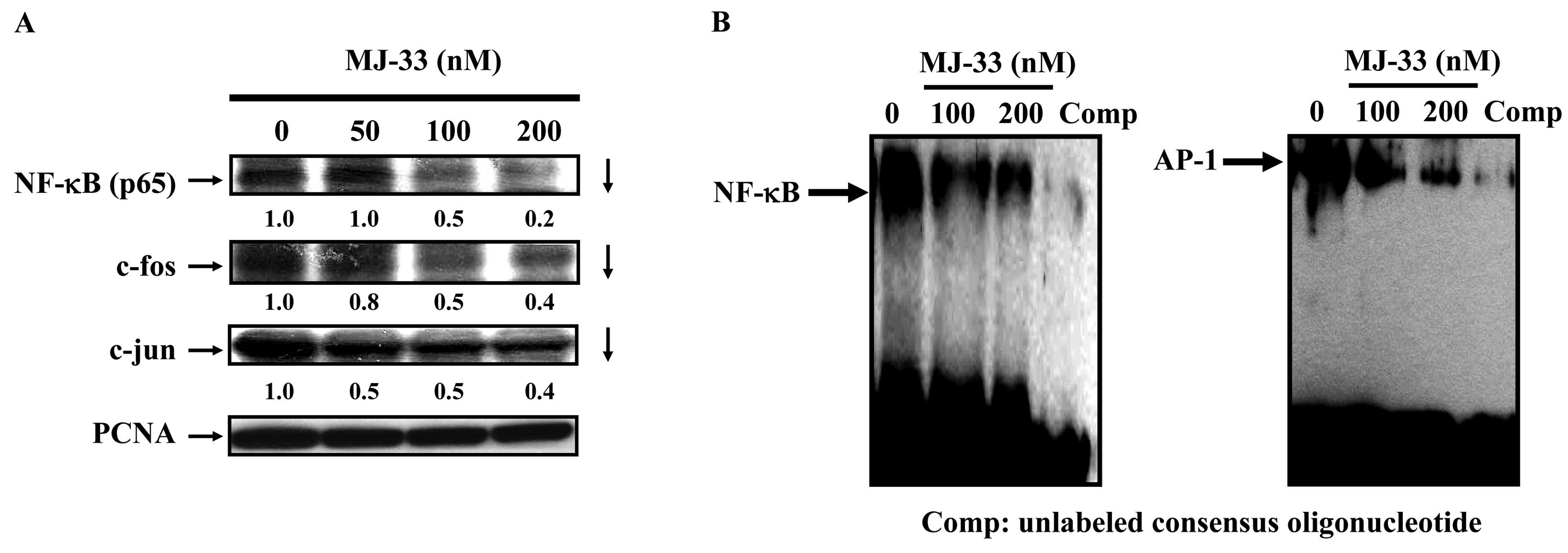
MJ-33 inhibits the AP-1 and NF-κB
signaling pathways in DU145 cells
Numerous studies have reported that MMP-9, MMP-2 and
u-PA promoters have several transcription binding motifs such as
NF-κB and AP-1. In order to clarify the involvement of NF-κB and
AP-1 proteins in the mechanisms of MJ-33′s action, we evaluated the
related protein levels in NF-κB, c-fos and c-Jun by western
blotting. In addition, the effects of MJ-33 on DNA binding of NF-κB
and AP-1 were determined using EMSA. We determined DU145 cells
after exposure to MJ-33 (0, 50, 100 and 200 nM) for 6 h. We found
that incubation of cells with MJ-33 reduced protein levels of
NF-κB, c-fos and c-Jun. The results shown in Fig. 6B demonstrate that MJ-33 inhibited
NF-κB and AP-1 DNA binding in a dose-dependent manner. Binding of
NF-κB and AP-1 were particularly inhibited by treatment with 100
and 200 nM of MJ-33. Therefore, our study proposes that MJ-33 might
block the invasion, migration and adhesion in DU145 cells by
inhibiting AKT and MAPKs as well as suppressing the NF-κB
signaling.
Discussion
Previous reports have found that quinazoline
derivatives exert antitumor activity against seven types of cancer
cells both in vitro and in vivo (18), and also induce apoptosis and
inhibit metastasis in the U-2 OS human osterogenic sarcoma cell
line (19). There is, however, no
information on the effects of MJ-33 on invasion, migration and
adhesion in human prostate cancer cells. Initially, three human
prostate cancer cell lines (DU145, LNCaP and PC-3) were examined
and it was observed that MJ-33 reduced DU145 cell viability more
compared with the other two cell lines. Based on these findings,
DU145 cells were used to examine the effects of MJ-33 on invasion,
migration and adhesion. We found that MJ-33 can induce growth
inhibition effects and inhibit invasion, migration and adhesion of
DU145 cells (Fig. 3). Furthermore,
these effects were associated with inactivation of the MAPKs (ERK,
JNK, p38) and AKT (Fig. 5),
inhibitory effects on NF-κB, c-fos, and c-Jun transcriptional
factors (Fig. 6). This effect on
AP-1 and NF-κB transcription factors was consistent with less DNA
binding of NF-κB and AP-1 DNA (Fig.
6B). MAPKs are intricately involved in the expression of the
components involved in MMPs or u-PA promoter induction through
NF-κB, AP-1 and its association with c-fos and c-Jun (20). Numerous studies from different cell
types have suggested the MAPKs play a central role in regulating
the activities of MMPs or u-PA (21–23).
Inhibition of the MAPKs pathway might have the potential of
preventing angiogenesis, proliferation, invasion, and migration
occurring with a wide range of tumors.
Our findings reinforce the potential of MJ-33 as a
new strategy for antitumor therapy, especially in the inhibition of
cancer metastasis which is a major cause of mortality in cancer
patients. MMP-2 and u-PA promoters have several transcription
factor binding motifs, including NF-κB and AP-1 (24). Thus, multiple pathways leading to
activation of NF-κB and AP-1 binding factors in tumor cells may
contribute to MMP-2, MMP-9 and u-PA transcription and metastatic
enhancement. We found that MJ-33 inhibited cell invasion, migration
and adhesion through the downregulation of MMP-2 and MMP-9 protein
abundance in DU145 cells. This is in agreement with our previous
study that LJJ-10 (a novel quinazoline derivative) inhibited the
invasion of human osteosarcoma U-2 OS cells through inhibition of
MMP-2 and MMP-9. There is evidence that growth factors and
cytokines affect MMP-9 expression through acting on the
transcription factors NF-κB and AP-1 through the Ras/MAPK and
PI3K/AKT signaling pathways (25).
NF-κB and AP-1 binding to the MMP-2 and MMP-9 promoter are
centrally involved in the induction of MMP-2 and
MMP-9 gene expression associated with tumor cell invasion
(26–28). To further explore how MJ-33
inhibits invasion, migration and adhesion, we used gelatin or
caseinplasminogen zymographic assays to detect activities of MMP-2,
MMP-9, and u-PA. In this study, MJ-33 significantly decreased the
levels of MMP-2, MMP-9 and u-PA activity (Fig. 4). These results indicate that the
anti-metastatic effect of MJ-33 is associated with the inhibition
of enzymatically degradative processes of tumor metastasis.
Furthermore, we used a wound-healing and a Boyden chamber assay to
quantify the migratory potential of DU145 cells.
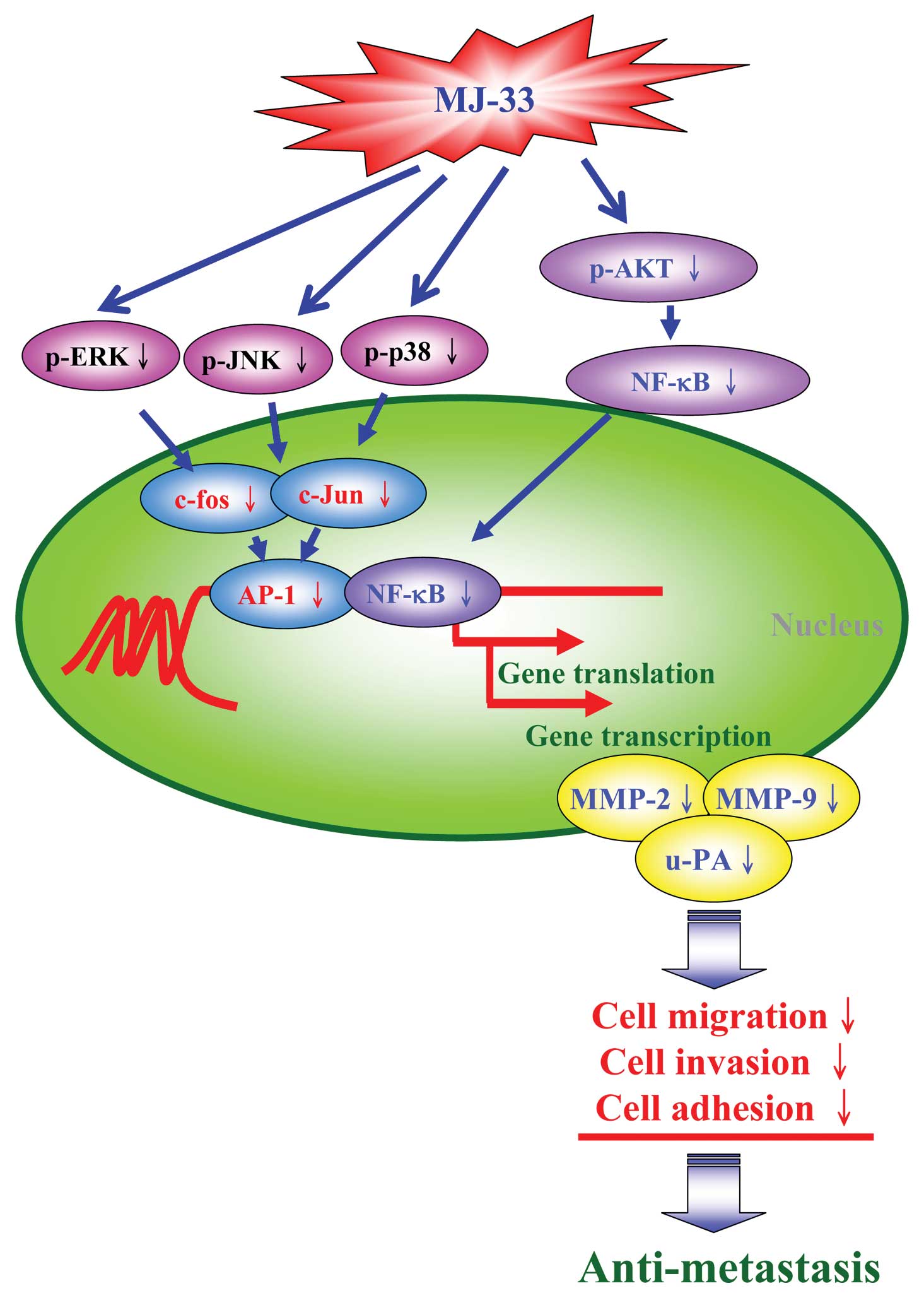
Taken together, these observations suggest that
MJ-33 significantly inhibits the invasion, migration and adhesion
of DU145 cells. MJ-33 acts as an anti-metastatic agent in prostate
cancer cells. Collectively, we have outlined the overall possible
signaling pathways for MJ-33-inhibited metastasis in DU145 cells
(Fig. 7). We explored for the
first time and investigated the roles of AP-1 and NF-κB in reducing
the levels and activities of MMP-2, MMP-9 and u-PA in human
prostate cancer cells.
Acknowledgements
This study was supported by a research
grant from the National Science Council of the Republic of China
(NSC 101-2313-B-039-008). We also thank Chi-Cheng Lu, Wen-Wen Huang
and Shu-Fen Peng (Department of Biological Science and Technology,
China Medical University) for their helpful suggestions and
technical support.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2
|
Petrylak DP: The current role of
chemotherapy in metastatic hormone-refractory prostate cancer.
Urology. 65:3–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chandrika PM, Yakaiah T, Rao AR, et al:
Synthesis of novel 4,6-disubstituted quinazoline derivatives, their
anti-inflammatory and anti-cancer activity (cytotoxic) against U937
leukemia cell lines. Eur J Med Chem. 43:846–852. 2008. View Article : Google Scholar
|
|
4
|
Chen Z, Huang X, Yang H, et al: Anti-tumor
effects of B-2, a novel 2,3-disubstituted
8-arylamino-3H-imidazo[4,5-g]quinazoline derivative, on the human
lung adenocarcinoma A549 cell line in vitro and in vivo. Chem Biol
Interact. 189:90–99. 2011.PubMed/NCBI
|
|
5
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
6
|
Chen KT, Hour MJ, Tsai SC, et al: The
novel synthesized
6-fluoro-(3-fluorophenyl)-4-(3-methoxyanilino)quinazoline (LJJ-10)
compound exhibits anti-metastatic effects in human osteosarcoma U-2
OS cells through targeting insulin-like growth factor-I receptor.
Int J Oncol. 39:611–619. 2011.
|
|
7
|
Liu KC, Huang AC, Wu PP, et al: Gallic
acid suppresses the migration and invasion of PC-3 human prostate
cancer cells via inhibition of matrix metalloproteinase-2 and -9
signaling pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
8
|
Ma CY, Ji WT, Chueh FS, et al: Butein
inhibits the migration and invasion of SK-HEP-1 human
hepatocarcinoma cells through suppressing the ERK, JNK, p38, and
uPA signaling multiple pathways. J Agric Food Chem. 59:9032–9038.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan YH, Wu YC, Wu KW, et al: Death
receptor 5-mediated TNFR family signaling pathways modulate
gamma-humulene-induced apoptosis in human colorectal cancer HT29
cells. Oncol Rep. 25:419–424. 2011.PubMed/NCBI
|
|
10
|
Huang WW, Ko SW, Tsai HY, et al:
Cantharidin induces G2/M phase arrest and apoptosis in human
colorectal cancer colo 205 cells through inhibition of CDK1
activity and caspase-dependent signaling pathways. Int J Oncol.
38:1067–1073. 2011.
|
|
11
|
Yu FS, Yang JS, Yu CS, et al: Safrole
induces apoptosis in human oral cancer HSC-3 cells. J Dent Res.
90:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
14
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danthron. Rep Carcinog. 12:128–129.
2011.
|
|
16
|
Lo C, Lai TY, Yang JS, et al: Gallic acid
inhibits the migration and invasion of A375.S2 human melanoma cells
through the inhibition of matrix metalloproteinase-2 and Ras.
Melanoma Res. 21:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo TC, Yang JS, Lin MW, et al: Emodin has
cytotoxic and protective effects in rat C6 glioma cells: roles of
Mdr1a and nuclear factor kappaB in cell survival. J Pharmacol Exp
Ther. 330:736–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SW, Pan SL, Huang YC, et al: CHM-1, a
novel synthetic quinolone with potent and selective antimitotic
antitumor activity against human hepatocellular carcinoma in vitro
and in vivo. Mol Cancer Ther. 7:350–360. 2008. View Article : Google Scholar
|
|
19
|
Hsu SC, Yang JS, Kuo CL, et al: Novel
quinolone CHM-1 induces apoptosis and inhibits metastasis in a
human osterogenic sarcoma cell line. J Orthop Res. 12:1637–1644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gondi CS and Rao JS: Therapeutic potential
of siRNA-mediated targeting of urokinase plasminogen activator, its
receptor, and matrix metalloproteinases. Methods Mol Biol.
487:267–281. 2009.PubMed/NCBI
|
|
21
|
Chen PN, Hsieh YS, Chiou HL and Chu SC:
Silibinin inhibits cell invasion through inactivation of both
PI3K-Akt and MAPK signaling pathways. Chem Biol Interact.
156:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
23
|
Aguirre Ghiso JA, Alonso DF, Farias EF,
Gomez DE and de Kier Joffe EB: Deregulation of the signaling
pathways controlling urokinase production. Its relationship with
the invasive phenotype. Eur J Biochem. 263:295–304. 1999.PubMed/NCBI
|
|
24
|
Peng PL, Hsieh YS, Wang CJ, Hsu JL and
Chou FP: Inhibitory effect of berberine on the invasion of human
lung cancer cells via decreased productions of
urokinase-plasminogen activator and matrix metalloproteinase-2.
Toxicol Appl Pharmacol. 214:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
26
|
Lin YT, Yang JS, Lin HJ, et al: Baicalein
induces apoptosis in SCC-4 human tongue cancer cells via a
Ca2+-dependent mitochondrial pathway. In Vivo.
21:1053–1058. 2007.PubMed/NCBI
|
|
27
|
Chuang JY, Huang YF, Lu HF, et al:
Coumarin induces cell cycle arrest and apoptosis in human cervical
cancer HeLa cells through a mitochondria- and caspase-3 dependent
mechanism and NF-kappaB down-regulation. In Vivo. 21:1003–1009.
2007.PubMed/NCBI
|
|
28
|
Huang YT, Hwang JJ, Lee LT, et al:
Inhibitory effects of a luteinizing hormone-releasing hormone
agonist on basal and epidermal growth factor-induced cell
proliferation and metastasis-associated properties in human
epidermoid carcinoma A431 cells. Int J Cancer. 99:505–513. 2002.
View Article : Google Scholar
|