Introduction
Malignant pleural mesothelioma (MPM) is a locally
aggressive disease characterized by a poor prognosis and an
increasing incidence (1–4). MPM is difficult to detect at an early
stage, and surgical and radiotherapeutic approaches are ineffective
when used independently, because MPM spreads diffusely in the
surrounding chest wall (5). No
universally accepted treatment approach currently exists. An
extrapleural pneumonectomy (EPP) with en bloc resection of the
lung, pleura, ipsilateral diaphragm, and pericardium is one of the
most invasive surgical procedures and is associated with a high
risk of local recurrence (6,7).
Recently, adjuvant radiation therapy to the ipsilateral hemithorax
after EPP has been reported to result in a dramatic reduction in
local relapse and the prolonged survival of patients with
early-stage disease (8).
Pemetrexed, a multi-target anti-folate, exhibits activity against
various tumors, but especially against MPM and non-small cell lung
cancer (NSCLC), for which it is routinely used (9). Pemetrexed inhibits at least three
kinds of enzymes involved in folate metabolism, and in pyrimidine
and purine biosynthesis: thymidylate synthase (TS), dihydrofolate
reductase (DHFR), and glycinamide ribonucleotide formyltransferase
(10). Combination of pemetrexed
and cisplatin has become the standard first-line regimen for MPM
based on the results of a phase III trial showing that this
combination improved survival compared with cisplatin treatment
alone (11). However, the impacts
of induction chemotherapy using pemetrexed and cisplatin, and of
adjuvant hemithoracic radiation therapy after EPP for MPM remain
controversial (12). Flores et
al reported that patients who underwent a
pleurectomy/decortication had a better survival outcome than those
who underwent EPP (13).
Photodynamic therapy (PDT) consists of the use of a
tumor-specific photosensitizer and laser irradiation to induce the
production of reactive oxygen species in cancer cells (14,15).
This treatment modality is used for many cancers and is widely used
as a treatment option for solid cancers (16–18).
The use of PDT as a treatment for MPM has been investigated under
both clinical and experimental conditions (19–21).
Friedberg et al reported a phase I clinical trial of
Foscan-mediated PDT and surgery in patients with MPM (20). They reported that Foscan-mediated
PDT afforded the option of accomplishing tumor debulking using a
lung-sparing pleurectom/decortication, rather than EPP. A phase III
randomized trial of surgery and chemotherapy with or without
intra-operative PDT using the first-generation photosensitizer
Photofrin, was reported in 1997 (22). The study concluded that PDT using
Photofrin did not prolong patient survival or increase local MPM
control. However, we recently reported that PDT using the
second-generation photosensitizer NPe6, has a strong antitumor
effect against large tumors, which are unsuitable for treatment
with Photofrin-PDT (23). NPe6 has
a major absorption band at 664 nm, which is longer than the
Photofrin band (630 nm), and NPe6-PDT can affect deeper lesions.
Therefore, in an attempt to establish a new treatment modality for
MPM, we examined the antitumor effect of combination therapy
consisting of pemetrexed chemotherapy and NPe6-PDT by comparing the
antitumor effects of pemetrexed administered before or after
NPe6-PDT in both in vitro and in vivo models.
Materials and methods
Cell cultures
The human mesothelioma cell lines, H28, H2452,
MSTO-211H, and H2052 were purchased from the American Type Culture
Collection (ATCC) (Manassas, VA, USA) (24,25).
These cell lines and human breast cancer MCF-7 cells transfected
with human procaspase-3 cDNA (MCF-7c3 cells) were cultured in
RPMI-1640 medium containing 10% fetal bovine serum (26).
Photosensitizer
NPe6 (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) is
a second-generation water-soluble photosensitizer with a molecular
weight of 799.69 and a chlorine annulus; its highest absorption
peak occurs at 407 nm, while a second peak occurs at 664 nm
(17,27).
Laser unit
A diode laser (Panasonic Healthcare Co., Ltd.,
Kanagawa, Japan) emitting continuous wave laser light at a
wavelength of 664 nm was used as the light source for the
excitation of NPe6 (28).
Measurement of the fluorescence intensity
of NPe6 in the cells
MSTO-211H cells were exposed to pemetrexed at an
IC50 dose of 1.2 μM for 48 h, and then were exposed to
NPe6 (15 μg/ml) for 4 h. The cells were washed with phosphate
buffered saline (PBS). The NPe6 remaining in the cells was excited
at 405 nm, and the fluorescence was detected with a charged coupled
device (CCD) camera system (Argus/HiSCa; Hamamatsu Photonics Co.
Ltd., Shizuoka, Japan) through a multilaminate interference filter
capable of selecting a fluorescence wavelength of 630 nm, as
previously reported (29).
Determination of cell viability
We evaluated the growth inhibitory effects using the
tetrazolium salt WST-1 assay according to the manufacturer’s
instructions, as described previously (29,30).
The effects of four different treatment schedules were examined.
For the treatment of PDT alone, cells were seeded into 96-well
microculture plates at a density of 1×104 cells/well and
allowed to adhere to the dish overnight. NPe6 was then added to the
medium in increasing concentrations, followed by incubation at 37°C
in the dark for 24 h. The cells were washed with PBS and the medium
was replaced; the cells were then irradiated with a laser (33
mW/cm2; total energy, 10 J/cm2) and cell
viability was measured 72 h later. For the treatment of PDT
followed by pemetrexed, the cells were incubated with NPe6 (10
μg/ml) for 24 h. Then, the cells were treated by PDT and the medium
was replaced with a medium containing pemetrexed, followed by
culturing for 48 h. For the treatment of pemetrexed alone, the
cells were incubated with pemetrexed for 48 h. For the treatment of
pemetrexed followed by PDT, the cells were incubated with
pemetrexed; 24 h later, NPe6 (10 μg/ml) was added. Forty-eight
hours later, the cells were treated by PDT then incubated for 24 h.
For each protocol, the cell viability was measured at 72 h after
the start of the treatment. Independent experiments were repeated
at least three times to confirm the data.
Nude mice
Five-week-old BALB/c nude mice weighing 20–30 g were
obtained from the Charles River Laboratories International, Inc.
(L’Abresele, France). The animal experiments were conducted in
accordance with the guidelines of the Animal Ethics Committee of
Tokyo Medical University, complying with the Guidelines for the
Welfare and Use of Animals in Cancer Research (31).
Protocol and therapeutic procedures
MSTO-211H cells were washed twice in Hank’s solution
(Invitrogen Life Technologies, Carlsbad, CA, USA), and
107 cells were injected subcutaneously into the right
thigh of individual nude mice. Treatments were initiated 7 days
after tumor cell implantation, when the MSTO-211H tumors were ~200
mm3 in volume. The tumor volumes were calculated using
the following formula: tumor volume = LD2/2 (L, long
diameter; D, short diameter) (32). For the pemetrexed followed by PDT
treatment, mice were intraperitoneally injected with pemetrexed
(150 mg/kg daily) on days 7–11; on day 12, the mice were
intravenously injected with NPe6 (10 mg/kg) and irradiated with a
664-nm laser (100 J/cm2) 2 h later. The irradiation time
was 16 min and 40 sec. For the PDT followed by pemetrexed
treatment, mice were intravenously injected with NPe6 (10 mg/kg)
and 2 h later irradiated with a 664-nm laser (100 J/cm2)
on day 7; on days 8–12, the mice were intraperitoneally injected
with pemetrexed (150 mg/kg daily). The progress of each tumor was
measured every day until day 28, and the ratio of the tumor volume
was calculated by comparing the volume with the tumor volume on day
7. All the in vivo studies were performed in accordance with
the Guidelines for the Welfare and Use of Animals in Cancer
Research (31).
Immunohistochemical analysis
Cells were grown on glass coverslips in 35-mm petri
dishes. To analyze the expression of TS, the coverslips were
removed from the petri dishes, washed with PBS, and fixed in 1%
formaldehyde for 30 min. After rinsing twice with PBS, the fixed
cells were incubated in IFA buffer (PBS containing 1% bovine serum
albumin, 0.1% Tween-20) for 10 min and then in IFA-containing mouse
anti-TS antibody (clone 8F1; Zymed Laboratories, Inc., San
Francisco, CA, USA) for 1 h at room temperature (26,30,32).
The MSTO-211H tumors in BALB/c nude mice were
collected before PDT and 24 h after PDT. We performed an
immunohistochemical analysis of these samples using anti-TS
antibody (clone 8F1; Zymed Laboratories, Inc.).
Results
NPe6-PDT alone, but not pemetrexed alone,
exerts a strong antitumor effect against human malignant
mesothelioma cell lines
We examined the antitumor effects of pemetrexed on
MSTO-211H, NCI-H2052, NCI-H2452, and NCI-H28 using the WST assay
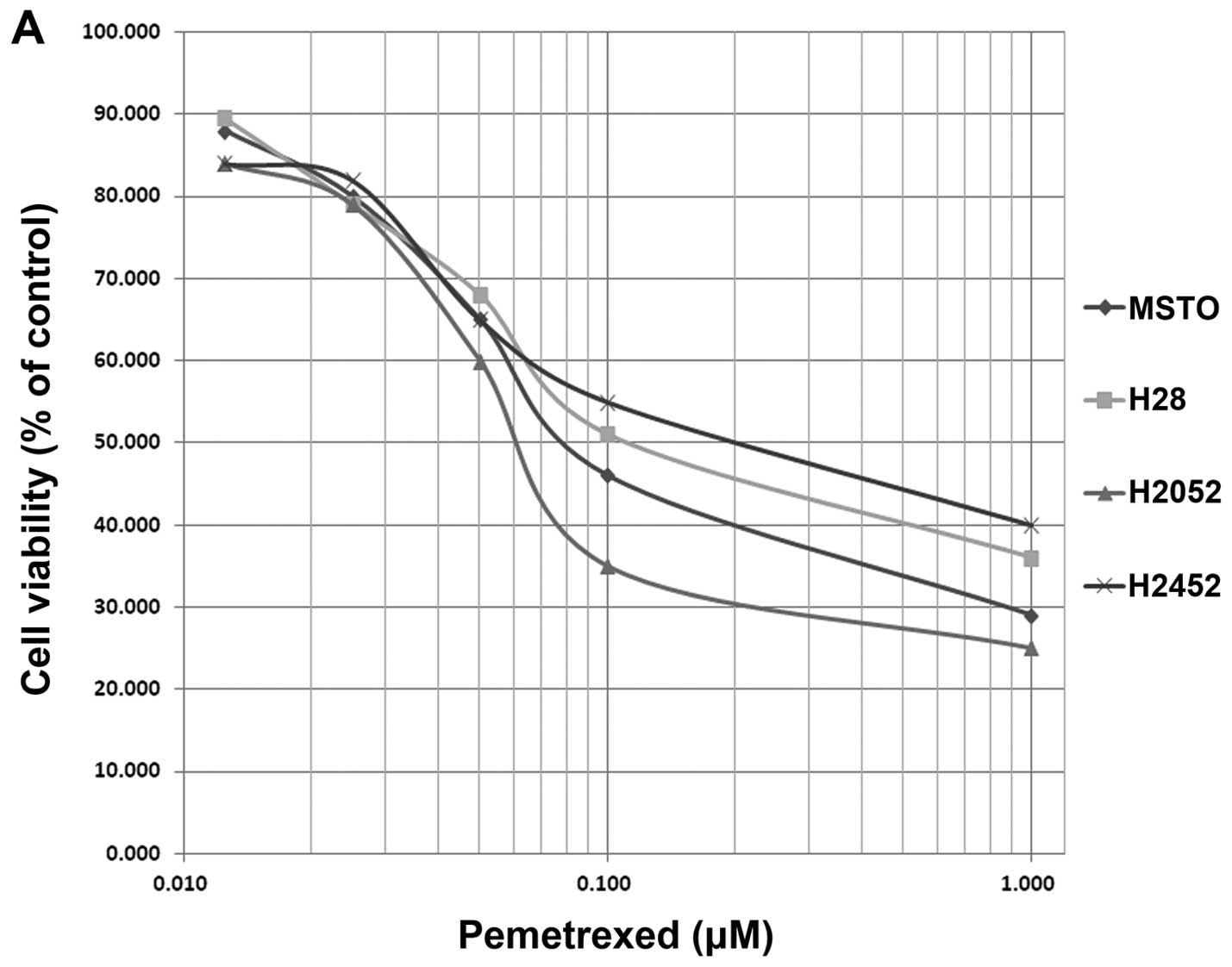
(Fig. 1A). The IC50
values of pemetrexed were 1.2 μM for MSTO-211H, 0.1 μM for
NCI-H2052, 10 μM for NCI-H2452, and 8.4 μM for NCI-H28; these
values were similar to those in a previous report (25). In MCF-7c3, the IC50
value was 5.5 μM (Fig. 1A).
Unfortunately, treatment using pemetrexed alone was not sufficient
to reach an LD90 in the NCI-H2452, and NCI-H28 cell
lines, as previously reported (25). On the other hand, NPe6-PDT caused
complete cell death in all four cell lines, and NPe6-PDT exerted a
strong antitumor effect against MPM in vitro, with an
LD90 being reached in all the cell lines (Fig. 1B). The IC50 values were
10 μg/ml of NPe6 and 10 J/cm2, 33 mW/cm2 of
laser irradiation.
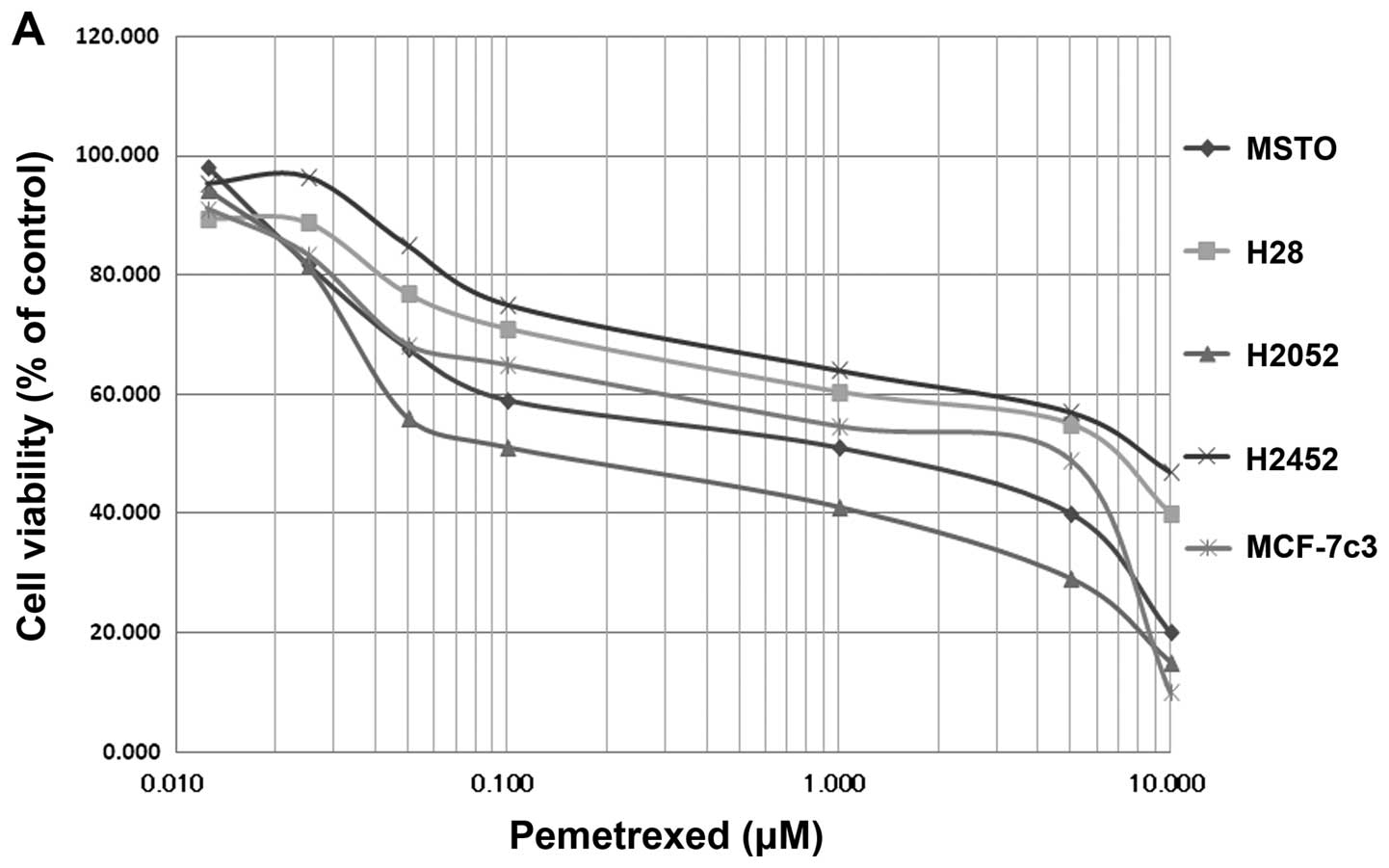 | Figure 1(A) Growth-inhibitory effect of
pemetrexed in MSTO-211H (◆), H28 (■), H2052 (▲), H2452 (×), and
MCF-7c3 cells (✳). Cells were exposed to pemetrexed for 96 h, and
the growth-inhibitory effect was measured using the WST assay. (B)
Growth-inhibitory effect of NPe6-PDT in MSTO-211H (◆), H28 (■),
H2052 (▲), H2452 (×), and MCF-7c3 cells (✳). Cells were exposed to
NPe6 for 24 h and then washed with phosphate buffered saline (PBS);
the medium was replaced. The cells were then irradiated with a
diode laser (33 mW/cm2, 10 J/cm2) and
cultured for another 48 h. The growth-inhibitory effect was
measured using the WST assay. |
Pemetrexed enhances the lethal effects of
NPe6-PDT against MPM cell lines
We examined the effects of combination therapy using
pemetrexed and NPe6-PDT. First, we evaluated whether pemetrexed
pre-treatment enhanced the antitumor effect of NPe6-PDT in MPM
cells. MPM cells were treated with pemetrexed for 48 h and then
were washed with PBS three times; the cells were then incubated for
4 h with NPe6 (10 μg/ml). After incubation, the cells were
irradiated with a diode laser (664 nm, 10 J/cm2), which
provided the IC50 dose of NPe6-PDT in MSTO-2511 cells.
As shown in Fig. 2A, the
IC50 values were 0.08 μM for the MSTO-211H cells, 0.14
μM for the H28 cells, 0.23 μM for the H2452 cells, and 0.06 μM for
the H2052 cells. Thus, pemetrexed treatment followed by NPe6-PDT
caused an initial decrease in viability, indicating that pemetrexed
enhances the lethal effect of NPe6 (Fig. 2A).
 | Figure 2(A) Growth-inhibitory effect of
pemetrexed followed by NPe6-PDT in MSTO-211H (◆), H28 (■), H2052
(▲), and H2452 (×) cells. Cells were incubated with 1.2 μM of
pemetrexed, which is the IC50 dose for MSTO-211H cells;
24 h later, 10 μg/ml of NPe6 was added. Forty-eight hours after the
start of treatment, the cells were irradiated with a diode laser
(33 mW/cm2, 10 J/cm2). These conditions for
NPe6-PDT correspond to the IC50 dose for MSTO-211H
cells. At 72 h after the start of treatment, the growth-inhibitory
effect was measured using the WST assay. (B) Growth-inhibitory
effect of NPe6-PDT followed by pemetrexed in MSTO-211H (◆), H28
(■), H2052 (▲), and H2452 (×) cells. Cells were incubated with NPe6
(10 μg/ml) for 24 h and then irradiated with a diode laser (33
mW/cm2, 10 J/cm2). These conditions for
NPe6-PDT correspond to the IC50 dose for MSTO-211H
cells. The cells were then washed with phosphate buffered saline
(PBS), and the medium was replaced with a medium containing
pemetrexed; the cells were then cultured for 48 h. At 72 h after
the start of treatment, the growth-inhibitory effect was measured
using the WST assay. |
NPe6-PDT followed by pemetrexed treatment
yielded no enhancement
We next examined whether NPe6-PDT followed by
pemetrexed treatment enhanced the antitumor effect. First, MPM
cells were treated with NPe6-PDT using the IC50
conditions (NPe6, 10 μg/ml; laser irradiation, 10
J/cm2). Then, the cells were treated with pemetrexed for
48 h. The survival curves indicated that NPe6-PDT followed by
pemetrexed treatment was incapable of obtaining an IC50
response except in the H2052 cells, and no enhancement of the
treatment effects was observed (Fig.
2B).
Pemetrexed treatment enhanced the
antitumor effect of NPe6-PDT against MPM tumors in vivo
We examined the efficacy of combination therapy with
NPe6-PDT and chemotherapy using pemetrexed for MPM tumors. We
transplanted MSTO-211H cells into nude mice as described in
previous reports (33), and then
treated the mice with pemetrexed for 5 days. On the sixth day of
treatment, we treated the tumors with NPe6-PDT using 10 mg/kg of
NPe6 and 10 J/cm2 of laser irradiation. Fig. 3 shows that pemetrexed pre-treatment
followed by NPe6-PDT enabled an 80% loss in the tumor volume and
inhibited the re-growth of the tumors. Using this dosage, NPe6-PDT
alone decreased the tumor volume by 50%; however, the tumor volume
increased once again, reaching the pre-treatment value 10 days
after PDT (Fig. 3).
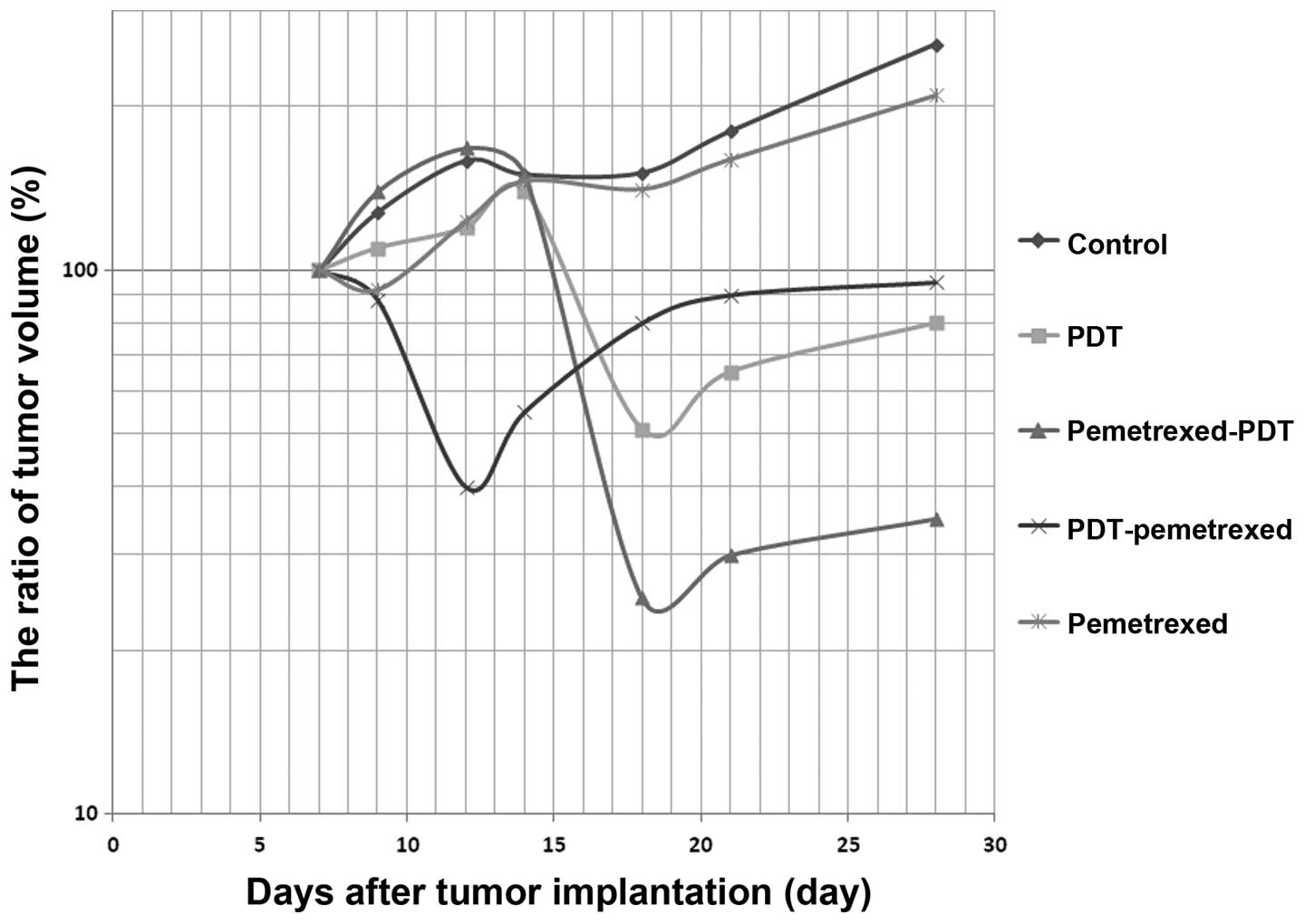 | Figure 3Nude mice transplanted with MSTO-211H
tumors measuring 5–7 mm in diameter were treated by pemetrexed
and/or NPe6-PDT. The tumor volumes were calculated using the
following formula: tumor volume = LD2/2 (L, long
diameter; D, short diameter). Untreated MSTO-211H tumors as control
(◆, N=10), treated by NPe6-PDT alone (■, N=10), treated by
pemetrexed followed by photodynamic therapy (PDT) (▲, N=10),
treated by PDT followed by pemetrexed (×, N=10), treated by
pemetrexed alone (✳, N=10). For the pemetrexed followed by PDT
treatment (▲), mice were intraperitoneally injected with pemetrexed
(150 mg/kg daily) on days 7–11; on day 12, the mice were
intravenously injected with NPe6 (10 mg/kg) and then irradiated
with a 664-nm laser (100 J/cm2) 2 h later. The laser
spot size was 14 mm in diameter, and the power output at the fiber
tip was 154 mW. The irradiation time was 16 min and 40 sec. For the
PDT followed by pemetrexed treatment (×), mice were intravenously
injected with NPe6 (10 mg/kg) and then 2 h later irradiated with a
664-nm laser (100 J/cm2) on day 7; on days 8–12, the
mice were intraperitoneally injected with pemetrexed (150 mg/kg
daily). Tumor response was monitored until day 28, and the ratio of
the tumor volume was calculated by comparing the volume with tumor
volume on day 7. |
We also evaluated the efficacy of NPe6-PDT followed
by pemetrexed treatment, but this treatment schedule did not
inhibit the re-growth of the tumor (Fig. 3). NPe6-PDT followed by pemetrexed
treatment yielded no enhancement in tumor cell lethality in the
in vivo experiments, similar to the results in vitro
(Fig. 3).
Pemetrexed did not stimulate the
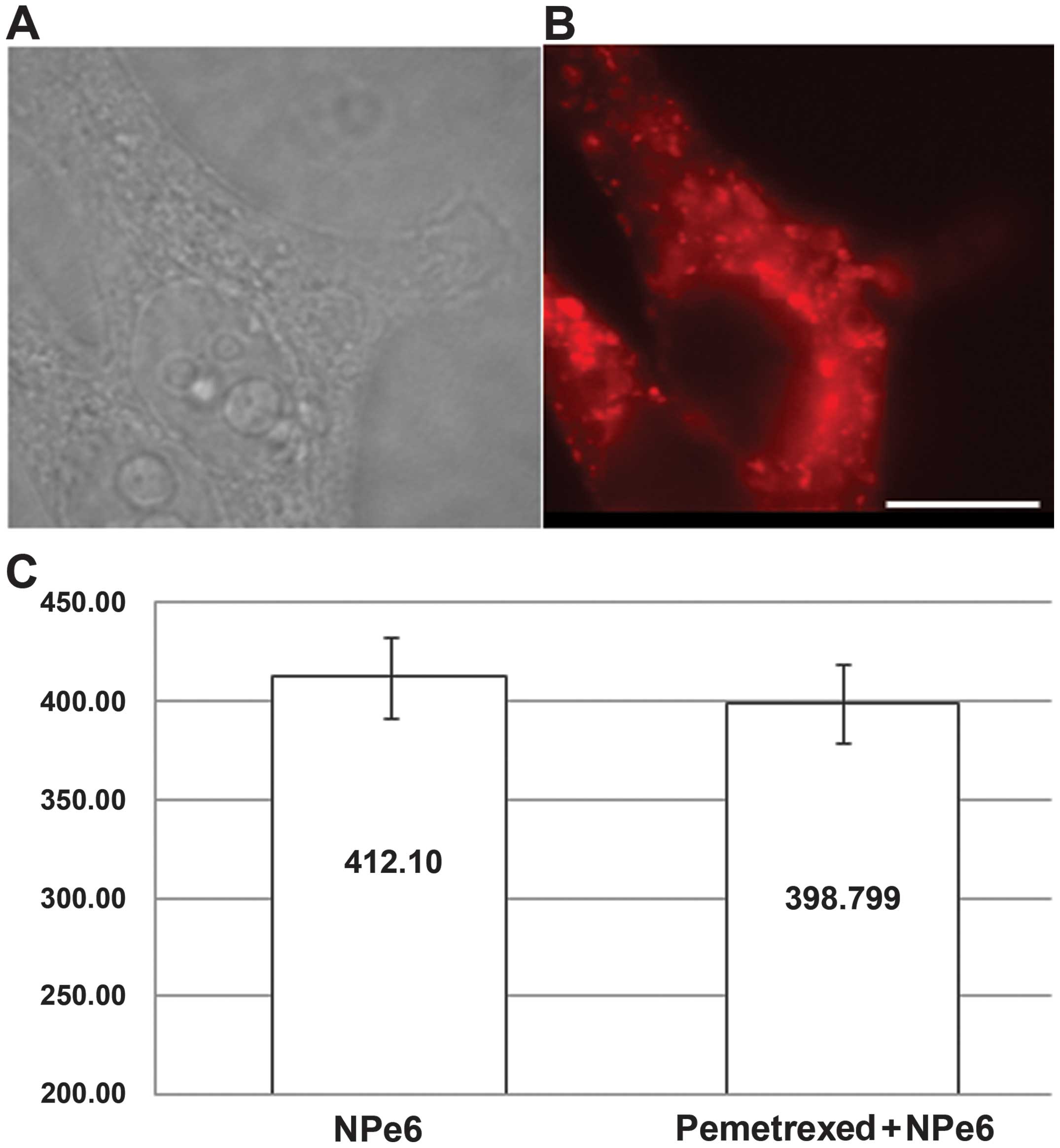
accumulation of intracellular NPe6 in MSTO-211 cells
To examine the mechanism responsible for the
enhancement in cell lethality enabled by pemetrexed pre-treatment
followed by NPe6-PDT, we investigated whether pemetrexed stimulates
the intracellular accumulation of NPe6 in MSTO-211 cells. MSTO-211
cells were pre-treated for 48 h with an IC50 dose of 1.2
μM, then exposed to NPe6 for 3 h. The resulting accumulation of
NPe6 was assessed by detecting red fluorescence using fluorescent
microscopy (29). No significant
difference in the intracellular accumulation of NPe6 was observed
between a group with pemetrexed pre-treatment and one without the
pre-treatment. Thus, pemetrexed pre-treatment did not enhance the
accumulation of intracellular NPe6.
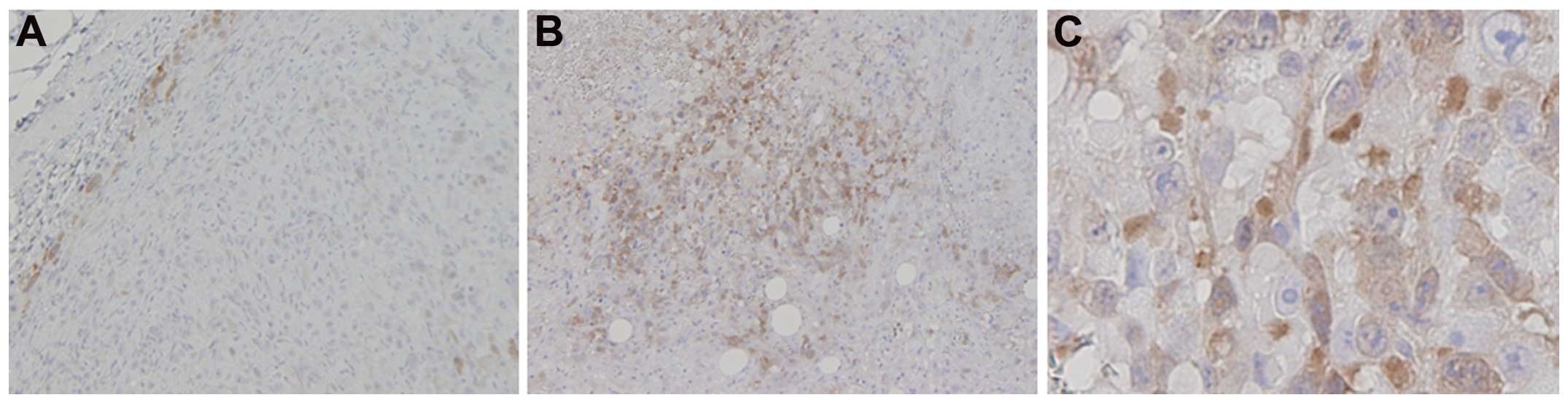
NPe6-PDT induced the expression of
TS
The inhibition of TS, resulting in a decrease in
thymidine available for DNA synthesis, is reportedly the primary
mechanism of pemetrexed (34,35).
Therefore, we hypothesized that TS expression may affect the
efficacy of combination therapy with pemetrexed and NPe6-PDT. We
examined TS protein expression in the MPM cell lines using an
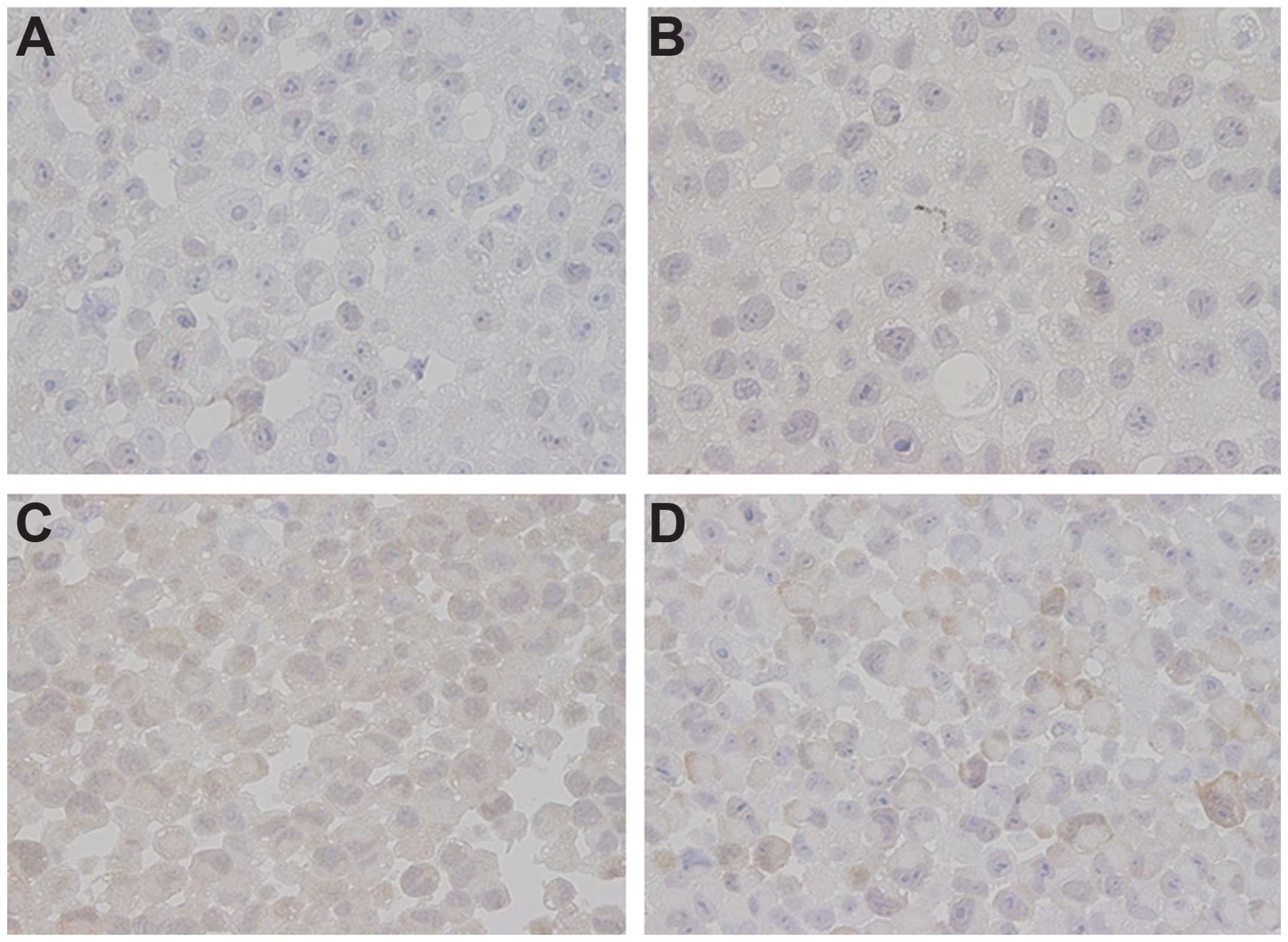
immunohistochemical analysis (Fig.
5). The expression of TS was relatively low in MSTO-211H and
NCI-H2052 cells but was relatively high in NCI-H2452 and NCI-H28
cells (Fig. 4). In the in
vivo model, NPe6-PDT induced TS expression in the MSTO-211H
tumors 24 h after the laser irradiation (Fig. 5). These results suggest that the
overexpression of TS protein induced by NPe6-PDT may be associated
with the failure of pemetrexed to exert a tumoricidal action.
Therefore, we concluded that NPe6-PDT followed by pemetrexed
treatment did not enhance tumor cell lethality in the in
vivo model.
Discussion
Recently, Debefve et al reported that PDT
affects vascular barrier function and thus increases vessel
permeability; this phenomenon may be exploited to facilitate
targeted drug delivery (36).
Snyder et al also reported that a direct vascular effect of
PDT at relatively low light doses may be exploited to increase the
uptake of systemically circulating drugs to tumors, and this new
treatment concept has been named ‘photodynamic drug delivery’
(37). They developed a novel PDT
treatment that enhances the delivery and efficacy of
macromolecule-based cancer therapy, such as a liposomally
encapsulated formulation of doxorubicin (37). Low-dose PDT reportedly increases
microvessel permeability, thereby promoting the controlled release
of circulating drugs into tissues; PDT additionally stimulates
leukocyte-endothelial cell interactions, mediating the effects of
PDT on improved drug delivery (38). Therefore, we hypothesized that
NPe6-PDT may enhance the delivery of pemetrexed to the tumors and
suspected that NPe6-PDT followed by pemetrexed treatment could
provide a synergistic or additive effect in vivo. However,
NPe6-PDT followed by pemetrexed did not enhance tumor cell
lethality, compared with NPe6-PDT alone, either in vitro
(Fig. 2B) or in vivo
(Fig. 3). These results indicated
that NPe6-PDT could not enhance the antitumor activity of
pemetrexed and in fact produced some resistance to treatment,
compared with PDT alone.
Pemetrexed reportedly inhibits multiple enzymes in
the folate metabolic pathway, with TS being the main target. In
NSCLC cell lines, high baseline TS expression levels confer
resistance to pemetrexed, and the TS level is correlated with
pemetrexed efficacy in a variety of solid tumors. As shown in
Fig. 5, the expression of TS was
relatively low in MSTO-211 and H2052 cells, which were somewhat
more sensitive to pemetrexed than the H2452 and H28 cells (as shown
in Fig. 1A). Based on our data
shown in Fig. 1A regarding the
growth inhibitory effects of pemetrexed, the H2052 cells were the
most sensitive to pemetrexed of all the cell lines examined, as in
a previous report, because the TS level was relatively low in the
H2052 cells, compared with in the H2454 and H28 cells (25). As shown in Fig. 6, NPe6-PDT at the IC50
dose induced the expression of TS in MSTO-211 cells. Therefore,
based on these results, we concluded that NPe6-PDT followed by
pemetrexed treatment did not enhance tumor cell lethality possibly
because of the NPe6-PDT-induced expression of TS. Moreover, we
previously reported that NPe6-PDT can damage the microvasculature
around tumors and induce a vascular shut-down effect, decreasing
blood flow to the tumors (17).
Sitnik et al also reported that PDT-induced microvasculature
damage is associated with a significant decrease in the blood flow
and severe hypoxia in the tumor (39). We suggested that NPe6-PDT does not
enhance the delivery of pemetrexed but may, in fact, obstruct the
delivery of pemetrexed to tumors.
Oleinick et al reported that photosensitizer
accumulation can influence cellular sensitivity to PDT (40). Robey et al reported that the
expression of ATP-binding cassette (ABC) transport proteins, which
render tumor cells resistant to chemotherapeutic drugs, decreases
the accumulation of photosensitizers and causes resistance to PDT
(41). We have also previously
reported that BCRP, a member of the ABC transporter family,
decreases the accumulation of Photofrin and may be a molecular
determinant (29).
Anand et al reported that methotrexate (MTX)
stimulated the accumulation of an intracellular photosensitizer,
protoporphyrin IX, and enhanced the antitumor effect of PDT using
5-aminolaevulinic acid (ALA), but that ALA-PDT followed by MTX
yielded no enhancement in tumor cell lethality (42,43).
In the present study, as shown in Fig.
4, pemetrexed pre-treatment did not enhance the accumulation of
intracellular NPe6. Further study is needed to explain why the
combination of pemetrexed pre-treatment and NPe6-PDT has an
additive effect on NPe6-PDT cytotoxicity both in vitro and
in vivo. In conclusion, combination therapy using pemetrexed
followed by NPe6 can enhance the cytotoxic effect of NPe6 and has
important clinical implications.
Pass et al reported that intraoperative PDT
did not prolong the survival of patients with MPM (22). However, we recently reported that
NPe6-PDT exerted a strong antitumor effect against cancer lesions
(29). Therefore, combination
treatment using pemetrexed followed by NPe6-PDT may become a new
treatment modality, and further combination with surgery may reduce
local recurrence and prolong the survival of patients with
malignant mesothelioma.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (C) from Japan Society for the Promotion of
Science (JSPS) (to J.U.) (KAKENHI 21591826).
References
|
1
|
Peto J, Decarli A, La Vecchia C, Levi F
and Negri E: The European mesothelioma epidemic. Br J Cancer.
79:666–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Price B: Analysis of current trends in
United States mesothelioma incidence. Am J Epidemiol. 145:211–218.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodgson JT, McElvenny DM, Darnton AJ,
Price MJ and Peto J: The expected burden of mesothelioma mortality
in Great Britan from 2002 to 2050. Br J Cancer. 92:587–593.
2005.PubMed/NCBI
|
|
4
|
McCormack V, Peto J, Byrnes G, Straif K
and Boffetta P: Estimating the asbestos-related lung cancer burden
from mesothelioma mortality. Br J Cancer. 106:575–584. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Ruth S, Bass P and Zoetmulder FA:
Surgical treatment of malignant pleural mesothelioma: a review.
Chest. 123:551–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Treasure T, Lang-Lazdunski L, Waller D,
Bliss JM, Tan C, Entwisle J, Snee M, O’Brien M, Thomas G, Senan S,
O’Byrne K, Kilburn LS, Spicer J, Landau D, Edwards J, Coombes G,
Darlison L and Peto J; for the MarS trialists. Extra-pleural
pneumonectomy versus no extra-pleural pneumonectomy for patients
with malignant pleural mesothelioma: clinical outcomes of the
mesothelioma and radical surgery (MARS) randomized feasibility
study. Lancet Oncol. 12:763–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pass HI, Kranda K, Temeck BK, Feuerstein I
and Steinberg SM: Surgically debulked malignant pleural
mesothelioma: results and prognostic factors. Ann Surg Oncol.
4:215–222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krug LM, Pass HI, Rusch VW, Kindler HL,
Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K,
Monberg M, Obasaju CK and Vogelzang NJ: Multicenter phase II trial
of neoadjuvant pemetrexed plus cisplatin followed by extrapleural
pneumonectomy and radiation for malignant pleural mesothelioma. J
Clin Oncol. 27:3007–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adjei AA: Pemetrexed (ALIMTA), a novel
multitargeted anti-neoplastic agent. Clin Cancer Res.
10:4276s–4280s. 2004. View Article : Google Scholar
|
|
10
|
Vogelzang NJ, Porta C and Mutti L: New
agents in the management of advanced mesothelioma. Semin Oncol.
32:336–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Maneqold C, Niyikiza C and Paoletti P: Phase III study of
pemetrexed in combination with cisplatin versus cisplatin alone in
patients with malignant pleural mesothelioma. J Clin Oncol.
21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rea F, Marulli G, Bortolotti L, Breda C,
Favaretto AG, Loreggian L and Sartori F: Induction chemotherapy,
extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic
radiation in malignant pleural mesothelioma (MPM): Feasibility and
results. Lung Cancer. 57:89–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flores RM, Pass HI, Seshan VE, Dycoco J,
Zakowski M, Carbone M, Bains MS and Rusch VW: Extrapleural
pneumonectomy versus pleurectomy/decortication in the surgical
management of malignant mesothelioma: results in 663 patients. J
Thorac Cardiovasc Surg. 135:620–626. 2008. View Article : Google Scholar
|
|
14
|
Dougherty TJ, Gomer CJ, Henderson BW, et
al: Photodynamic therapy. J Natl Cancer Inst. 90:889–905. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dougherty TJ: An update on photodynamic
therapy applications. J Clin Laser Med Surg. 20:3–7. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edell ES and Cortese DA: Photodynamic
therapy in the management of early superficial squamous cell
carcinoma as an alternative to surgical resection. Chest.
102:1319–1322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato H, Usuda J, Okunaka T, Furukawa K,
Honda H, Sakaniwa N, Suga Y, Hirata T, Ohtani K, Inoue T, Maehara
S, Kubota M, Yamada K and Tsuitsui H: Basic and clinical research
on photodynamic therapy at Tokyo Medical University Hospital.
Lasers Surg Med. 38:371–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kennedy TC, McWilliams A, Edell E, Sutedja
T, Downie G, Yung R, Gazdar A and Mahur PN; American College of
Chest Physicians. Bronchial intraepithelial neoplasia/early central
airways lung cancer: ACCP evidence-based clinical practice
guidelines (2nd edition). Chest. 132(Suppl 3): S221–S233. 2007.
View Article : Google Scholar
|
|
19
|
Krueger T, Altermatt HJ, Mettler D, Scholl
B, Magnusson L and Ris HB: Experimental photodynamic therapy for
malignant pleural mesothelioma with pegylated mTHPC. Laser Surg
Med. 32:61–68. 2003. View Article : Google Scholar
|
|
20
|
Friedberg JS, Mick R, Stevenson J, Metz J,
Zhu T, Buyske J, Sterman DH, Pass HI, Glatstein E and Hahn SM: A
phase I study of Foscan-mediated photodynamic therapy and surgery
in patients with mesothelioma. Ann Thorac Surg. 75:952–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ris HB: Photodynamic therapy as an adjunct
to surgery for malignant pleural mesothelioma. Lung Cancer.
49(Suppl 1): S65–S68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pass HI, Temeck BK, Kranda K, Thomas G,
Russo A, Smith P, Friauf W and Steinberg SM: Phase III randomized
trial of surgery with or without intraoperative photodynamic
therapy and postoperative immunochemotherapy for malignant pleural
mesothelioma. Ann Surg Oncol. 4:628–633. 1997. View Article : Google Scholar
|
|
23
|
Usuda J, Ichinose S, Ishizumi T, Hayashi
H, Ohtani K, Maehara S, Ono S, Honda H, Kajiwara N, Uchida O,
Tsutsui H, Ohira T, Kato H and Ikeda N: Outcome of photodynamic
therapy using NPe6 for bronchogenic carcinomas in central airways
>1.0 cm in diameter. Clin Cancer Res. 16:2198–2204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozasa H, Oguri T, Uemura T, Miyazaki M,
Maeno K, Sato S and Ueda R: Significance of thymidylate synthase
for resistance to pemetrexed in lung cancer. Cancer Sci.
101:161–166. 2010. View Article : Google Scholar
|
|
25
|
O’Kane SL, Eagle GL, Greenman J, Lind MJ
and Gawkwell L: COX-2 specific inhibitors enhance the cytotoxic
effects of pemetrexed in mesothelioma cell lines. Lung Cancer.
67:160–165. 2010. View Article : Google Scholar
|
|
26
|
Usuda J, Chiu SM, Murphy ES, Lam M,
Nieminen AL and Oleinick NL: Domain-dependent photodamage to Bcl-2.
A membrane anchorage region is needed to form the target of
phthalocyanine photosensitization. J Biol Chem. 278:2021–2029.
2003. View Article : Google Scholar
|
|
27
|
Usuda J, Hirata T, Ichinose S, Ishizumi T,
Inoue T, Ohtani K, Maehara S, Yamada M, Tsutsui H, Okunaka T, Kato
H and Ikeda N: Tailor-made approach to photodynamic therapy in the
treatment of cancer based on Bcl-2 photodamage. Int J Oncol.
33:689–696. 2008.PubMed/NCBI
|
|
28
|
Kato H, Furukawa K, Sato M, Okunaka T,
Kusunoki Y, Kawahara M, et al: Phase II clinical study of
photodynamic therapy using mono-L-aspartyl chlorine e6 and diode
laser for early superficial squamous cell carcinoma of the lung.
Lung Cancer. 42:103–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Usuda J, Tsunoda Y, Ichinose S, Ishizumi
T, Ohtani K, Maehara S, Ono S, Tsutsui H, Ohira T, Okunaka T,
Furukawa K, Sugimoto Y, Kato H and Ikeda N: Breast cancer resistant
protein (BCRP) is a molecular determinant of the outcome of
photodynamic therapy (PDT) for centrally located early lung cancer.
Lung Cancer. 67:198–204. 2010. View Article : Google Scholar
|
|
30
|
Xue LY, Chiu SM and Oleinick NL:
Photodynamic therapy-induced death of MCF-7 human breast cancer
cells: a role for caspase-3 in the late steps of apoptosis but not
for the critical lethal event. Exp Cell Res. 263:145–155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson
S, Wedge SR and Eccles SA; Committee of the National Cancer
Research Institute. Guidelines for the welfare and use of animals
in cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohtani K, Usuda J, Ichinose S, Ishizumi T,
Hirata T, et al: High expression of GADD-45α and VEGF induced tumor
recurrence via upregulation of IL-2 after photodynamic therapy
using NPe6. Int J Oncol. 32:397–403. 2008.PubMed/NCBI
|
|
33
|
Van TT, Hanibuchi M, Kakiuchi S, Sato S,
Kuramoto T, Goto H, Mitsuhashi A, Nishioka Y, Akiyama S and Sone S:
The therapeutic efficacy of S-1 against orthotopically implanted
human pleural mesothelioma cells in severe combined immunodeficient
mice. Cancer Chemother Pharmacol. 68:497–504. 2011. View Article : Google Scholar :
|
|
34
|
Cappia S, Righi L, Mirabelli D, Ceppi P,
Bacillo E, Ardissone F, Molinaro L, Scagliotti GV and Papotti M:
Prognostic role of osteopontin expression in malignant pleural
mesothelioma. Am J Clin Pathol. 130:58–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Righi L, Papotti MG, Ceppi P, Bille A,
Bacillo E, Molinaro L, Ruffini E, Scagliotti GV and Selvaggi G:
Thymidylate synthase but not excision repair cross-complementation
group 1 tumor expression predicts outcome in patients with
malignant pleural mesothelioma treated with pemetrexed-based
chemotherapy. J Clin Oncol. 28:1534–1539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Debefve E, Mithieux F, Perentes JY, Wang
Y, Cheng C, Schaefer SC, Ruffieux C, Ballini JP, Gonzalez M, van
den Bergh H, Ris HB, Lehr HA and Krueger T: Leukocyte-endothelial
cell interaction is necessary for photodynamic therapy induced
vascular permeabilization. Lasers Surg Med. 43:696–704.
2011.PubMed/NCBI
|
|
37
|
Snyder JW, Greco WR, Bellnier DA, Vaughan
L and Henderson BW: Photodynamic therapy: a means to enhanced drug
delivery to tumors. Cancer Res. 63:8126–8131. 2003.PubMed/NCBI
|
|
38
|
Wang Y, Perentes JY, Schäfer SC, Gonzalez
M, Debefve E, Lehr HA, van den Bergh H and Krueger T: Photodynamic
drug delivery enhancement in tumors does not depend on
leukocyte-endothelial interaction in a human mesothelioma xenograft
model. Eur J Cardiothorac Surg. 42:348–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sitnik TM, Hampton JA and Henderson BW:
Reduction of tumor oxygenation during and after photodynamic
therapy in vivo: effects of fluence rate. Br J Cancer.
77:1386–1394. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oleinick NL, Morris RL and Belichenko I:
The role of apoptosis in response to photodynamic therapy: what,
where, why, and how. Photochem photobiol Sci. 1:1–21. 2002.
View Article : Google Scholar
|
|
41
|
Robey RW, Steadman K, Polgar O and Bates
SE: ABCG2-mediated transport of photosensitizers: potential impact
on photodynamic therapy. Cancer Biol Ther. 4:187–194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anand S, Honari G, Hasan T, Elson P and
Maytin EV: Low-dose methotrexate enhances aminolevulinate-based
photodynamic therapy in skin carcinoma cells in vitro and in vivo.
Clin Cancer Res. 15:3333–3343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sinha AK, Anand S, Ortel BJ, Chang Y, Mai
Z, Hasan T and Maytin EV: Methotrexate used in combination with
aminolaevulinic acid for photodynamic killing of prostate cancer
cells. Br J Cancer. 95:485–495. 2006. View Article : Google Scholar : PubMed/NCBI
|