Introduction
Conventional cancer treatments preferentially
destroy non-stem cancer cells within tumors, whereas cancer stem
cells (CSCs) are more resistant and survive, which can subsequently
cause a relapse, and in some cases, life-threatening metastasis
(1,2). Identification of a regulatory
mechanism, such as a functional cell surface marker, would be
useful for distinguishing CSCs from non-stem cancer cells, which
would allow for reduction of the dosage of chemo- and radiotherapy
and maximize tumor targeting (3).
Previously, we identified CD13/aminopeptidase as a cell surface
marker, which is preferentially expressed in CSCs of
gastrointestinal organs. CD13/aminopeptidase has a functional role
in the reduction of reactive oxygen species (ROS) in tumor cells by
modulating glutathione synthesis, thereby contributing to the
survival of CSCs after chemo- and radiotherapy (4). CD13 is also upregulated during the
epithelial-mesenchymal transition (EMT), a phenotype important for
cancer metastasis (5). Recent
studies suggest that susceptibility to EMT can serve as a marker of
CSCs (6). Because CSCs are a
heterogeneous cell population (7),
further research is necessary for identification of CSC
markers.
Since the identification of rare CSCs in leukemia
(8–10), molecular markers for detection of
CSCs have been reported in solid tumors of the head and neck
(11), gastrointestinal system
(12), colon (13,14),
breast (15) and brain (16,17).
CD44 (hyaluronic acid receptor) is one of the most commonly studied
surface markers, which is expressed by almost all cancer stem
cells, and CD24 (heat-stable antigen) is another surface marker
expressed in many tumor types (18). Although
CD44+CD24− cell populations have been
identified as CSCs in breast cancer (19), expression of CD133, CD166, CD44,
CD29, CD24, Lgr5, and nuclear β-catenin has been suggested to mark
the CSC population in the colon (20).
Subsequent studies showed that although it is
unclear whether CD133 is a marker of colon CSCs, other cell surface
markers, such as epithelial-specific antigen, CD44, CD166,
Musashi-1, CD29, CD24, leucine-rich repeat-containing G
protein-coupled receptor 5, and aldehyde dehydrogenase 1, have been
shown to be promising candidates (21). A recent study on mice narrowed down
the targets and demonstrated that CD24 can be used to isolate
Lgr5+ putative colonic epithelial stem cells; their data
suggest that the presence of CD24 expression in normal colonic
epithelium may have important implications in the use of colorectal
cancer therapies targeting CD24 (22). Here we studied the responsiveness
to TGF-β of CSCs carrying various markers (TGF-β is an effective
EMT inducer). The data on CD24 demonstrated that CD24+
cells are susceptible to EMT induction and are associated with
tumorigenesis in mice.
Materials and methods
Cell culture
Human CRC cell lines were obtained from American
Type Culture Collection (ATCC) and cultured in minimum essential
medium (MEM; Invitrogen, CA, USA) containing 10% fetal bovine serum
(FBS; Gibco, CA, USA) at 37°C in a humidified atmosphere containing
5% CO2. For flow cytometry and cell sorting, an
allophycocyanin (APC)-conjugated anti-human CD44 antibody, a
fluorescein isothiocyanate (FITC)-conjugated anti-human CD24
antibody, and a phycoerythrin (PE)-conjugated anti-human N-CAD
antibody (BD Bioscience, San Jose, CA, USA) were used for
characterization of cancer cells. Labeled cells were analyzed on a
BD FACS Aria II Cell Sorter System (Becton-Dickinson, Franklin
Lakes, NJ, USA), followed by data analysis using the Diva program
(Becton-Dickinson), as described previously (4,5).
The expression study
Total RNA was extracted from cells,
reverse-transcribed to cDNA, and subjected to PCR analysis using
specific primers as described previously (4,5).
Animal experiments
Cells were injected subcutaneously into NOD/SCID
mice as described previously (4,5).
These mice were monitored for up to 10 weeks and sacrificed when
the tumors reached a maximum diameter of 15 mm. All animal studies
were approved by the Animal Experiments Committee of Osaka
University.
Statistical analysis
For continuous variables used in an in vitro
analysis, the data were calculated as mean ± SD and were analyzed
using the Wilcoxon rank test. The relationship between mRNA
expression and clinicopathological factors was analyzed using the
χ2 test and Student’s t-test. Kaplan-Meier survival
curves were plotted and compared using the generalized log-rank
test. Univariate and multivariate analyses for identification of
factors prognostic of overall survival were performed using the Cox
proportional hazards regression model. All calculations were
performed using the JMP software (SAS Institute, Cary, NC, USA).
Differences with a p-value of <0.05 were considered
statistically significant.
Results
TGF-β stimulates EMT
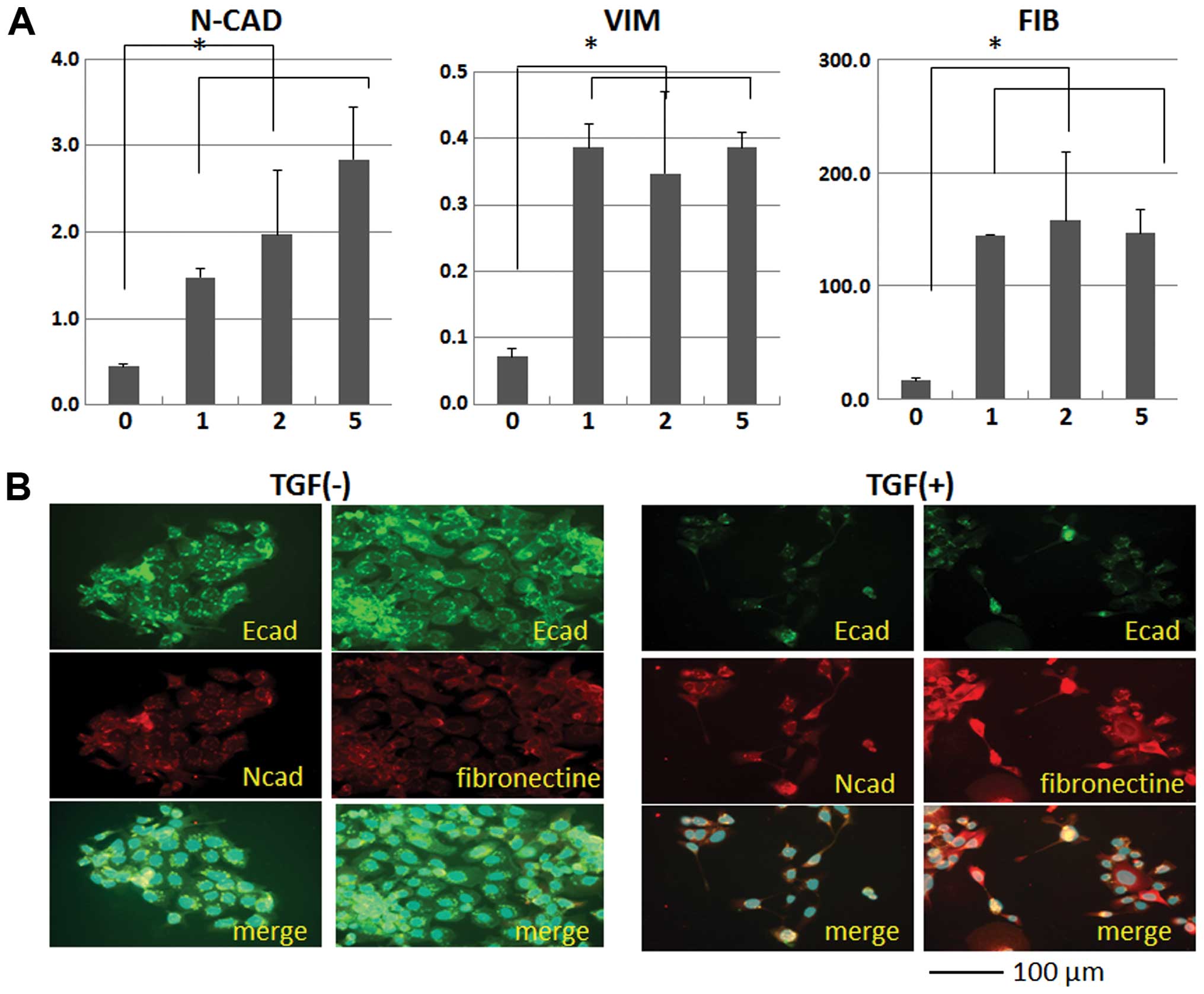
To study the EMT mechanism, we cultured the
colorectal cancer cell lines CaR-1 and CCK81 in the medium
containing TGF-β. The data from quantitative PCR indicated that
expression of EMT markers such as N-cadherin, vimentin, and
fibronectin was increased in a dose-dependent manner with TGF-β
concentration in the culture medium (Fig. 1A). Immunohistochemical analysis
indicated that expression of these genes was increased after
exposure to TGF-β (Fig. 1B). The
data show that EMT was induced in the cell lines we examined under
these conditions.
TGF-β increases CD24 expression in
colorectal cancer cells
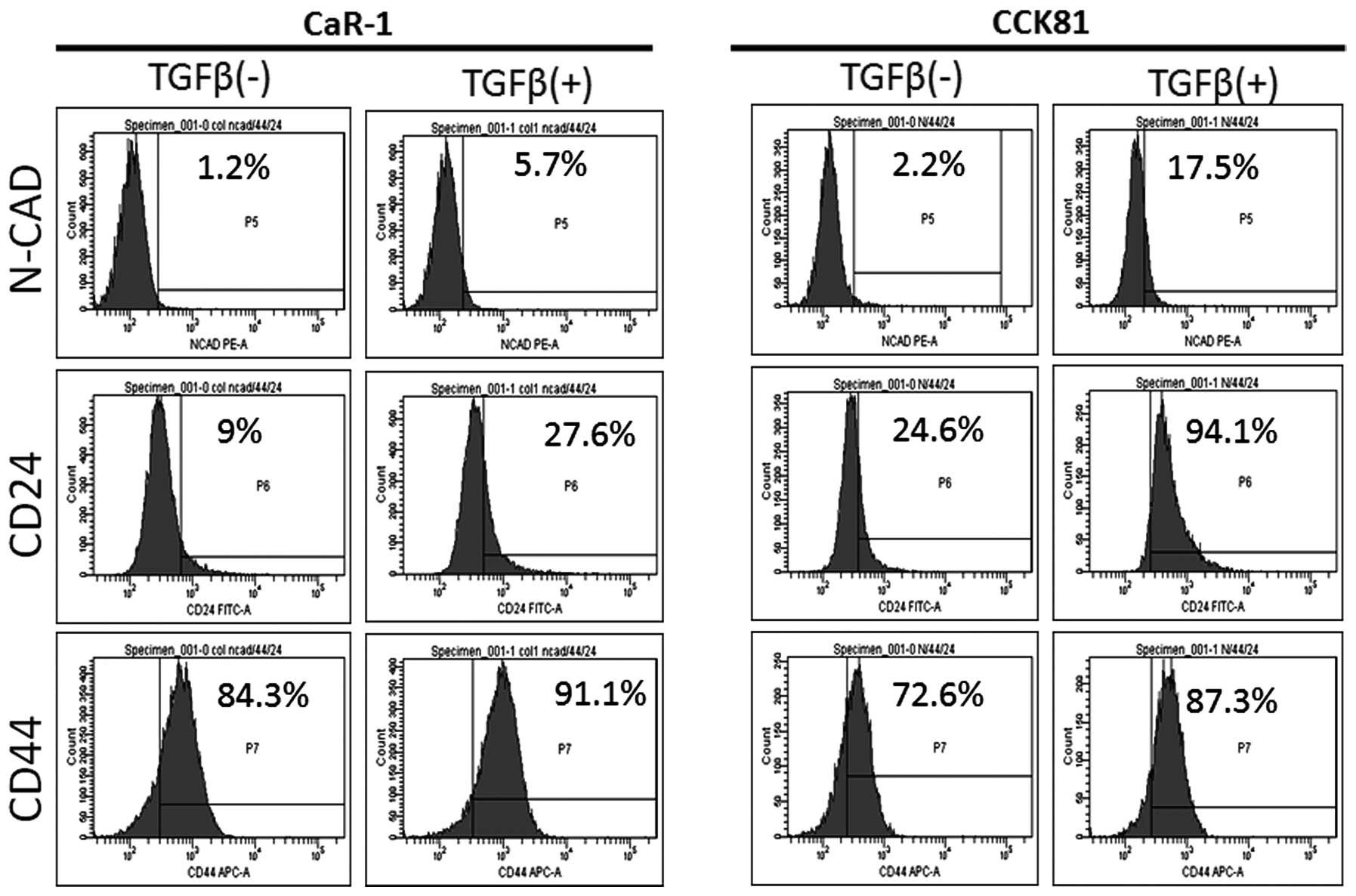
To study the effect of TGF-β, we investigated the
expression of CSC markers CD44, CD24, and N-cadherin. The data
indicated that the expression of these markers was increased after
TGF-β exposure; the effect was strong in CD24 compared with the
other two markers (Fig. 2).
Accordingly, in subsequent experiments, we focused on CD24.
CD24 is enriched in the EMT cells
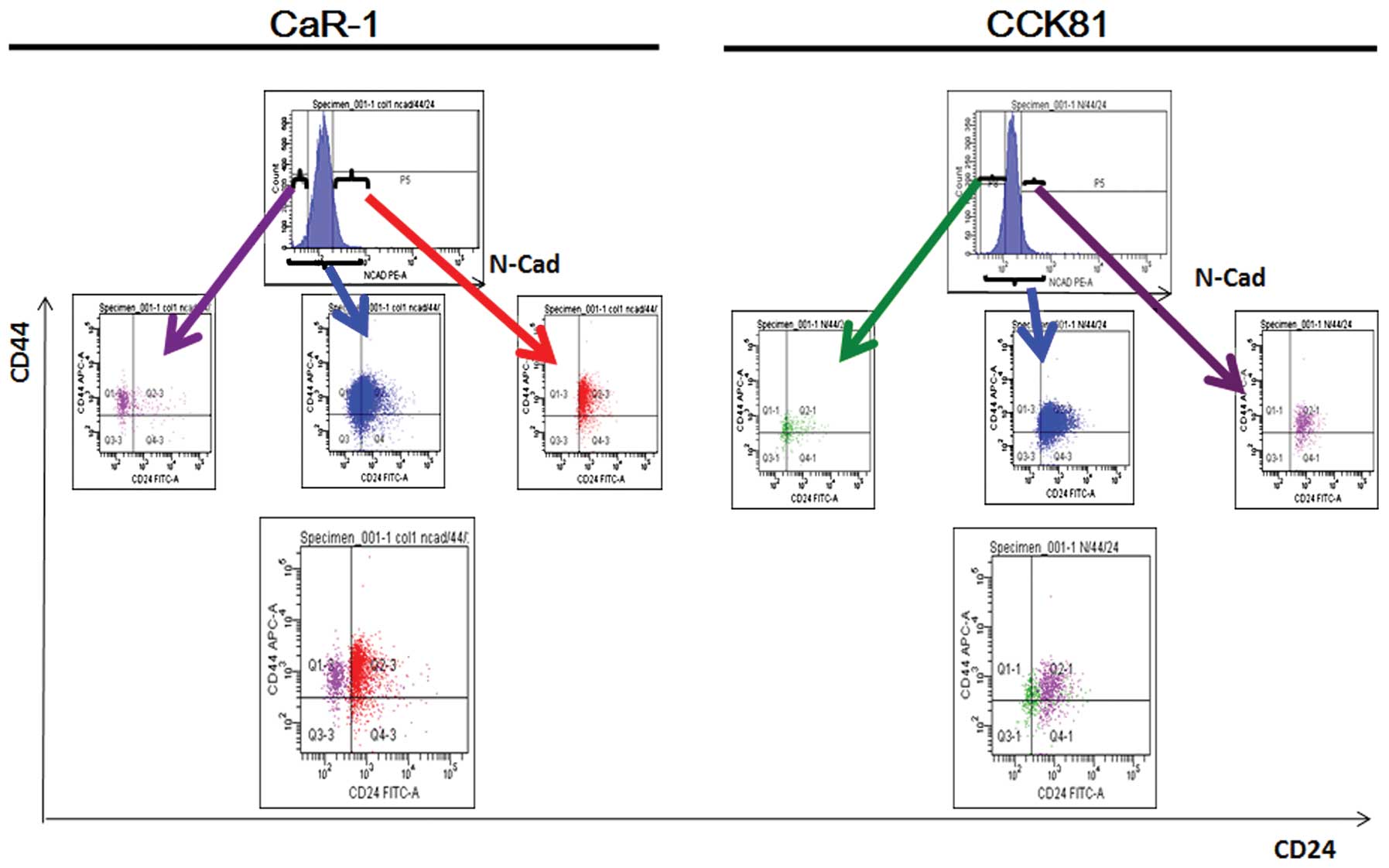
We were interested in whether CD24+ cells
are susceptible to TGF-β-induced EMT. To this end, we subjected the
colorectal cancer cell lines CaR-1 and CCK81, to
fluorescence-activated cell sorting (FACS) and analyzed the
results. The data showed that EMT-primed cells, which were marked
by the expression of N-cadherin, were enriched in CD24+
cells (Fig. 3). The data were
consistent between the two cell lines, suggesting that CD24 is a
marker of EMT.
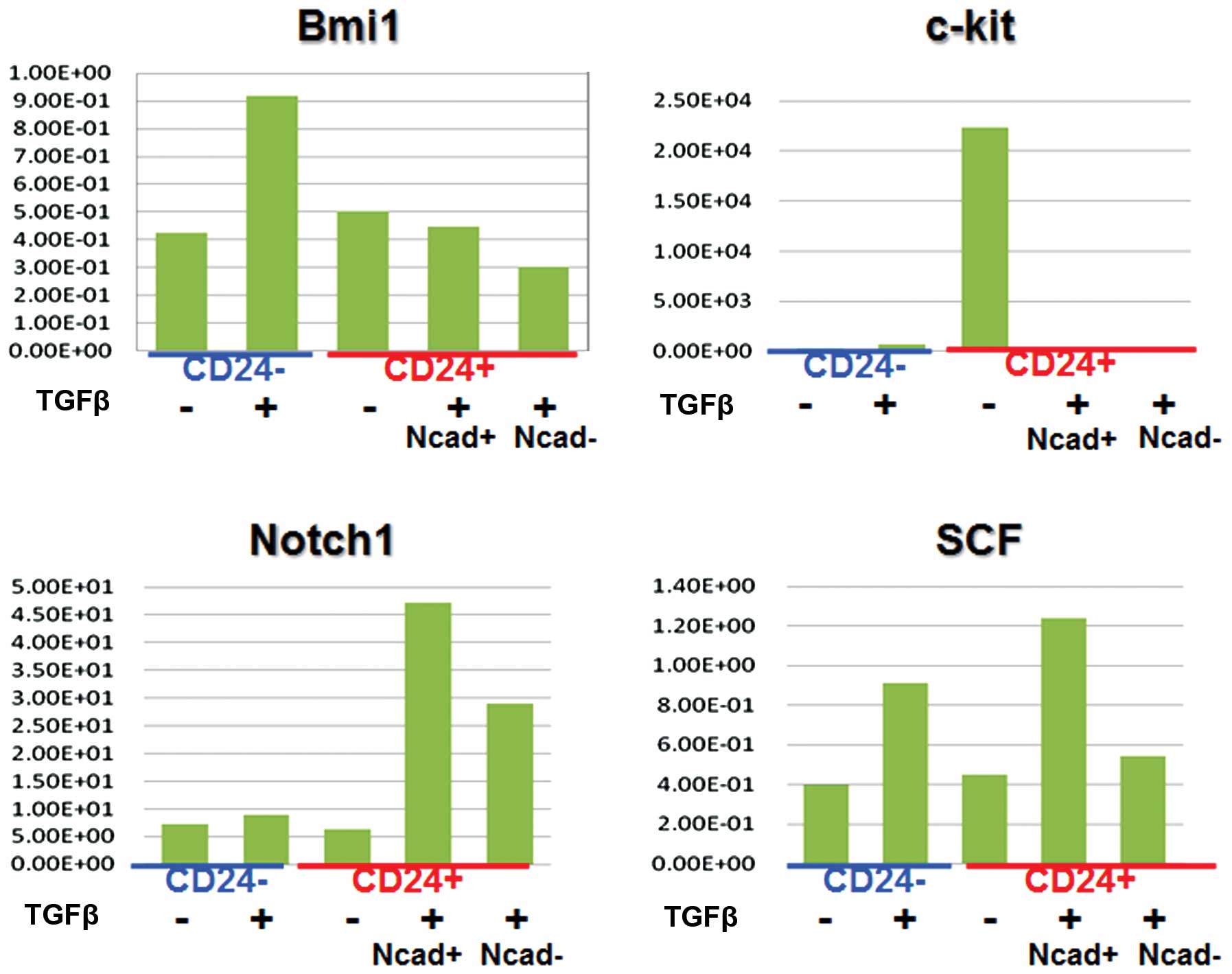
CD24 stem markers
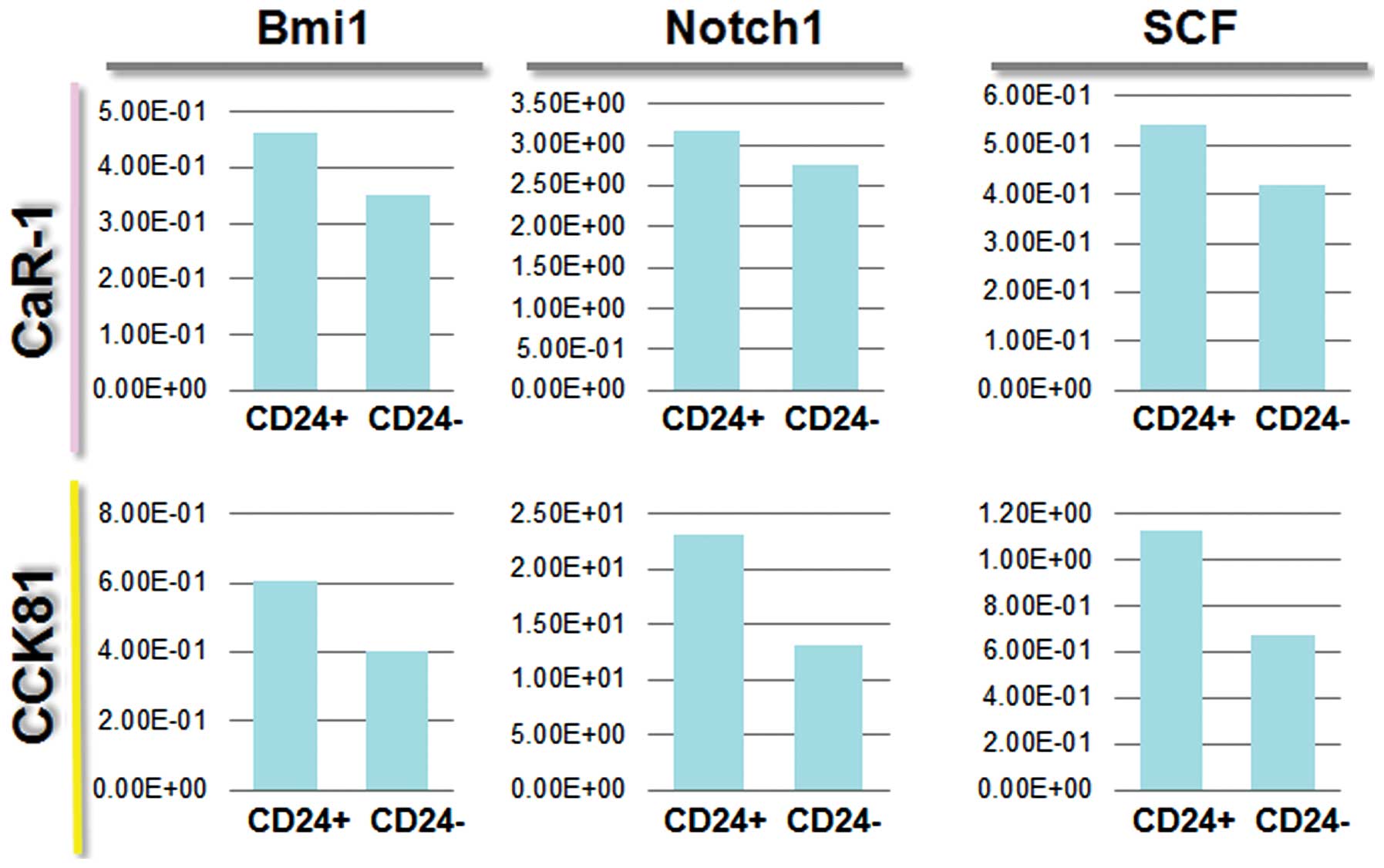
We wanted to identify the molecules expressed in
CD24+ cells, which could be associated with cancer
stemness. We analyzed the expression of several markers, including
c-kit, Bmi1, SCF, and Notch 1 (Fig.
4). Then we assessed the effect of adding TGF-β (EMT inducer)
to cell culture medium on each separate cell population by FACS
sorting. The data on Notch 1 expression indicated that
CD24+ cells are likely to be CSCs. CD24+
cells responded to TGF-β, and this effect was more appreciable in
N-cadherin+ cells (Fig.
5), suggesting that CD24+ cells are prone to EMT,
and CD24+ N-cadherin+ cells are more
sensitive to TGF-β than are CD24+N-cadherin−
cells. The data showed that the expression of Notch 1 correlated
with expression of CD24, suggesting that there was a link between
the CD24+ CSCs and the Notch 1 pathway.
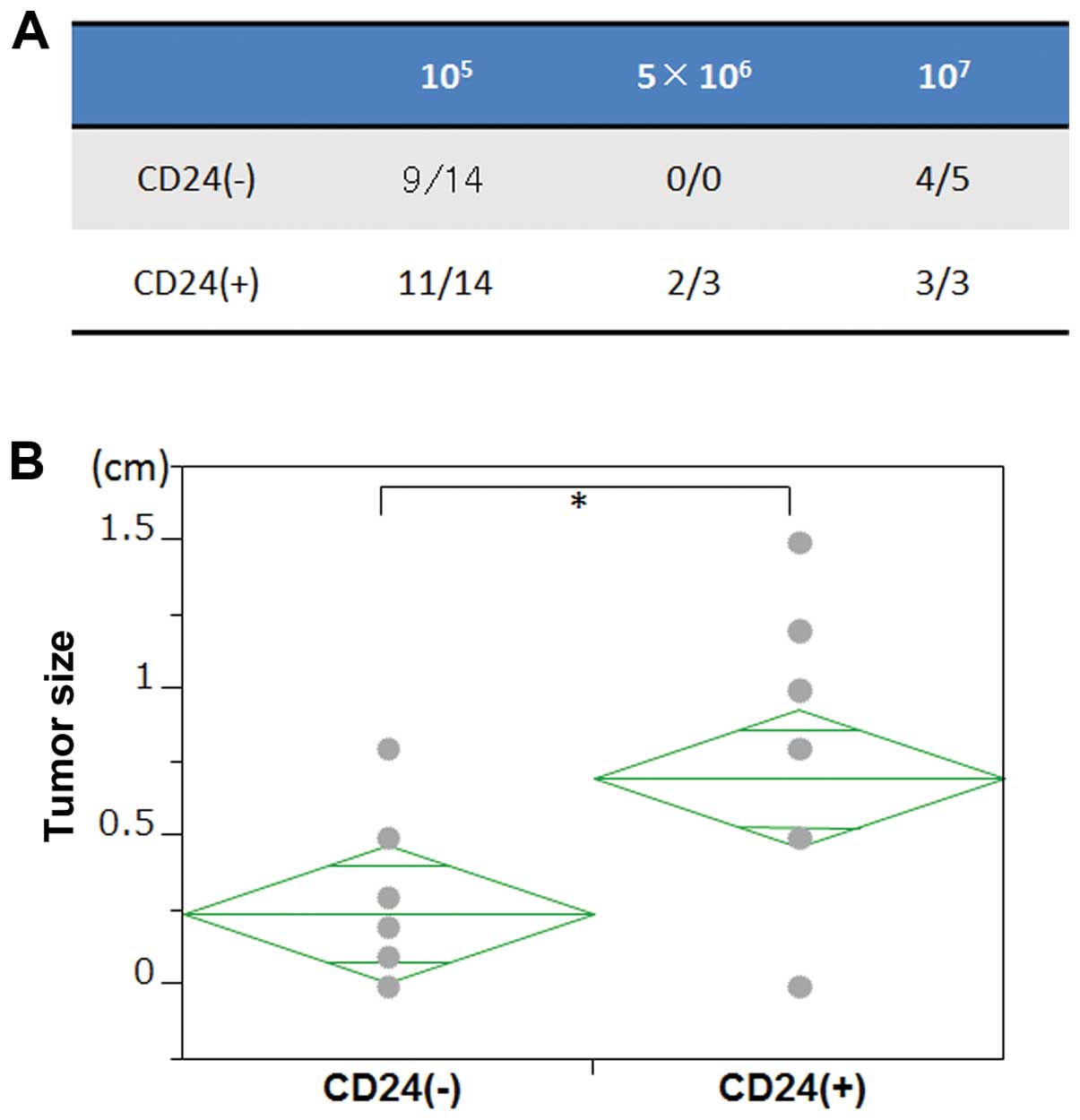
CD24+ cells show high
tumorigenic activity
To assess the tumorigenic potential of
CD24+ cells, we injected the cancer cells into
immunocompetent NOD/SCID mice subcutaneously. The results showed
that CD24+ cancer cells had a higher tumorigenic
potential compared with CD24− cells with respect to both
tumor frequency and tumor size (Fig.
6). These data suggest that in our experimental model,
CD24+ cells drive tumorigenicity, which is one of the
characteristics of CSCs.
Discussion
Previous studies pointed to the candidacy of CD24 as
a CSC marker in colorectal cancer (20.21). The present study shows
that CD24+ colonic cancer cells increase in number after
exposure to TGF-β in culture, compared with N-CAD+ and
CD44+ cells, suggesting that CD24+ cells are
susceptible to EMT, a cellular trait important for cancer
metastasis. The data were consistent between the two cell lines
that were studied, CaR-1 and CCK81. The cell sorting experiment
indicated that CD24 is a more useful marker than CD44, for
separation of EMT-prone cells, according to assessment of the
expression of N-CAD, a marker of EMT. The present study indicates
that CD24 is associated with EMT, and the association is more
pronounced compared with other possible markers, such as CD44. The
findings are compatible with the data from other cell lines
(23).
Because CD24+ cancer cells formed larger
tumors in immunocompetent NOD/SCID mice, we determined if any
stemness markers were expressed preferentially in CD24+
cells. The FACS experiment indicated that exposure to TGF-β in
culture resulted in increased Notch 1 expression. Recent studies
have indicated that Notch signaling has a critical role at the
intersection of EMT and cancer stemness and that Notch inhibition
is an attractive strategy for the treatment of several cancers, at
least in part because of its ability to reverse or prevent EMT
(24).
A previous study indicated that TGF-β is a possible
niche signal in the bone marrow to induce hibernation of
hematopoietic stem cells (25), a
dormant phenotype of cancer cells, showing resistance to
chemotherapy. The hibernation state is associated with inhibition
of lipid raft clustering; this change results in inhibition of
signaling of growth factors or cytokines through cell surface
receptors (25). The study of
Listeria monocytogens indicated that CD14 and CD24, which normally
exhibit uniform distribution on cells undergo clustering upon
treatment with the stimulation (26); the phenomenon is suggestive of
lipid raft clustering and signaling through CD24. A recent study of
protein clustering showed enrichment of CD24 in lipid rafts and a
more random distribution of CD44 in the plasma membrane (27). Taken together, the data are
indicative of the significance of CD24 as a functional marker of
CSCs and suggest that this protein is a possible therapeutic target
in colorectal cancer.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science, and Technology; a Grant-in-Aid from the Third
Comprehensive 10-year Strategy for Cancer Control, Ministry of
Health, Labor and Welfare; a grant from the Kobayashi Cancer
Research Foundation; a grant from the Princess Takamatsu Cancer
Research Fund; a grant from the Senshin Medical Research
Foundation; a grant from the National Institute of Biomedical
Innovation, Japan.
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dewi DL, Ishii H, Kano Y, Nishikawa S,
Haraguchi N, Sakai D, Satoh T, Doki Y and Mori M: Cancer stem cell
theory in gastrointestinal malignancies: recent progress and
upcoming challenges. J Gastroenterol. 46:1145–1157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishii H, Iwatsuki M, Ieta K, Ohta D,
Haraguchi N, Mimori K and Mori M: Cancer stem cells and
chemoradiation resistance. Cancer Sci. 99:1871–1877. 2008.
View Article : Google Scholar
|
|
4
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard
GF, Doki Y and Mori M: CD13 is a therapeutic target in human liver
cancer stem cells. J Clin Invest. 120:3326–3339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HM, Haraguchi N, Ishii H, Ohkuma M,
Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, Doki
Y and Mori M: Increased CD13 expression reduces reactive oxygen
species, promoting survival of liver cancer stem cells via an
epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol.
(Suppl 3): S539–S548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell
LL, Polyak K, Brisken C, Yang J and Weinberg RA: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wulf GG, Wang RY, Kuehnle I, Weidner D,
Marini F, Brenner MK, Andreeff M and Goodell MA: A leukemic stem
cell with intrinsic drug efflux capacity in acute myeloid leukemia.
Blood. 98:1166–1173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
15
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piccirillo SG, Reynolds BA, Zanetti N,
Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi
AL: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: an enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponti D, Zaffaroni N, Capelli C and
Daidone MG: Breast cancer stem cells: an overview. Eur J Cancer.
42:1219–12124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
King JB, von Furstenberg RJ, Smith BJ,
McNaughton KK, Galanko JA and Henning SJ: CD24 can be used to
isolate Lgr5+ putative colonic epithelial stem cells in
mice. Am J Physiol Gastrointest Liver Physiol. 303:G443–G452. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke J, Wu X, Wu X, He X, Lian L, Zou Y, He
X, Wang H, Luo Y, Wang L and Lan P: A subpopulation of
CD24+ cells in colon cancer cell lines possess stem cell
characteristics. Neoplasma. 59:282–288. 2012.
|
|
24
|
Espinoza I and Miele L: Deadly crosstalk:
Notch signaling at the intersection of EMT and cancer stem cells.
Cancer Lett. 341:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamazaki S, Iwama A, Takayanagi S, Eto K,
Ema H and Nakauchi H: TGF-beta as a candidate bone marrow niche
signal to induce hematopoietic stem cell hibernation. Blood.
113:1250–1256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gekara NO and Weiss S: Lipid rafts
clustering and signalling by listeriolysin O. Biochem Soc Trans.
32:712–714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu X, Wang J, Feizpour A and Reinhard BM:
Illuminating the lateral organization of cell-surface CD24 and CD44
through plasmon coupling between Au nanoparticle immunolabels. Anal
Chem. 85:1290–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|