Telomerase is a reverse transcriptase that carries
its own template and synthesizes DNA telomere repeats to maintain
telomere length. These repeats are composed of 1000–2000 non-coding
tandem repeats of the TTAGGG sequence and serve as protective
‘caps’ at the ends of chromosomes, protecting the chromosomes from
degradation and thereby maintaining chromosome stability, enhancing
cell proliferation and promoting cell immortality (1–5). In
most cell types, after each round of DNA replication, the telomeres
are shortened. However, telomere length is stabilized by the
telomerase enzyme in some stem cells, and telomerase activation is
a very common occurrence in tumor cells (6–9). In
humans, the active telomerase is composed of two components: i)
human telomerase RNA (hTR), which contains the template for reverse
transcription and is expressed in most cells; and ii) human
telomerase reverse transcriptase (hTERT), which is a reverse
transcriptase that catalytically synthesizes telomere DNA. hTERT
expression seems to be restricted to telomerase-positive tissues,
which indicates that hTERT is the limiting factor for telomerase
activity (10–13). Recently, evidence was shown that
hTERT alone is sufficient to restore telomerase activity and this
restoration results in tumorigenesis in telomerase negative cells,
such as epithelial cells and human fibroblasts (14–16).
Tumors express high levels of hTERT (80–90%) (17), suggesting that the reverse
transcriptase activity of hTERT plays an important role in tumor
occurrence and development.
Most research on hTERT has been focused on its
crucial function of telomere maintenance. However, there are many
phenomena that cannot be explained by its reverse transcriptase
activity. Recent research has discovered that hTERT has other
functions unrelated to its reverse transcriptase activity, such as
increasing the anti-apoptotic capacity of cells, enhancing DNA
repair, maintaining stem cells and regulating gene expression
(18). Non-canonical roles of
hTERT have also been revealed (19). These non-canonical roles of hTERT
are referred to as its non-reverse transcriptase activity. We
review the role and mechanisms of the non-reverse transcriptase
activity of hTERT in tumor progression.
Cellular immortalization is recognized as a major
hallmark of cancer and it is generally accepted as a necessary step
in the cancer initiation process (20). Mounting evidence suggests that
telomerase plays an important role in cellular immortalization and
oncogenesis. The number of telomeres determines the proliferative
capacity of the cell and hTERT plays a key role in maintaining
telomere length. Cellular senescence is due to telomere shortening,
and immortalization strategies typically include forced expression
of hTERT (21). In a mouse model,
mouse telomerase reverse transcriptase (mTert) could immortalize
wild-type (WT) and Nmp4-deletion bone marrow stromal cells, causing
them to exhibit sustained growth. In other mammals and in humans,
expression of exogenous hTERT in bone marrow mesenchymal stem cells
(MSCs) resulted in immortalization (22–26).
A recent study indicated that introduction of hTERT alone was
sufficient for the immortalization of human mammary epithelial
cells (hMECs) grown in specialized media (27).
These previous studies demonstrate that cellular
immortalization is a result of hTERT extending telomere length
through its reverse transcriptase activity and telomere maintenance
is an important aspect of the biological process of
immortalization. Nonetheless, recent data have shown that the
reverse transcriptase activity of hTERT is not necessary for cell
proliferation and immortalization. Stewart et al showed that
ectopic expression of hTERT in the GM847 immortal cell line
imparted a tumorigenic phenotype. This outcome was also observed
after introduction of a mutant hTERT that was incapable of
maintaining telomere length. This indicates that hTERT has an
additional function that is required for tumorigenesis but does not
depend on its ability to maintain telomeres (28).
In 2004, it was shown that in a maturation-resistant
acute promyelocytic leukemia (APL) cell line, overexpression of
hTERT imparted protection from apoptosis induced by tumor necrosis
factor (TNF) or TNF-related apoptosis-inducing ligand (TRAIL)
following all-trans retinoic acid (ATRA) treatment, and this
function was independent of telomerase activity on telomeres
(29). Beliveau et al found
that the enhanced cell proliferation ability of HMECs after hTERT
overexpression does not rely on its reverse transcriptase function,
but on its ability to modulate the DNA damage response (DDR), which
in turn suppresses apoptosis (30). These findings provide a previously
unknown mechanistic explanation for the observation that
exogenously expressed hTERT offers growth advantages to cells
without the basic functions of its enzyme activity, indicating that
hTERT has growth regulatory properties independent of its role in
telomere maintenance.
hTERT has also been shown to be involved in
mitochondrial apoptosis induced by targeted inhibition of Bcl-2. In
addition, hTERT mutants, which are catalytically and biologically
inactive, showed similar behavior as the wild-type form, indicating
that hTERT inhibited apoptosis regardless of its telomerase
activity and its ability to lengthen telomeres (31). hTERT has also been found to
activate Wnt signaling, therefore causing target genes to promote
cell proliferation and induce carcinogenesis in normal epithelia
(32,33). Carcinogenesis was chemically
induced in TERT-positive and TERT-negative mice and their risk of
skin cancer was analysed. The mice with high levels of TERT
expression had a significantly higher risk of skin cancer than the
hTERT-negative mice, but the length of their telomeres was not
changed (34). Mukherjee et
al found that the ability of the hTERT to enhance cell
proliferation can be uncoupled not only from telomere elongation
but also from other telomerase activities. The cellular lifespan
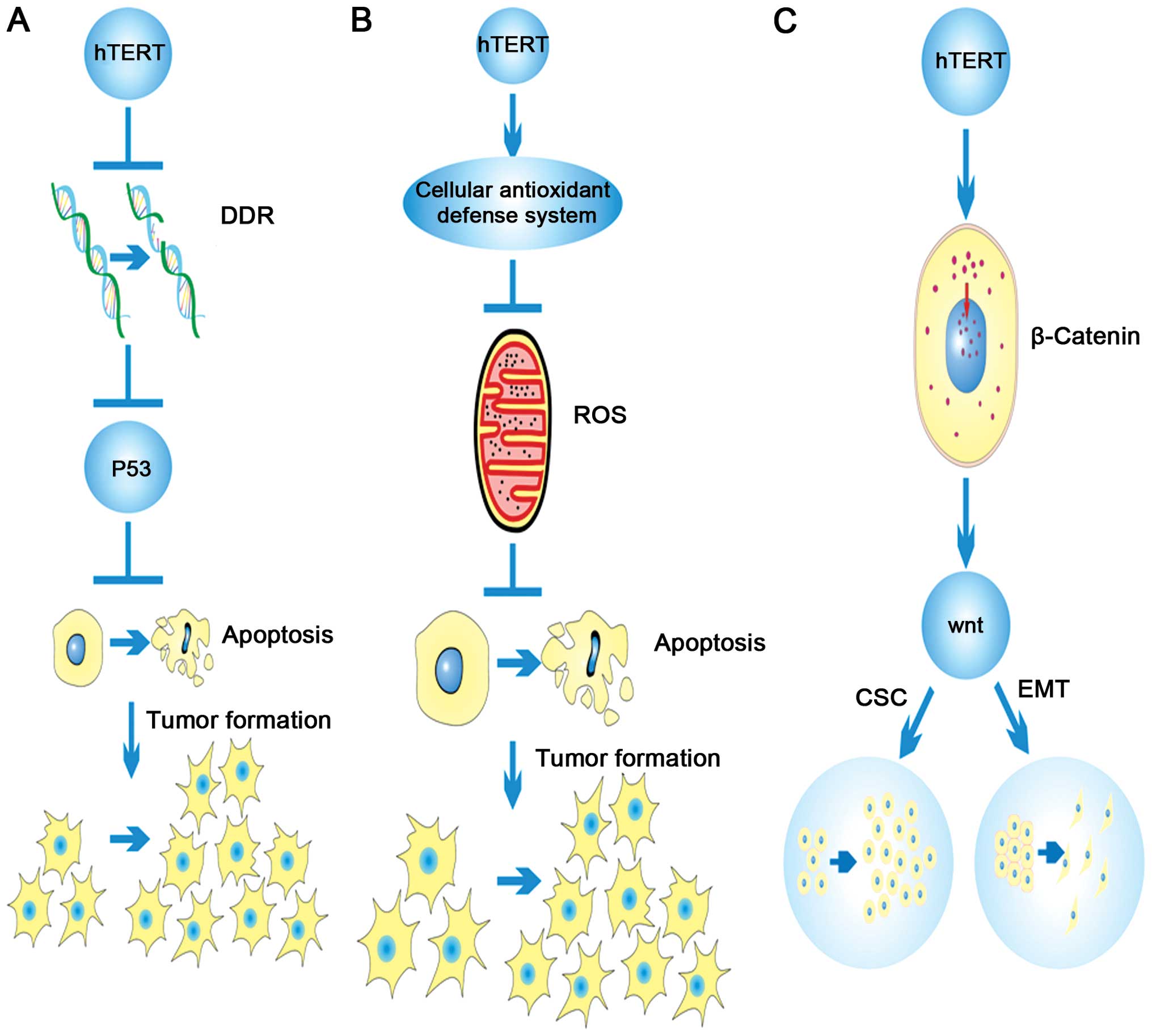
extension was found to be due to hTERT regulating DNA damage
responses (Fig. 1A) and reducing
RNA processing endoribonucleases (RMRP) (35). hTERT can also alleviate basal
cellular reactive oxygen species (ROS) levels by potentiating the
cellular antioxidant defense systems, (Fig. 1B), thus allowing cancer cells to
evade death stimuli (36).
The data presented here, together with other recent
evidence underscore that there are broad biological consequences of
hTERT expression aside from its essential function in telomere
maintenance. The non-reverse transcriptase activity of hTERT plays
a very important role in tumor formation; this effect is
independent of the reverse transcriptase activity of hTERT, and
telomere extension is not necessary for cell immortalization and
tumor formation.
If superior proliferation ability is the main
feature of early primary tumors, then metastasis is the main
feature of end-stage cancer. Metastasis directly threatens the
lives of cancer patients and is the cause of 90% of cancer deaths
(37). The multi-step process of
tumor invasion and metastasis, referred to as the
invasion-metastasis cascade, includes loss of cellular adhesion,
increased motility, entry into and survival in the circulation,
exit into new tissue and eventual colonization at a distant site
(38,39). Tumor invasion and metastasis are
associated with a variety of factors and processes, including: the
epithelial mesenchymal transition (EMT), heterotypic contributions
of stromal cells and plasticity in the invasive growth program. EMT
plays a critical role in cancer metastatic progression and it has
been postulated to be an absolute requirement for tumor invasion
and metastasis (40–43). EMT refers to the physiological and
pathological situations occurring during cell
epithelial-mesenchymal transition, accompanied by cell morphology
and gene expression changes. It is characterized by the loss of
epithelial proteins, including E-cadherin, γ-catenin and β-catenin,
and is often accompanied by the increase of mesenchymal proteins
such as vimentin, fibronectin and smooth muscle actin (19,44).
E-cadherin expression is a marker of epithelial
cells and it is an initiating factor for EMT. The downregulation,
inhibition, or loss of function of E-cadherin can activate EMT.
E-cadherin also helps maintain cancer cell adhesion to prevent
tumor invasion and metastasis. A variety of factors have been shown
to regulate E-cadherin, including somatic mutations, promoter
hypermethylation, the Snail protein and the ZEB family (45).
Evidence shows that hTERT can promote the metastasis
of cells and this capability may be independent of its non-reverse
transcriptase activity. Upon hTERT transfection into U2OS
osteosarcoma cells, a telomerase-negative cell line, the invasion
and metastasis of tumor cells were increased (46). In human esophageal squamous cell
cancer, hTERT activation increased migration and invasion when
compared with control cells. It has been shown that hTERT regulates
the glycolytic pathway in melanoma cells, improving the energy
supply state of the tumor cells thus contributing to tumor invasion
and metastasis (47,48). Recent studies have indicated that
exogenous expression of hTERT also leads to upregulation of MMP9
and RhoC and promotes the invasiveness and metastasis of HepG2
cells in vitro (49).
hTERT promotes not only tumor formation, but also
tumor metastasis. Therefore, it is possible that hTERT promotes
tumor metastasis through the EMT pathway. Transfection of TERT into
Xenopus caused faster embryonic limb and neuron development
compared to controls, and promoting embryonic development is one of
the three main functions of EMT, which also plays a central role in
embryogenesis (32,40,50).
It has also been demonstrated that hTERT can affect TGF-β1-mediated
β-catenin induction and nuclear accumulation, which enhances Wnt
signaling pathway activation and promotes EMT (51) (Fig.
1C). hTERT can form a complex with the brahma-related gene 1
(BRG1) and nucleostemin (NS) through upregulation of Twist to
increase EMT and this complex does not directly contribute to
telomere maintenance (52)
(Fig. 1C). In summary, hTERT plays
a role in tumor invasion and metastasis by promoting EMT and this
function is independent of its reverse transcriptase activity.
In recent years, the theory of cancer stem cells
(CSCs) has provided a more reasonable explanation for the formation
and recurrence of malignant tumor metastasis and chemotherapy
resistance. CSCs are a subset of tumor cells that have the ability
to self-renew and generate the diverse cells that form the tumor
(53,54). Evidence suggests that most solid
tumors are hierarchically organized and sustained by CSCs (55). Some scholars believe that the
existence of CSCs leads to the failure of cancer treatment.
Therefore, studying the mechanisms of regulation of CSCs and
targeting CSCs for therapy may be a promising area for finding a
cure for cancer (56). Park et
al showed that transduction of hTERT, SV40 large T antigen and
four transcription factors (OCT4, SOX2, MYC and KLF4) resulted in a
higher frequency of human pluripotent stem (iPS) cell colony
formation (57). A study showed
that human mammary progenitor cells rendered immortal using hTERT
retain both self-renewal and differentiation capacity along the
luminal and myoepithelial lineages (58). It is known that EMT and CSCs have
some common features, EMT generates cancer cells with stem
cell-like characteristics; stem-like cells express markers
associated with EMT; and the diversity and abundance of CSCs in
solid tumors allows cells the ability to undergo EMT. Mainly by
non-cancer stem cells, but under certain conditions non-tumor stem
cells can also adopt cancer stem cell characteristics via EMT
(43,59–61).
Because hTERT can promote EMT through its
non-reverse transcriptase activity, it may contribute to CSC
maintenance. CD133, a marker of CSCs, was found to be more highly
expressed in hTERT-immortalized cells than in primary prostate
cells. Stem cell properties were increased when SV40ER and hTERT
were introduced into breast cancer cells (62,63).
Castelo-Branco et al found that CSCs had significantly
higher levels of hTERT expression than normal tissue stem cells,
but Southern blot analysis revealed that CSCs had extremely short
telomeres compared with the normal tissue stem cells (64). In a gastric cancer (GC) model,
hTERT has been shown to induce stem-like activity of cancer cells,
and this activity is independent of its telomere-lengthening
function (51). Further research
defines a complex composed of TERT, BRG1 and NS that maintains the
function of CSCs, and this interaction is independent of telomerase
activity (52). Wnt signaling
activity functionally designates the colon CSC population and is a
marker for colon CSCs. As described previously, hTERT can activate
the Wnt signaling pathway. Therefore, hTERT regulates tumor stem
cell maintenance through the Wnt signaling pathway (32,65)
(Fig. 1C). The above data
demonstrate that hTERT contributes to the maintenance of CSCs
through its non-reverse transcriptase activity.
It is known that the majority of tumor cells express
high levels of hTERT and that normal somatic cells do not express
hTERT. Therefore, telomerase has been considered as a tumor marker
and an attractive target for anticancer therapy for many types of
cancer (66). When the hTERT
promoter is replaced by an adenoviral promoter to construct
cytolytic adenovirus, it can efficiently infect tumor cells and
significantly inhibit the growth of hepatoma cells. Experiments in
nude mice showed that this adenovirus can reduce the formation of
tumor nodules by lung cancer cells, and this was associated with
low liver toxicity (67). In
addition, because of the tumor-specific expression of hTERT, some
researchers believe that hTERT is a tumor-associated antigen.
Studies have shown that hTERT fragments act as antigens in mice,
and CD8+ and CD4+ are stimulated for
expansion (68,69). GV1001, which is a 16-amino acid MHC
class II-restricted hTERT peptide vaccine, consists of amino acids
611–626 (EARPALLTSRLRFIPK) of the hTERT active site (70,71).
GV1001 has shown good antitumor efficacy in patients in phase I and
II clinical trials (72,73). In addition, a potent hTERT
inhibitor 2-[(E)-3-naphthalene-2-yl-but-2-enoylamino]-benzoic acid
(BIBR1532) specifically blocks the elongation of telomerase DNA,
therefore resulting in cellular senescence and inhibition of
proliferation (74,75).
Because hTERT promoter regulation very tightly
controls telomerase activity, directly targeting the hTERT promoter
may be an effective method for tumor therapy (76,77).
When telomerase-positive cells were treated with an hTERT-driven
prodrug-activating enzyme which could repress the hTERT promoter,
the cells became apoptotic (78–80).
However, targeting hTERT has some problems: i) hTERT activity may
not be detected in the whole of the tumor, therefore it may not be
sensitive to targeted therapy; ii) When hTERT is inhibited, the
telomere length shortens over a period of time and tumor apoptosis
may have a lag; iii) Despite inhibition of hTERT reverse
transcriptase activity, some tumors evade apoptosis through other
mechanisms, such as using the alternative lengthening of telomeres
(ALT) pathway or activating mitochondrial adaptive mechanisms
(81,82).
Targeting the non-reverse transcriptase activity of
hTERT may solve the problems noted above. First, the hTERT
non-reverse transcriptase activity is unrelated to its telomerase
activity, so if the cell has no telomerase activity, the hTERT
non-reverse transcriptase activity can be targeted for tumor
therapy. Second, because targeting the non-reverse transcriptase
activity of hTERT does not shorten telomere length, there would be
no lag effect. Finally, targeting the hTERT non-reverse
transcriptase activity will not activate other pathways that
promote tumor proliferation and metastasis. Telomerase
immunotherapy is currently an area of active research focus.
Therapeutic resistance is an issue to be considered, especially
because of the existence of ALT mechanisms to maintain telomeres
(83). Inhibition of the
non-reverse transcriptase activity of hTERT for anticancer therapy
can be used as a supplement for telomerase therapy and may even
completely replace it in some tumors.
Telomerase plays an important role in the
maintenance, protection and stabilization of chromosomes, but these
diverse roles can lead to opportunities for cancers to activate
hTERT reverse transcriptase activity during tumorigenesis and
escape cell senescence (84). Most
cancer cells express hTERT, underscoring the importance of efforts
to understand its mechanisms of regulation, its implications for
cell survival and cancer therapy resistance, and its interaction
with other signaling pathways.
In recent years, studying the reverse transcriptase
activity of hTERT has been a prime research area and inhibition of
telomerase activity has become a popular treatment. hTERT
expression provides valuable information for early tumor diagnosis,
staging and prognosis. However, some studies show that hTERT has
novel functions that are independent of its reverse transcriptase
activity. These include inducing tumor formation, increasing cell
proliferation, promoting tumor metastasis and maintaining CSCs.
These new findings will allow us to better understand the function
of hTERT, and its alternative functions help clarify the
unexplained phenomena that are not due to its reverse transcriptase
activity.
The hTERT non-reverse transcriptase activity
mechanism will open up new avenues for tumor therapy. Inhibition of
hTERT non-reverse transcriptase activity has the potential to be an
efficient and low toxicity method of cancer treatment. However,
hTERT activity and its regulatory mechanisms and pathways are
complex and diverse. Therefore, we still face many problems and
challenges. Research on the non-reverse transcriptase activity of
hTERT is still in its early stages, and there are many unanswered
questions remaining, such as how the non-reverse transcriptase
activity of hTERT affects tumor occurrence, proliferation,
metastasis and CSC maintenance. Its mechanisms will require further
study. For example, it is unknown whether the non-reverse
transcriptase activity of hTERT is involved in normal cell division
and proliferation, stem cell differentiation and embryonic
development. The hTERT non-reverse transcriptase activity promotes
tumor development through multiple mechanisms, so the development
of targeted therapy is a complex issue that merits further
study.
In summary, there is mounting evidence that hTERT
has different roles when it associates with different factors or is
targeted to different cellular locations away from telomeres. New
functions of hTERT are only beginning to be elucidated. We plan to
further study the non-reverse transcriptase activity of hTERT and
determine its pro-cancer development mechanism and how it can be
therapeutically targeted. We hope this research would help improve
the efficiency of cancer treatment, reduce drug doses to lower the
cytotoxicity in normal cells and eventually lead to a cancer
cure.
This work was supported by the National Nature
Science Foundation of China (No. 81272689).
|
1
|
Liu L, Lai S, Andrews G and Tollefsbol TO:
Genetic and epigenetic modulation of telomerase activity in
development and disease. Gene. 340:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu L, Saldanha SN, Pate MS, Andrews LG
and Tollefsbol TO: Epigenetic regulation of human telomerase
reverse transcriptase promoter activity during cellular
differentiation. Genes Chromosomes Cancer. 41:26–37. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blackburn EH: Telomerase and cancer: Kirk
A. Landon - AACR prize for basic cancer research lecture. Mol
Cancer Res. 3:477–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blackburn EH, Greider CW and Szostak JW:
Telomeres and telomerase: the path from maize, Tetrahymena and
yeast to human cancer and aging. Nat Med. 12:1133–1138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shay JW, Zou Y, Hiyama E and Wright WE:
Telomerase and cancer. Hum Mol Genet. 10:677–685. 2001. View Article : Google Scholar
|
|
8
|
Greider CW: Telomerase activation. One
step on the road to cancer? Trends Genet. 15:109–112. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nugent CI and Lundblad V: The telomerase
reverse transcriptase: components and regulation. Genes Dev.
12:1073–1085. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng J, Funk WD, Wang SS, Weinrich SL,
Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC and Yu J: The
RNA component of human telomerase. Science. 269:1236–1241. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura TM, Morin GB, Chapman KB,
Weinrich SL, Andrews WH, Lingner J, Harley CB and Cech TR:
Telomerase catalytic subunit homologs from fission yeast and human.
Science. 277:955–959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smogorzewska A and de Lange T: Regulation
of telomerase by telomeric proteins. Annu Rev Biochem. 73:177–208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang JT, Chen YL, Yang HT, Chen CY and
Cheng AJ: Differential regulation of telomerase activity by six
telomerase subunits. Eur J Biochem. 269:3442–3450. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi DL, Ohhira T, Fujisaki C, Inoue T, Ohta
T, Osaki M, Ohshiro E, Seko T, Aoki S and Oshimura M:
Identification of PITX1 as a TERT suppressor gene located on human
chromosome 5. Mol Cell Biol. 31:1624–1636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gonzalez-Suarez E, Flores JM and Blasco
MA: Cooperation between p53 mutation and high telomerase transgenic
expression in spontaneous cancer development. Mol Cell Biol.
22:291–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahn WC, Counter CM, Lundberg AS,
Beijersbergen RL, Brooks MW and Weinberg RA: Creation of human
tumour cells with defined genetic elements. Nature. 400:464–468.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harley CB: Telomerase and cancer
therapeutics. Nat Rev Cancer. 8:167–179. 2008. View Article : Google Scholar
|
|
18
|
Cong Y and Shay JW: Actions of human
telomerase beyond telomeres. Cell Res. 18:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kan CY, Wen VW, Pasquier E, Jankowski K,
Chang M, Richards LA, Kavallaris M and MacKenzie KL: Endothelial
cell dysfunction and cytoskeletal changes associated with
repression of p16(INK4a) during immortalization. Oncogene.
31:4815–4827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tátrai P, Szepesi Á, Matula Z, Szigeti A,
Buchan G, Mádi A, Uher F and Német K: Combined introduction of
Bmi-1 and hTERT immortalizes human adipose tissue-derived stromal
cells with low risk of transformation. Biochem Biophys Res Commun.
422:28–35. 2012.PubMed/NCBI
|
|
22
|
Simonsen JL, Rosada C, Serakinci N,
Justesen J, Stenderup K, Rattan SI, Jensen TG and Kassem M:
Telomerase expression extends the proliferative life-span and
maintains the osteogenic potential of human bone marrow stromal
cells. Nat Biotechnol. 20:592–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao K, Lu YR, Wei LL, Lu XF, Li SF, Wan L,
Li YP and Cheng JQ: Immortalization of mesenchymal stem cells from
bone marrow of rhesus monkey by transfection with human telomerase
reverse transcriptase gene. Transplant Proc. 40:634–637. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang GP, Pan ZJ, Huang JP, Yang JF, Guo
CJ, Wang YG, Zheng Q, Chen R, Xu YL, Wang GZ, Xi YM, Shen D, Jin J
and Wang JF: Proteomic analysis of human bone marrow mesenchymal
stem cells transduced with human telomerase reverse transcriptase
gene during proliferation. Cell Prolif. 41:625–644. 2008.
View Article : Google Scholar
|
|
25
|
Wei LL, Gao K, Liu PQ, Lu XF, Li SF, Cheng
JQ, Li YP and Lu YR: Mesenchymal stem cells from Chinese Guizhou
minipig by hTERT gene transfection. Transplant Proc. 40:547–550.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alvarez MB, Childress P, Philip BK,
Gerard-O’Riley R, Hanlon M, Herbert BS, Robling AG, Pavalko FM and
Bidwell JP: Immortalization and characterization of osteoblast cell
lines generated from wild-type and Nmp4-null mouse bone marrow
stromal cells using murine telomerase reverse transcriptase
(mTERT). J Cell Physiol. 227:1873–1882. 2012. View Article : Google Scholar
|
|
27
|
Zhao XS, Malhotra GK, Lele SM, Lele MS,
West WW, Eudy JD, Band H and Band V: Telomerase-immortalized human
mammary stem/progenitor cells with ability to self-renew and
differentiate. Proc Natl Acad Sci USA. 14146–14151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stewart SA, Hahn WC, O’Connor BF, Banner
EN, Lundberg AS, Modha P, Mizuno H, Brooks MW, Fleming M, Zimonjic
DB, Popescu NC and Weinberg RA: Telomerase contributes to
tumorigenesis by a telomere length-independent mechanism. Proc Natl
Acad Sci USA. 12606–12611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dudognon C, Pendino F, Hillion J, Saumet
A, Lanotte M and Ségal-Bendirdjian E: Death receptor signaling
regulatory function for telomerase: hTERT abolishes TRAIL-induced
apoptosis, independently of telomere maintenance. Oncogene.
23:7469–7474. 2004. View Article : Google Scholar
|
|
30
|
Beliveau A, Bassett E, Lo AT, Garbe J,
Rubio MA, Bissell MJ, Campisi J and Yaswen P: p53-dependent
integration of telomere and growth factor deprivation signals. Proc
Natl Acad Sci USA. 104:4431–4436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Del Bufalo D, Rizzo A, Trisciuoglio D,
Cardinali G, Torrisi MR, Zangemeister-Wittke U, Zupi G and Biroccio
A: Involvement of hTERT in apoptosis induced by interference with
Bcl-2 expression and function. Cell Death Differ. 12:1429–1438.
2005.PubMed/NCBI
|
|
32
|
Park JI, Venteicher AS, Hong JY, Choi J,
Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M,
Veenstra TD, Nusse R, McCrea PD and Artandi SE: Telomerase
modulates Wnt signalling by association with target gene chromatin.
Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi JK, Southworth LK, Sarin KY,
Venteicher AS, Ma WX, Chang W, Cheung P, Jun SH, Artandi MK and
Shah N: TERT promotes epithelial proliferation through
transcriptional control of a Myc- and Wnt-related developmental
program. PLoS Genet. 4:e102008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
González-Suárez E, Samper E, Ramírez A,
Flores JM, Martín-Caballero J, Jorcano JL and Blasco MA: Increased
epidermal tumors and increased skin wound healing in transgenic
mice overexpressing the catalytic subunit of telomerase, mTERT, in
basal keratinocytes. EMBO J. 20:2619–2630. 2001.PubMed/NCBI
|
|
35
|
Mukherjee S, Firpo EJ, Wang Y and Roberts
JM: Separation of telomerase functions by reverse genetics. Proc
Natl Acad Sci USA. 108:1363–1371. 2011. View Article : Google Scholar
|
|
36
|
Indran IR, Hande MP and Pervaiz S: hTERT
overexpression alleviates intracellular ROS production, improves
mitochondrial function, and inhibits ROS-mediated apoptosis in
cancer cells. Cancer Res. 71:266–276. 2011. View Article : Google Scholar
|
|
37
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Talmadge JE and Fidler IJ: AACR centennial
series: the biology of cancer metastasis: historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
40
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu ST, Chen L, Wang HJ, Tang XD, Fang DC
and Yang SM: hTERT promotes the invasion of telomerase-negative
tumor cells in vitro. Int J Oncol. 35:329–336.
2009.PubMed/NCBI
|
|
47
|
Okawa T, Michaylira CZ, Kalabis J, Stairs
DB, Nakagawa H, Andl CD, Johnstone CN, Klein-Szanto AJ, El-Deiry WS
and Cukierman E: The functional interplay between EGFR
overexpression, hTERT activation, and p53 mutation in esophageal
epithelial cells with activation of stromal fibroblasts induces
tumor development, invasion, and differentiation. Genes Dev.
21:2788–2803. 2007. View Article : Google Scholar
|
|
48
|
Bagheri S, Nosrati M, Li S, Fong S,
Torabian S, Rangel J, Moore DH, Federman S, Laposa RR, Baehner FL,
Sagebiel RW, Cleaver JE, Haqq C, Debs RJ, Blackburn EH and
Kashani-Sabet M: Genes and pathways downstream of telomerase in
melanoma metastasis. Proc Natl Acad Sci USA. 103:11306–11311. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen PC, Peng JR, Huang L, Li WX, Wang WZ,
Cui ZQ, Han H, Gong L, Xiang DP, Qiao SS, Yu X, Wei YH, Ma LP, Li
N, Zhu JY and Leng XS: Overexpression of human telomerase reverse
transcriptase promotes the motility and invasiveness of HepG2 cells
in vitro. Oncol Rep. 30:1157–1164. 2013.PubMed/NCBI
|
|
50
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Z, Li Q, Li K, Chen L, Li W, Hou M,
Liu T, Yang J, Lindvall C, Björkholm M, Jia J and Xu D: Telomerase
reverse transcriptase promotes epithelial-mesenchymal transition
and stem cell-like traits in cancer cells. Oncogene. 32:4203–4213.
2012. View Article : Google Scholar
|
|
52
|
Okamoto N, Yasukawa M, Nguyen C, Kasim V,
Maida Y, Possemato R, Shibata T, Ligon KL, Fukami K, Hahn WC and
Masutomi K: Maintenance of tumor initiating cells of defined
genetic composition by nucleostemin. Proc Natl Acad Sci USA.
108:20388–20393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
54
|
Guo W, Lasky JL and Wu H: Cancer stem
cells. Pediatr Res. 59:59–64. 2006. View Article : Google Scholar
|
|
55
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia J, Chen C, Chen Z, Miele L, Sarkar FH
and Wang Z: Targeting pancreatic cancer stem cells for cancer
therapy. Biochim Biophys Acta. 1826:385–399. 2012.PubMed/NCBI
|
|
57
|
Park IH, Zhao R, West JA, Yabuuchi A, Huo
H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of
human somatic cells to pluripotency with defined factors. Nature.
45:141–146. 2008. View Article : Google Scholar
|
|
58
|
Zhao X, Malhotra GK, Band H and Band V: A
block in lineage differentiation of immortal human mammary
stem/progenitor cells by ectopically-expressed oncogenes. J
Carcinog. 10:392011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell
LL, Polyak K, Brisken C, Yang J and Weinberg RA: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rosen JM and Jordan CT: The increasing
complexity of the cancer stem cell paradigm. Science.
324:1670–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Miki J, Furusato B, Li H, Gu Y, Takahashi
H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S and Rhim JS:
Identification of putative stem cell markers, CD133 and CXCR4, in
hTERT-immortalized primary nonmalignant and malignant tumor-derived
human prostate epithelial cell lines and in prostate cancer
specimens. Cancer Res. 67:3153–3161. 2007. View Article : Google Scholar
|
|
63
|
Paranjape AN, Mandal T, Mukherjee G, Kumar
MV, Sengupta K and Rangarajan A: Introduction of SV40ER and hTERT
into mammospheres generates breast cancer cells with stem cell
properties. Oncogene. 31:1896–1909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Castelo-Branco P, Zhang C, Lipman T,
Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D,
Walker E, Kaplan DR, Dirks P and Tabori U: Neural tumor-initiating
cells have distinct telomere maintenance and can be safely targeted
for telomerase inhibition. Clin Cancer Res. 17:111–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G and
Medema JP: Wnt activity defines colon cancer stem cells and is
regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lü MH, Liao ZL, Zhao XY, Fan YH, Lin XL,
Fang DC, Guo H and Yang SM: hTERT-based therapy: a universal
anticancer approach (Review). Oncol Rep. 28:1945–1952.
2012.PubMed/NCBI
|
|
67
|
Kuppuswamy M, Spencer JF, Doronin K,
Tollefson AE, Wold WS and Toth K: Oncolytic adenovirus that
overproduces ADP and replicates selectively in tumors due to hTERT
promoter-regulated E4 gene expression. Gene Ther. 12:1608–1617.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu JP, Chen W, Schwarer AP and Li H:
Telomerase in cancer immunotherapy. Biochim Biophys Acta.
1805:35–42. 2010.PubMed/NCBI
|
|
69
|
Nair SK, Heiser A, Boczkowski D, Majumdar
A, Naoe M, Lebkowski JS, Vieweg J and Gilboa E: Induction of
cytotoxic T cell responses and tumor immunity against unrelated
tumors using telomerase reverse transcriptase RNA transfected
dendritic cells. Nat Med. 6:1011–1017. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kyte JA: Cancer vaccination with
telomerase peptide GV1001. Expert Opin Investig Drugs. 18:687–694.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nava-Parada P and Emens LA: GV-1001, an
injectable telomerase peptide vaccine for the treatment of solid
cancers. Curr Opin Mol Ther. 9:490–497. 2007.PubMed/NCBI
|
|
72
|
Mavroudis D, Bolonakis I, Cornet S,
Myllaki G, Kanellou P, Kotsakis A, Galanis A, Nikoloudi I,
Spyropoulou M, Menez J, Miconnet I, Niniraki M, Cordopatis P,
Kosmatopoulos K and Georgoulias V: A phase I study of the optimized
cryptic peptide TERT(572y) in patients with advanced malignancies.
Oncology. 70:306–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Menez-Jamet J and Kosmatopoulos K:
Development of optimized cryptic peptides for immunotherapy.
IDrugs. 12:98–102. 2009.PubMed/NCBI
|
|
74
|
Pascolo E, Wenz C, Lingner J, Hauel N,
Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K and
Schnapp A: Mechanism of human telomerase inhibition by BIBR1532, a
synthetic, non-nucleosidic drug candidate. J Biol Chem.
277:15566–15572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Philippi C, Loretz B, Schaefer UF and Lehr
CM: Telomerase as an emerging target to fight cancer -
opportunities and challenges for nanomedicine. J Control Release.
146:228–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Cong YS, Wright WE and Shay JW: Human
telomerase and its regulation. Microbiol Mol Biol Rev. 66:407–425.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Buseman CM, Wright WE and Shay JW: Is
telomerase a viable target in cancer? Mutat Res. 730:90–97. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schepelmann S, Ogilvie LM, Hedley D,
Friedlos F, Martin J, Scanlon I, Chen P, Marais R and Springer CJ:
Suicide gene therapy of human colon carcinoma xenografts using an
armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer
Res. 67:4949–4955. 2007. View Article : Google Scholar
|
|
79
|
Majumdar AS, Hughes DE, Lichtsteiner SP,
Wang Z, Lebkowski JS and Vasserot AP: The telomerase reverse
transcriptase promoter drives efficacious tumor suicide gene
therapy while preventing hepatotoxicity encountered with
constitutive promoters. Gene Ther. 8:568–578. 2001. View Article : Google Scholar
|
|
80
|
Zhou JH, Tang B, Liu XL, He DW and Yang
DT: hTERT-targeted E. coli purine nucleoside phosphorylase
gene/6-methylpurine deoxyribose therapy for pancreatic cancer. Chin
Med J (Engl). 120:1348–1352. 2007.PubMed/NCBI
|
|
81
|
Xue Y, Li L, Zhang D, Wu K, Chen Y, Zeng
J, Wang X and He D: Twisted epithelial-to-mesenchymal transition
promotes progression of surviving bladder cancer T24 cells with
hTERT-dysfunction. PLoS One. 6:e277482011. View Article : Google Scholar
|
|
82
|
Hu J, Hwang SS, Liesa M, Gan B, Sahin E,
Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, Johnson S,
Ivanova E, Kost-Alimova M, Protopopov A, Wang YA, Shirihai OS, Chin
L and DePinho RA: Antitelomerase therapy provokes ALT and
mitochondrial adaptive mechanisms in cancer. Cell. 148:651–663.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bechter OE, Zou Y, Walker W, Wright WE and
Shay JW: Telomeric recombination in mismatch repair deficient human
colon cancer cells after telomerase inhibition. Cancer Res.
64:3444–3451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cukusić A, Skrobot Vidacek N, Sopta M and
Rubelj I: Telomerase regulation at the crossroads of cell fate.
Cytogenet Genome Res. 122:263–272. 2008.PubMed/NCBI
|