Introduction
MET tyrosine kinase receptor is overexpressed in a
variety of cancers (1–3), which could be the result of the
MET gene amplification, which has been observed,
particularly in transcriptional activation in a small subset of
cancers (4–8), and more recently in lung cancers
(9,10). Signaling by MET and its ligand,
hepatocyte growth factor (HGF) (11), is known to play an important role
in increased cell proliferation, reduced apoptosis, invasion,
scattering, increased metastasis, and angiogenesis (12,13).
The dimerization and autophosphorylation of MET
receptors occur following HGF binding, leading to the activation of
pathways such as mitogen-activated protein kinase (MAPK),
phosphatidylinositol-3-kinase (PI3K)-serine/threonine kinase AKT
(AKT), and the signal transducers and activator of transcription
(STAT) signaling (14–16). Then, the HGF-induced MET receptors
are internalized and trafficked to the early endosomes commonly
referred to as the sorting endosomes. In the sorting endosomes, MET
receptors are ubiquitinated and are recognized by the endosomal
sorting complex required for transport (ESCRT) machinery that
generates multivesicular bodies (MVBs) by packaging molecular cargo
into small vesicles that bud off from the limiting membrane into
the lumen of the endosomes (17).
Accordingly, endocytosis and downregulation of HGF-MET complexes
are closely related to the attenuation of intracellular MET
signaling (18,19). Alternatively, MET is recycled to
the plasma membrane by endosomal recycling pathways (20).
Maoto, a traditional Kampo medicine, has been
suggested to prevent cancer metastasis by directly suppressing the
metastatic ability of cancer cells, based on its inhibition of the
serum-induced motility of human breast cancer and mouse
osteosarcoma cells (21–23). Maoto is composed of four
ingredients, namely Ephedrae herba, Armeniacae semen, Cinnamomi
cortex, and Glycyrrhizae radix. Furthermore, extracts of maoto and
Ephedrae herba were both previously shown to prevent HGF-induced
cancer cell motility by inhibiting HGF/MET/AKT signaling through
the suppression of MET phosphorylation kinase (23,24).
In addition, Ephedrae herba directly inhibited the tyrosine-kinase
activity of MET as well as phosphorylation of AKT, a downstream
target of MET. Moreover, MET protein and gene expression was
considerably reduced following 24-h treatment with maoto or
Ephedrae herba exracts, and these inhibitory effects were abrogated
by the removal of Ephedrae herba from the incubation medium
(23,24). Thus, these suppressive effects may
be attributed to Ephedrae herba. Further, it has recently been
demonstrated that herbacetin glycosides (25), which are active molecules found in
Ephedrae herba and their aglycon, herbacetin, inhibits HGF-MET-AKT
signaling in human MDA-MB-231 breast cancer cells (26). However, it was also shown that
herbacetin treatment does not affect MET protein expression in
these cells. Accordingly, we surmised that some other components of
Ephedrae herba extract might have a stimulatory influence on the
HGF-induced MET endocytosis via the early/late endocytic
pathways.
Therefore, in this study, we investigated the
molecular mechanisms by which Ephedrae herba inhibits the
phosphorylation of MET and decreases MET protein expression.
Specifically, we examined the effect of Ephedrae herba on
HGF-stimulated MET endocytosis and its downregulation via the
early/late endocytic pathways in the H1993 NSCLC cell line,
harboring MET gene amplification and MET over-expression
(10), using immunofluorescence
microscopy and western blot analysis. We found that the
pretreatment of cells with Ephedrae herba not only led to a
considerably suppressed MET/AKT phosphorylation, but also plasma
membrane-associated MET was rapidly endocytosed and subsequently
downregulated via the early/late endocytic pathways in the cells.
Taken together, our results led us to infer that components of
Ephedrae herba might play a novel role in promoting HGF-stimulated
MET/p-MET endocytosis and subsequent degradation in the early/late
endocytic pathways.
Materials and methods
Materials
Ephedrae herba was purchased from Tsumura & Co.
(Tokyo, Japan) and was mixed with RPMI medium to a concentration of
10 mg/ml at 37°C for 30 min. Recombinant human HGF was purchased
from PeproTech (London, UK). Bafilomycin A1, cycloheximide (CHX),
and DAPI were obtained from Sigma (St. Louis, MO, USA). SlowFade
anti-fade reagent was purchased from Molecular Probes (Eugene, OR,
USA). Other chemicals were of reagent grade and were obtained from
commercial sources.
Cell culture
The human H1993 non-small cell lung cancer cell line
was obtained from the American Type Culture Collection. Cells were
cultured at 37°C and 5% CO2 in RPMI-1640 containing 10%
fetal bovine serum (FBS) in a humid environment. The serum-starved
cells were preincubated with Ephedrae herba extract (100 μg/ml) at
37°C for 2 h and 0.17 μM bafilomycin A1 for 30 min, and then
stimulated with HGF (50 ng/ml) at 37°C for the indicated times,
after which further analysis was performed.
Antibodies
Alexa 488-labeled or Texas red-labeled goat
anti-mouse and goat anti-rabbit secondary antibodies were obtained
from Molecular Probes. Normal rabbit IgG and normal mouse
monoclonal IgG1 were purchased from Imgenex (San Diego, CA, USA)
and Angio-proteomie (Boston, MA, USA), respectively. Normal goat
serum was purchased from Sigma. Anti-MET, phospho-MET (p-MET), AKT,
phospho-AKTS473 (p-AKTS473), p44/42 MAPK
(MAPK), phospho-p44/42 MAPK (p-MAPK), EEA1, and LAMP1 antibodies
were obtained from Cell Signaling Technology (Beverly, MA, USA),
and anti-β-actin antibody was obtained from Sigma. Mouse monoclonal
antibody to SNX1 was purchased from BD Biosciences (San Jose, CA,
USA). Mouse monoclonal anti-HGF α-chain antibody was purchased from
Institute of Immunology (Tokyo, Japan). Anti-cathepsin D was
affinity-purified by protein A Sepharose CL-4B (Sigma), followed by
immunoaffinity chromatography using antigen-conjugated Sepharose 4B
as previously described (27,28).
Immunofluorescence microscopy (General
procedures)
Immunofluorescence microscopy was described
previously (29–32). H1993 cells were grown for 2 days on
glass coverslips in 6-well plates in RPMI with 10% fetal bovine
serum. Cells pretreated with or without Ephedrae herba extract (100
μg/ml) at 37°C for 4 h were fixed with 3.7% formaldehyde in
phosphate-buffered saline (PBS), pH 7.4, permeabilized in PBS
containing 0.1% saponin. After washing with PBS, cells were blocked
with PBS-10% normal goat serum. All subsequent antibody and wash
solutions contained 0.1% saponin. H1993 cells were then
double-stained for MET with anti-MET antibody and SNX1 with
anti-SNX1 antibody or LAMP1 with anti-LAMP1 antibody, or for p-MET
with anti-p-MET antibody and SNX1 with anti-SNX1 antibody or LAMP1
with anti-LAMP1 antibody. Early endosomes were stained with
anti-SNX1 antibody, since SNX1 protein is a family of sorting nexin
proteins, and are localized to early endosomes (33,34).
Late endosomes/lysosomes were stained with
anti-LAMP1 antibody, since the LAMP1 protein is distributed within
endocytic organelles and is at its highest concentration in the
late endosomes/lysosomes, as observed for other lysosomal
glycoproteins, namely, lysosomal-associated membrane protein-1
(LAMP-1) and LAMP-2 (35,36). The cells were then incubated for 1
h with the secondary antibodies at 20 μg/ml and was further stained
with DAPI to reveal nuclei. Controls for antibody specificity were
non-immune normal mouse IgG1 or non-immune normal rabbit IgG. The
distribution of the labeled proteins was then analyzed by confocal
immunofluorescence microscopy of the fixed cells. Slides were
mounted with SlowFade anti-fade reagent and observed on a Zeiss LSM
510 META confocal laser scanning microscope (Carl Zeiss,
Oberkochen, Germany), equipped with krypton/argon laser sources.
Colocalization of MET and SNX1 or LAMP1, p-MET and SNX1 or LAMP1
was quantified using ImageJ software and the MacSCOPE X software
(Mitani Corporation, Osaka, Japan).
Immunofluorescence microscopy
(Pretreatment of cells with Ephedrae herba)
To clarify MET internalization, we followed the
uptake of HGF with time in H1993 cell line (19). To minimize the contribution of
recycling and/or lysosomal degradation of the internalized HGF, we
quantified the HGF uptake in each cell for time periods of up to 60
min. H1993 cells were starved for 3 h with RPMI without FBS at
37°C, and the serum-starved cells pretreated with or without
Ephedrae herba extract (100 μg/ml) for 2 h were then incubated with
HGF (50 ng/ml) at 37°C for 15, 30, or 60 min. The distribution of
internalized HGF stained with anti-HGF α-chain antibody and early
endosomes with EEA1 antibody or lysosomes stained with
anti-cathepsin D antibody was then assessed by confocal
immunofluorescence microscopy. Early endosomes and lysosomes were
stained with anti-EEA1 antibody and anti-cathepsin D antibody,
respectively. In another case, cells pretreated with or without
Ephedrae herba extract were starved for 3 h with RPMI without FBS
at 37°C and then the phosphorylation of MET was induced with HGF
for 15, 30, or 60 min. The fixed cells were double-stained for
p-MET with anti-p-MET antibody and LAMP1 with anti-LIMP1 antibody.
In some cases, cells pretreated with Ephedrae herba extract were
incubated with 0.17 μM bafilomycin A1 for 30 min and then
stimulated with HGF at 37°C for the indicated times, after which
further analysis was performed.
Western blot analysis
Protein samples were separated by sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). Following blocking, the membrane was blotted
with the appropriate antibody, and subsequently, horseradish
peroxidase-conjugated anti-mouse or anti-rabbit IgG (GE Healthcare
Bioscience, Tokyo, Japan) was applied. The final signal was
revealed by ECL chemiluminescence (Pierce, Rockford, IL, USA).
Digital images were analyzed with NIH Image software to measure the
density of each band without a saturated signal.
HGF-stimulated phosphorylated MET
degradation
H1993 cells were starved for 12 h with RPMI without
FBS at 37°C. The serum-starved cells pretreated with or without
Ephedrae herba extract (100 μg/ml) for 2 h were preincubated for 30
min in the presence of CHX (20 μg/ml), and then the cells were
incubated with HGF at 37°C for the indicated times. The cells were
washed with ice-cold-PBS and lysed, followed by SDS-PAGE and
western blot analysis.
Statistical analysis
Data are expressed as mean ± SD unless otherwise
noted. Significance (P<0.05) was determined by using Student's
t-test, since all data met all the assumptions for parametric
statistical analysis.
Results
Alteration of intracellular distribution
of MET and phosphorylated MET in Ephedrae herba-pretreated NSCLC
cells
To examine the effect of Ephedrae herba on the
intracellular distribution of MET and phosphorylated (p)-MET in
H1993 NSCLC cells harboring MET gene amplification and MET
overexpression (10), cells were
pretreated with Ephedrae herba extract (100 μg/ml) for 4 h at 37°C
as described in the Materials and methods. The control and Ephedrae
herba extract-pretreated cells were fixed, double-labeled with
antibodies against to MET or p-MET and sorting nexin-1 (SNX1) or
lysosomal integral membrane protein (LAMP1), and then analyzed by
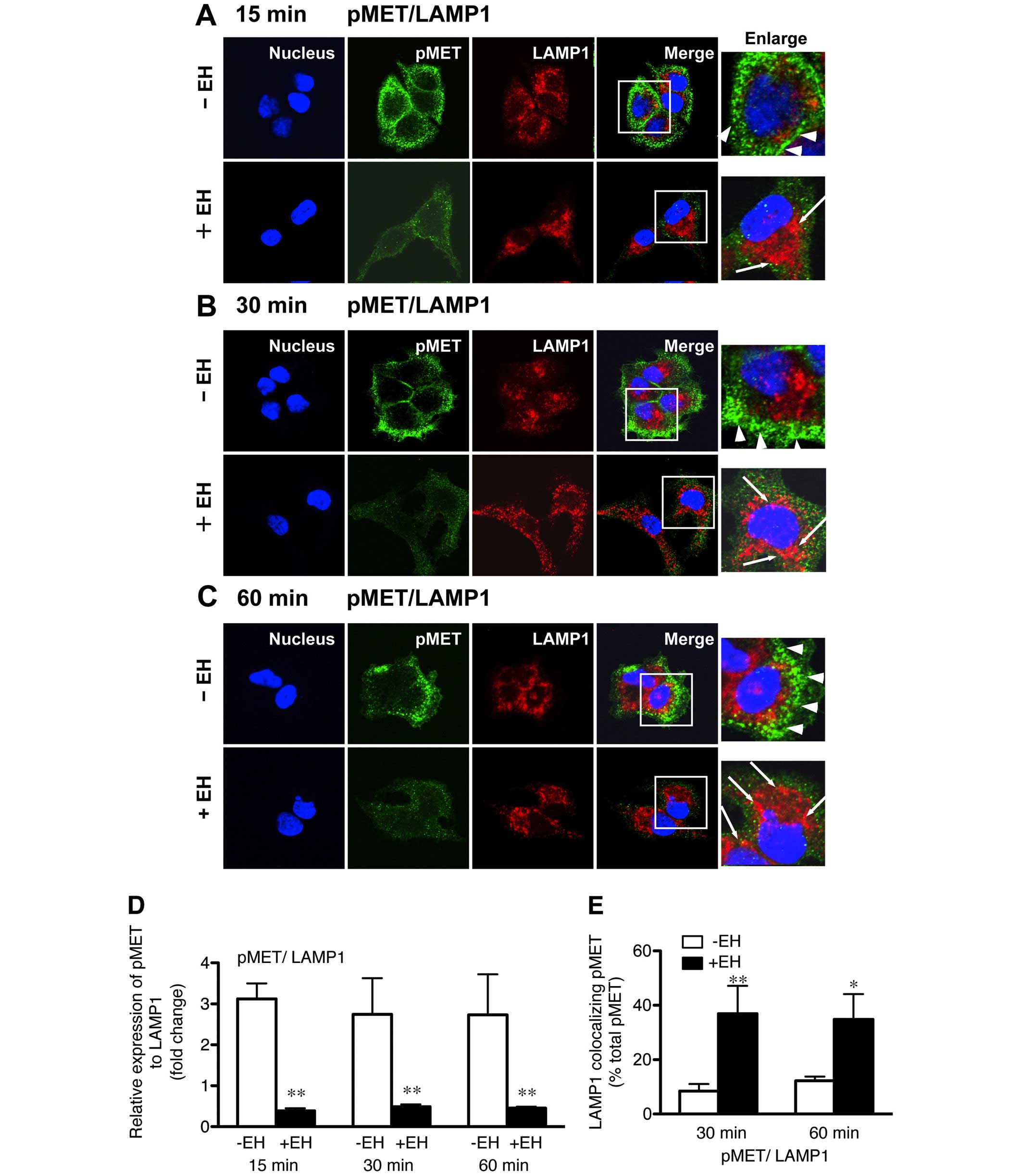
using confocal immunofluorescence microscopy (Fig. 1A–D). We examined the intracellular
distribution of MET or p-MET with SNX1 as a marker of early
endosomes, since SNX1 consists of a family of sorting nexin
proteins, and approximately 25 human sorting nexins have been
identified (33). In addition,
SNX1 is known to interact with epidermal growth factor receptor
(EGFR) (34) and are localized in
early endosomes (33). We also
determined the intracellular distribution of late
endosomes/lysosomes by using an antibody specific to LAMP1, which
is distributed in the endocytic organelles at concentrations that
are highest in late endosomes/lysosomes, as has been reported for
other lysosomal glycoproteins (35,36).
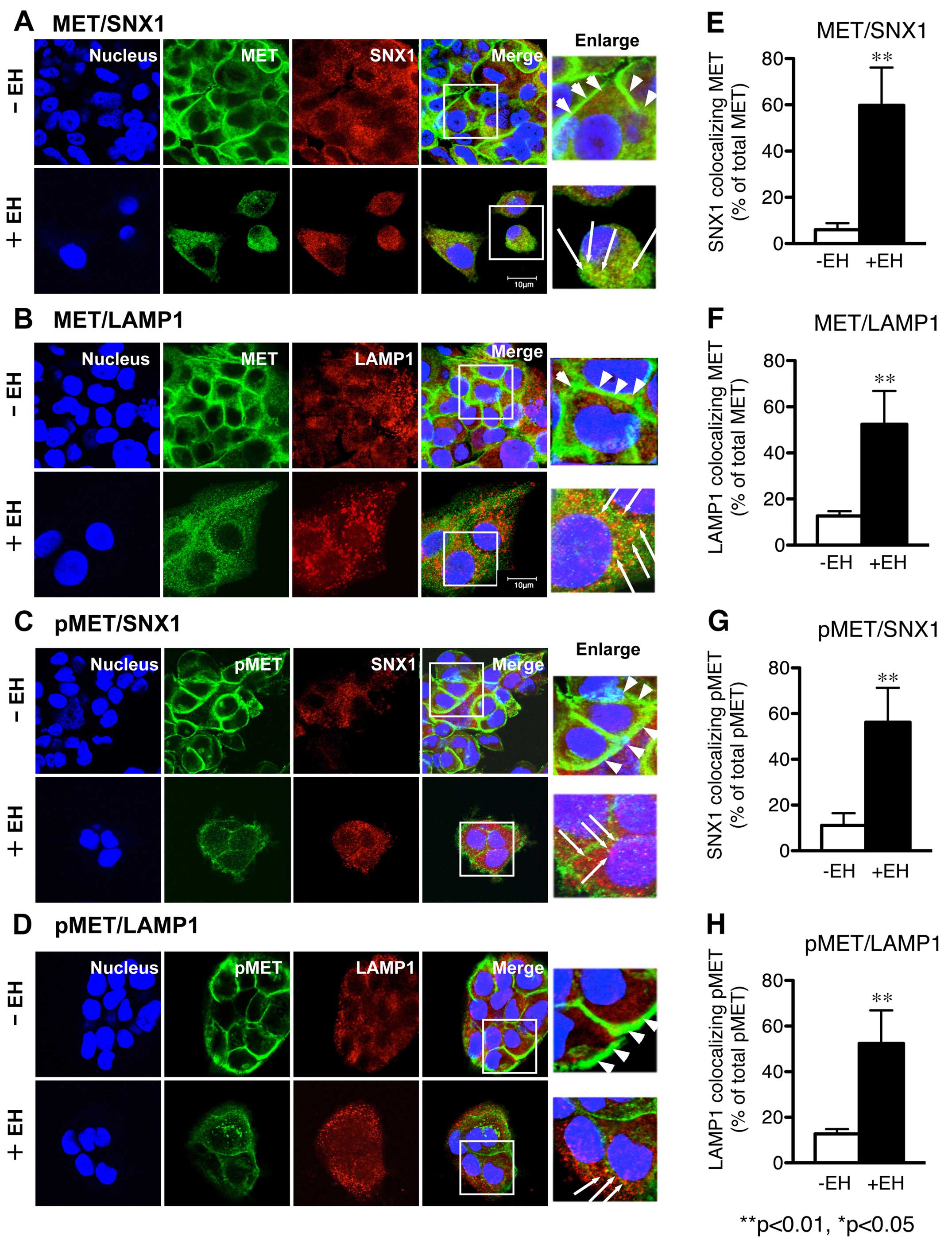 | Figure 1Effect of Ephedrae herba on
intracellular distribution of MET and phosphorylated MET in the
human H1993 gefitinib-resistant NSCLC cell line. The H1993 cells,
harboring MET overexpression, were pretreated with Ephedrae
herba extract (EH; 100 μg/ml) for 4 h at 37°C. The cells in the
control (-EH) or the Ephedrae herba-pretreated groups (+EH)
were fixed, double-labeled with antibodies specific to MET and SNX1
(A) or lysosomal integral membrane protein (LAMP1) (B), p-MET and
SNX1 (C) or LAMP1 (D), and then analyzed by using confocal
immunofluorescence microscopy as described in Materials and
methods. Superimposed images of MET or p-MET and each organelle
marker protein are also shown. Each cell was stained with DAPI
(blue) to reveal nuclei. Bar, 10 μm. Right column shows the merged
images of MET and SNX1 in (A), MET and LAMP1 (B), p-MET and SNX1
(C), or p-MET and LAMP1 (D) in H1993 cells pretreated with or
without Ephedrae herba extract, and white squares indicate
enlarged regions. The white arrowheads indicate the MET-positive
staining distributed in the plasma membranes of H1993 cells, and
the long white arrows indicate the merged confocal images as yellow
color of MET or p-MET and each organelle marker proteins spread
throughout the cytoplasm of each cell. The bar diagrams in (E, F,
G, and H) indicate the increase of colocalized confocal images of
MET or p-MET and each organelle marker proteins in H1993 cells
pretreated with (black columns) or without (white columns)
Ephedrae herba extract. Values are the percentage of the
integrated density of SNX1 or LAMP1-colocalizing MET compared to
that of total MET in (E and F), and the percentage of the
integrated density of SNX1 or LAMP1-colocalizing p-MET compared to
that of total p-MET in (G and H). The error bar denotes SD from
three separate experiments, and significance was determined using
Student's t-test. Significant difference between the values
*P<0.05; **P<0.01. In H1993 cells, MET
and p-MET is exclusively localized in the plasma membrane at a
cell-cell contact sites, however, no colocalization with organelle
marker proteins such as SNX1 and LAMP1 is observed, suggesting that
MET and p-MET is mainly associated with plasma membrane, but not
with the early endosomes or late endosomes. In contrast, the
pretreatment of cells with Ephedrae herba extract led to a
rapid loss of MET and p-MET in the cells, and the resultant MET and
p-MET staining was distributed throughout the cytoplasm. |
Confocal immunofluorescence microscopy studies
revealed that MET was exclusively localized on the plasma membrane
at a cell-cell contact sites of H1993 cells; however, no
colocalization with organelle marker proteins such as SNX1 or LAMP1
was observed. These results indicate that MET is mostly associated
with the plasma membrane but not with early endosomes or late
endosomes in H1993 cells (Fig. 1A and
B). Further, an increased level of p-MET was associated with
the plasma membranes, and p-MET-positive staining was not
colocalized with other organelle marker proteins (Fig. 1C and D). In contrast, the
pretreatment of cells with Ephedrae herba extract strongly
decreased the intracellular distribution of the plasma
membrane-associated MET and p-MET while the resultant MET and p-MET
staining was spread throughout the cytoplasm (Fig. 1A–D). Ephedrae herba extract also
led to the gradual disappearance of MET and phosphorylated MET in
the cells. Quantitative analysis of the colocalization rate was
performed and expressed as the percentage of the integrated density
of SNX1-positive early endosomes (Fig.
1E) or LAMP1-positive late endosome/lysosomes (Fig. 1F) colocalized with MET relative to
total MET (% total MET) in H1993 cells pretreated with or without
Ephedrae herba extract. Our data verified that colocalization of
MET with SNX1 (59.8±16.3 vs. 6.0±2.9% total MET) or LAMP1
(52.4±14.5 vs. 12.7±2.1% total MET) was markedly higher in the
Ephedrae herba extract-pretreated cells than it was in the control
cells. In addition, we observed that the colocalization of p-MET
and SNX1 (49.1±5.4 vs. 12.9±4.8% total MET) or LAMP1 (47.3±5.4 vs.
14.2±4.7% total MET) was also greater in the Ephedrae herba extract
pre-treated cells than it was in the control cells (Fig. 1G and H). These results indicate
that Ephedrae herba extract pretreatment abrogated the expression
of MET and p-MET in the H1993 cells and, therefore, suggests that
plasma membrane-associated MET or p-MET might be rapidly
endocytosed and sorted to the late endosomes/lysosomes in Ephedrae
herba extract-pretreated H1993 cells.
Ephedrae herba stimulates HGF-induced MET
and p-MET endocytosis and subsequent degradation in human NSCLC
cells
We made a novel discovery in the present study that
the pretreatment of H1993 cells with Ephedrae herba extract altered
the intracellular distribution of plasma membrane-associated MET
and p-MET, which led to their gradual disappearance from the cells.
Therefore, we inferred that some components of Ephedrae herba
extract might have impaired the mechanisms of MET endocytosis that
is tightly regulated by the early/late endocytic pathways.
Accordingly, to further substantiate the effect of
Ephedrae herba on the HGF-mediated disappearance of MET, we
investigated the endocytosis of HGF-induced MET in H1993 cells
pretreated with or without Ephedrae herba extract. The Ephedrae
herba extract pretreated cells were stimulated with HGF for 15, 30,
or 60 min, and then double-stained for the internalized HGF (with
anti-HGF α-chain antibody), which is regarded as HGF receptor (MET)
and EEA1 (with anti-EEA1 antibody), which is a marker of early
endosomes. Following its stimulation, HGF is recognized by the HGF
receptor (MET) and then endocytosed via an endocytic pathway.
Confocal immunofluorescence analysis confirmed that a large
proportion of the intracellular HGF-positive staining disappeared
in the Ephedrae herba extract-pretreated cells after 15-min of
HGF-stimulation (Fig. 2A),
indicating that Ephedrae herba extract pretreatment caused the
gradual disappearance of MET in the cells. Furthermore, confocal
immunofluorescence studies demonstrated the rapid endocytosis of
HGF and small punctate vesicles, which were positively stained for
internalized HGF and mostly overlapped with EEA1, and were
distributed in the cytoplasm of the cells pretreated with Ephedrae
herba extract for 15 min (Fig.
2A). The results of the quantitative analysis of the relative
expression levels of HGF-positive to endogenous EEA1 staining in
H1993 cells pretreated with or without Ephedrae herba extract
showed fold changes of 0.73±0.1 vs. 2.2±0.5, 0.7±0.1 vs. 2.7±0.9,
and 0.5±0.1 vs. 2.5±0.2 after 15, 30, 60 min of HGF-stimulation,
respectively (Fig. 2D).
In contrast, endocytosis of HGF was considerably
suppressed, and the internalized HGF remained localized in the
plasma membranes after control cells were stimulated for 15 min
with HGF (Fig. 2A). Furthermore,
the endocytosed HGF-positive staining was still accumulated and
colocalized with EEA1-positive early endosomes 60 min after
internalization (Fig. 2C). The
colocalization rate results confirmed that EEA1-colocalized MET was
higher in the cells pretreated with Ephedrae herba extract than it
was in the untreated cells after 30 and 60 min (36.5±12.8 vs.
29.2±5.4% total MET and 52.3±8.3 vs. 30.4±2.4% total MET) of HGF
stimulation, respectively (Fig.
2E). These results show that Ephedrae herba extract
pretreatment stimulated cellular HGF-induced MET endocytosis and
subsequent degradation, resulting in decreased MET protein
expression.
We also examined the effect of Ephedrae herba
extract on the HGF-induced p-MET endocytosis likely mediated by the
early/late endocytic pathways in H1993 cells. The cells were
stimulated with HGF for 15, 30, or 60 min, and then double-stained
for the internalized p-MET and LAMP1 (late endosome/lysosome
marker) with anti-p-MET and anti-LAMP1 antibodies, respectively.
The results revealed that a large proportion of p-MET was mostly
associated with the plasma membranes of the non-pretreated cells
and colocalization of p-MET with LAMP1 was not discernible even
after stimulation for 60 min (Fig.
3). However, a considerable proportion of the intracellular
p-MET-positive staining disappeared in the Ephedrae herba
extract-pretreated cells after 15-min HGF-stimulation (Fig. 3A–C), indicating that the
pretreatment of cells with Ephedrae herba extract almost depleted
cellular p-MET.
Noteworthy, we observed a rapid endocytosis of p-MET
that clearly overlapped with LAMP1 staining in the cytoplasm of the
cells pretreated with Ephedrae herba extract after 15- and 30-min
HGF stimulation (Fig. 3A and B).
In contrast, p-MET endocytosis was inhibited and a considerable
amount of p-MET staining remained associated with the plasma
membranes even 60 min after HGF stimulation of the control cells
(Fig. 3C). The Ephedrae herba
extract-induced endocytic trafficking of p-MET observed was
consistent with the effect of the extract on MET endocytosis in
H1993 cells. The results of the quantitative analysis of the
expression of p-MET staining relative to endogenous LAMP1 with or
without Ephedrae herba extract pretreatment showed fold changes of
0.4±0.1 vs. 3.1±0.4, 0.5±0.1 vs. 2.7±0.9, and 0.5±0.1 vs. 2.7±0.9
after 15, 30, and 60 min of HGF-stimulation, respectively (Fig. 3D). The colocalization rate
determination further confirmed that LAMP1-colocalized p-MET is
greater in the cells pretreated with Ephedrae herba extract than it
was in the untreated cells after both 30 and 60 min HGF stimulation
(36.9±10.3 vs. 8.5±2.6 and 34.9±9.3 vs. 12.2±1.6% total p-MET,
respectively, Fig. 3E). These
results show that some components of Ephedrae herba extract might
have stimulatory activity on both HGF-induced MET and p-MET
endocytosis and subsequently induce their degradation in human lung
cancer cells.
Lysosomal inhibitor attenuates Ephedrae
herba-stimulated MET endocytosis and MET degradation in human NSCLC
cells
To further explore the mechanism by which Ephedrae
herba stimulates the endocytosis of MET or p-MET and their
subsequent degradation in H1993 cells, we determined if the
lysosomal inhibitor, bafilomycin A1, prevented the accelerated
degradation of MET or p-MET in cells pretreated with or without
Ephedrae herba extract. Cells were pretreated with or without
Ephedrae herba extract for 2 h, incubated with bafilomycin A1 for
30 min, and then were stimulated with HGF for 30 or 60 min followed
by co-staining for HGF and cathepsin D or p-MET and LAMP1. The
results illustrated in the confocal images showed an apparent
increase in the colocalization of HGF and cathepsin D as well as
p-MET and LAMP1 in the presence of bafilomycin A1 in the Ephedrae
herba extract-pretreated cells after 30 or 60 min of HGF
stimulation (Fig. 4A and B). The
determination of the colocalization rate confirmed that cathepsin
D-colocalized HGF (60.2±8.1% total HGF) and LAMP1-colocalized p-MET
(39.2±6.5% total p-MET) were apparently higher in the cells in the
presence of bafilomycin A1 and Ephedrae herba extract-pretreatment
after 60 min of HGF stimulation (Fig.
4C) than in cells not treated with the lysosomal inhibitor.
These results indicate that baflilomycin A1 treatment considerably
blocked the HGF-induced MET and p-MET endocytosis, leading to the
increased amounts of colocalized staining of both with
LAMP1-positive late endosomes/lysosomes in the cells. Furthermore,
this observation suggests that some components of Ephedrae herba
extract might have stimulatory activity on the HGF-induced MET and
p-MET endocytosis and subsequent degradation likely was mediated
via the early/late endocytic pathways.
Activation of MET and its downstream
signaling are inhibited by Ephedrae herba following HGF stimulation
of human NSCLC cells
Our confocal immunofluorescence microscopy study
described above demonstrated that Ephedrae herba extract
considerably stimulated the endocytosis of MET and p-MET and
subsequent degradation via the early/late endocytic pathways in
H1993 cells following HGF stimulation. Therefore, we hypothesized
that Ephedrae herba might suppress MET activation and subsequently
inhibit the successive PI3K-AKT signaling pathway.
To investigate this hypothesis, we analyzed the
effect of Ephedrae herba extract pretreatment on the
phosphorylation of AKT or p44/42 MAPK. Cells pretreated with or
without Ephedrae herba extract were stimulated with HGF at 37°C for
the indicated times. The lysates were analyzed with anti-p-MET,
anti-p-AKTS473, or anti-p-p44/42 MAPK antibodies by
western blot analysis.
As shown in Fig. 5A and
B, we observed an increase in MET phosphorylation even without
HGF stimulation (0 h), and the increased levels of p-MET protein
persisted in the cells over the 4-h incubation. The human NSCLC
cell line H1993, which harbors MET gene amplification, was
reported to overexpress MET receptors that were constitutively
phosphorylated (10). Therefore,
the increased MET phosphorylation we observed even without HGF
stimulation is consistent with the previous findings (10). On the contrary, Ephedrae herba
extract pretreatment markedly suppressed the HGF-induced MET
phosphorylation and p-MET was decreased by 35, 43, and 71% at 0, 1,
and 4 h, respectively after HGF stimulation (Fig. 5A and B).
 | Figure 5Effect of Ephedrae herba on
the activation of MET tyrosine kinase receptor and downstream
signaling in human H1993 NSCLC cell line. (A) Expression of p-MET,
p-AKT (Ser473), p-MAPK (p-p44/42 MAPK), total MET, total AKT, and
total p44/42 MAPK (MAPK) before and after 0.5, 1, 2, or 4 h of HGF
stimulation in H1993 cells pretreated with or without Ephedrae
herba extract was determined by western blotting. β-actin was
used as a loading control. (B) The quantitative analysis of the
western blots is shown. Relative expression levels of p-MET, p-AKT
(Ser473), p-MAPK (p-p44/42 MAPK), total MET, total AKT, and total
p44/42 MAPK (MAPK) are shown and the values are normalized to each
phosphorylated or unphosphorylated protein before HGF stimulation
in H1993 cells. In the case of p-MAPK, the relative expression
levels of phosphorylated form of p44/42 MAPK (upper band) was
determined. The results shown are representative of three
independent experiments. p-MET, phosphorylated human MET tyrosine
kinase receptor; p-AKT, phosphorylated AKT (protein kinase B);
p-MAPK, phosphorylated p44/42 MAPK. |
In addition, a rapid increase in
p-AKTS473 was observed in the untreated cells, and the
level increased up to 24-fold that of the basal level after 2 h of
HGF stimulation. In addition, this effect was sustained during the
following 4-h incubation period (Fig.
5A and B). However, Ephedrae herba extract pretreatment
significantly suppressed AKTS473 phosphorylation, and
p-AKTS473 was decreased by 95, 80, and 94% at 1, 2, and
4 h, respectively after HGF stimulation (Fig. 5A and B). Furthermore, an increase
in p-p44/42 MAPK was observed in the untreated cells, with the
level attaining a 4.3-fold increase compared to the basal level
after at 0.5 h of HGF stimulation; however, this effect gradually
decreased during the 4-h incubation period (Fig. 5A and B). The inhibitory effect of
Ephedrae herba extract on the expression of p-p44/42 MAPK was also
evident in the cells, and p-p44/42 MAPK was decreased by 93, 88,
and 93% at 0.5, 1, and 4 h, respectively after HGF stimulation
(Fig. 5A and B). These results
indicate that Ephedrae herba extract had a strong inhibitory effect
on MET phosphorylation and subsequent activation of the PI3K-AKT
activation pathway in H1993 cells.
Next, we further analyzed the effect of Ephedrae
herba extract on the HGF-induced degradation of MET in H1993 cells
using western blot. As shown in Fig.
5, we found a gradual HGF-dependent degradation of MET in the
Ephedrae herba extract pretreated cells, and MET expression
decreased by 8, 23, and 33% at 1, 2, and 4 h after HGF stimulation
(Fig. 5A and B). In contrast,
HGF-dependent MET degradation was not seen in the untreated cells.
Furthermore, it should be noted that the protein expression levels
of AKT and p44/42 MAPK were not changed by Ephedrae herba extract
pretreatment during the 4-h incubation with HGF, indicating that
Ephedrae herba might selectively stimulate the HGF-dependent
downregulation of MET/p-MET via the early/late endocytic
pathways.
Discussion
In the present study, we evaluated the novel role of
Ephedrae herba and demonstrated that it exerted a considerable
suppressive effect on the phosphorylation of MET, leading to the
loss of MET and p-MET in human H1993 NSCLC cells. Using confocal
immunofluorescence microscopy, we found a significant reduction of
endogenous MET and p-MET protein expression in H1993 cells
pretreated with Ephedrae herba extract for 4 h. Furthermore, MET
and p-MET was exclusively localized in the plasma membrane at a
cell-cell contact sites in H1993 cells and no colocalization was
observed with the early endosomes or late endosomes/lysosomes. The
pretreatment of cells with Ephedrae herba extract markedly
decreased the levels of plasma membrane-associated MET and p-MET,
and the resultant intracellular MET/p-MET staining was distributed
throughout the cytoplasm, and large amounts were clearly
colocalized with the early/late endosome marker proteins (SNX1 and
LAMP1). These results demonstrate that pretreatment of cells with
Ephedrae herba extract inhibited MET phosphorylation and induced
the loss of MET. Moreover, the results also suggest that plasma
membrane-associated MET and p-MET might be rapidly endocytosed in
the extract-treated cells following HGF-stimulation, and then
subsequently sorted to endosomes/lysosomes. This assumption was
further confirmed by the observation that bafilomycin A1 treatment
considerably blocked HGF-induced MET and p-Met endocytosis followed
by their degradation in the Ephedrae herba extract-pretreated
cells.
We further used western blot analysis to demonstrate
that Ephedrae herba extract pretreatment markedly suppressed MET
phosphorylation following HGF stimulation during the 4-h incubation
(Fig. 5). We also showed evidence
that MET protein gradually disappeared from the cells during the
4-h stimulation with HGF. In contrast, HGF-dependent MET
degradation was not observed in the non-pretreated cells. Previous
studies showed MET gene amplification and overexpression of
MET receptors, which were constitutively phosphorylated and
activated in a human H1993 NSCLC cell line (10). Therefore, our results showing the
suppression of MET phosphorylation by Ephedrae herba during HGF
stimulation might be attributable to the enhanced endocytic
translocation of MET/p-MET from the plasma membranes via early
endosomes to late endosomes/lysosomes. Alternatively, we also
postulated that Ephedrae herba might be able to suppress MET
phosphorylation followed by its downregulation without HGF
stimulation in H1993 cells. Here, we also showed that Ephedrae
herba significantly suppressed the phosphorylation of
AKTS473 and p44/42 MAPK during the 4-h incubation with
HGF (Fig. 5). These results
indicate that Ephedrae herba might have a selective inhibitory
effect on MET phosphorylation and subsequent activation of the
PI3K-AKT and RAS/MAPK activation pathways in H1993 cells.
Furthermore, our data also support previous findings that Ephedrae
herba inhibits HGF-induced cancer cell motility via suppression of
HGF/Met-Akt signaling through the reduction of MET tyrosine kinase
activity in human MDA-MB-231 breast cancer cells (23,24).
We recently reported the novel observation that
large amounts of SNX1, a family of sorting nexin proteins that are
believed to play a role in the endocytic trafficking of EGFR
(33,34), are localized in the aggregated
vesicular structures of early endosomes where the internalized
p-EGFR is also accumulated (30,31).
Furthermore, we reported that the depletion of endogenous SNX1 by
siRNA stimulates endocytosis and the ligand-induced downregulation
of MET/p-MET and EGFR/p-EGFR while it increases p-MET and p-EGFR
protein expression in gefitinib-resistant cells (19,32).
Accordingly, we postulate that SNX1 might negatively regulate
ligand-dependent downregulation of MET and EGFR as well as their
phosphorylation via the early/late endocytic pathways in human lung
cancer cells. Therefore, our present data demonstrating the
suppressive role of Ephedrae herba on the phosphorylation of MET
and its subsequent enhanced downregulation appear consistent with
those demonstrating the role of SNX1 in HGF-induced phosphorylation
and endocytosis of MET via the endocytic pathway in human lung
cancer cells (19,32). Although the detailed mechanisms
underlying the effect of Ephedrae herba on endocytic trafficking of
transmembrane tyrosine kinase receptors such as MET remain unclear,
we inferred that some of its components might perturb the tightly
regulated processes involved in the ligand-induced MET endocytosis
via the early/late endocytic pathways in the cells.
Engelman et al (9) previously reported that MET
amplification leads to acquired resistance to gefitinib and
erlotinib in patients with lung cancer by activating erb-b2
receptor tyrosine kinase 3 (ERBB3) signaling. In addition, another
study reported that MET amplification occurs with or without a
representative secondary T790M mutation in EGFR mutant lung tumors
with acquired resistance to gefitinib or erlotinib (37). Accordingly, we inferred that
pretreatment of the cells with Ephedrae herba extract enhanced the
downregulation of HGF-stimulated MET, which might have affected the
sensitivity of the human NSCLC cells to the EGFR-tyrosine kinase
inhibitor (TKI), gefitinib. In the present study, the
identification and characterization of the main biologically active
constituents of the Ephedrae herba extract proved to be difficult
due to its complex composition. Further studies to identify and
investigate the compounds in Ephedrae herba that are biologically
active against HGF/MET activation and signaling in NSCLC cell lines
are now in progress.
Abbreviations:
|
HGF
|
hepatocyte growth factor
|
|
p-MET
|
phosphorylated MET
|
|
EGFR
|
epidermal growth factor receptor
|
|
p-EGFR
|
phosphorylated EGFR
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Di Renzo MF, Narsimhan RP, Olivero M,
Bretti S, Giordano S, Medico E, Gaglia P, Zara P and Comoglio PM:
Expression of the Met/HGF receptor in normal and neoplastic human
tissues. Oncogene. 6:1997–2003. 1991.PubMed/NCBI
|
|
2
|
Comoglio PM and Boccaccio C: The HGF
receptor family: Unconventional signal transducers for invasive
cell growth. Genes Cells. 1:347–354. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeffers M, Rong S and Vande Woude GF:
Hepatocyte growth factor/scatter factor-Met signaling in
tumorigenicity and invasion/metastasis. J Mol Med (Berl).
74:505–513. 1996. View Article : Google Scholar
|
|
4
|
Pennacchietti S, Michieli P, Galluzzo M,
Mazzone M, Giordano S and Comoglio PM: Hypoxia promotes invasive
growth by transcriptional activation of the met protooncogene.
Cancer Cell. 3:347–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuniyasu H, Yasui W, Kitadai Y, Yokozaki
H, Ito H and Tahara E: Frequent amplification of the c-met gene in
scirrhous type stomach cancer. Biochem Biophys Res Commun.
189:227–232. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kijima Y, Hokita S, Yoshinaka H, Itoh T,
Koriyama C, Eizuru Y, Akiba S and Aikou T: Amplification and
overexpression of c-met gene in Epstein-Barr virus-associated
gastric carcinomas. Oncology. 62:60–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakazawa K, Dobashi Y, Suzuki S, Fujii H,
Takeda Y and Ooi A: Amplification and overexpression of c-erbB-2,
epidermal growth factor receptor, and c-met in biliary tract
cancers. J Pathol. 206:356–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller CT, Lin L, Casper AM, Lim J, Thomas
DG, Orringer MB, Chang AC, Chambers AF, Giordano TJ, Glover TW, et
al: Genomic amplification of MET with boundaries within fragile
site FRA7G and upregulation of MET pathways in esophageal
adenocarcinoma. Oncogene. 25:409–418. 2006.
|
|
9
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lutterbach B, Zeng Q, Davis LJ, Hatch H,
Hang G, Kohl NE, Gibbs JB and Pan BS: Lung cancer cell lines
harboring MET gene amplification are dependent on Met for growth
and survival. Cancer Res. 67:2081–2088. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura T, Teramoto H and Ichihara A:
Purification and characterization of a growth factor from rat
platelets for mature parenchymal hepatocytes in primary cultures.
Proc Natl Acad Sci USA. 83:6489–6493. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Birchmeier C and Gherardi E: Developmental
roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends
Cell Biol. 8:404–410. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ullrich A and Schlessinger J: Signal
transduction by receptors with tyrosine kinase activity. Cell.
61:203–212. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodrigues GA and Park M:
Autophosphorylation modulates the kinase activity and oncogenic
potential of the Met receptor tyrosine kinase. Oncogene.
9:2019–2027. 1994.PubMed/NCBI
|
|
16
|
Ma PC, Tretiakova MS, Nallasura V,
Jagadeeswaran R, Husain AN and Salgia R: Downstream signalling and
specific inhibition of c-MET/HGF pathway in small cell lung cancer:
Implications for tumour invasion. Br J Cancer. 97:368–377. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henne WM, Buchkovich NJ and Emr SD: The
ESCRT pathway. Dev Cell. 21:77–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yarden Y: The EGFR family and its ligands
in human cancer signaling mechanisms and therapeutic opportunities.
Eur J Cancer. 37:3–8. 2001. View Article : Google Scholar
|
|
19
|
Nishimura Y, Takiguchi S, Ito S and Itoh
K: Evidence that depletion of the sorting nexin 1 by siRNA promotes
HGF-induced MET endocytosis and MET phosphorylation in a
gefitinib-resistant human lung cancer cell line. Int J Oncol.
44:412–426. 2014.
|
|
20
|
Nishimura Y, Takiguchi S, Ito S and Itoh
K: EGF-stimulated AKT activation is mediated by EGFR recycling via
an early endocytic pathway in a gefitinib-resistant human lung
cancer cell line. Int J Oncol. 46:1721–1729. 2015.PubMed/NCBI
|
|
21
|
Hyuga S, Hyuga M, Yamagata S, Yamagata T
and Hanawa T: Mao-to, a Kampo medicine, inhibits migration of
highly metastatic osteosarcoma cells. J Trad Med. 21:174–181.
2004.
|
|
22
|
Hyuga S, Hyuga M, Nakanishi H, Ito H,
Watanabe K, Oikawa T and Hanawa T: Maoto, Kampo medicine,
suppresses the metastatic potential of highly metastatic
osteosarcoma cells. J Trad Med. 24:51–58. 2007.
|
|
23
|
Hyuga S, Shiraishi M, Hyuga M, Goda Y and
Hanawa T: Ephedrae herba, a major component of maoto, inhibits the
HGF-induced motility of human breast cancer MDA-MB-231 cells
through suppression of c-Met tyrosine phosphorylation and c-Met
expression. J Trad Med. 28:128–138. 2011.
|
|
24
|
Hyuga S and Hanawa T: Basic research on
the use of Kampo medicines to protect against cancer recurrence and
metastasis. J Trad Med. 30:19–26. 2013.
|
|
25
|
Amakura Y, Yoshimura M, Yamakami S,
Yoshida T, Wakana D, Hyuga M, Hyuga S, Hanawa T and Goda Y:
Characterization of phenolic constituents from ephedra herb
extract. Molecules. 18:5326–5334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hyuga S, Hyuga M, Yoshimura M, Amakura Y,
Goda Y and Hanawa T: Herbacetin, a constituent of ephedrae herba,
suppresses the HGF-induced motility of human breast cancer
MDA-MB-231 cells by inhibiting c-Met and Akt phosphorylation.
Planta Medica. 79:1525–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura Y, Higaki M and Kato K:
Identification of a precursor form of cathepsin D in microsomal
lumen: Characterization of enzymatic activation and proteolytic
processing in vitro. Biochem Biophys Res Commun. 148:335–343. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishimura Y, Kawabata T and Kato K:
Identification of latent procathepsins B and L in microsomal lumen:
Characterization of enzymatic activation and proteolytic processing
in vitro. Arch Biochem Biophys. 261:64–71. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishimura Y, Bereczky B and Ono M: The
EGFR inhibitor gefitinib suppresses ligand-stimulated endocytosis
of EGFR via the early/late endocytic pathway in non-small cell lung
cancer cell lines. Histochem Cell Biol. 127:541–553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishimura Y, Yoshioka K, Bereczky B and
Itoh K: Evidence for efficient phosphorylation of EGFR and rapid
endocytosis of phosphorylated EGFR via the early/late endocytic
pathway in a gefitinib-sensitive non-small cell lung cancer cell
line. Mol Cancer. 7:422008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishimura Y, Yoshioka K, Takiguchi S,
Bereczky B, Nakabeppu Y and Itoh K: A role for SNX1 in the
regulation of EGF-dependent phosphorylated EGFR endocytosis via the
early/late endocytic pathway in a gefitinib-sensitive human lung
cancer cells. Curr Signal Transduct Ther. 6:383–395. 2011.
View Article : Google Scholar
|
|
32
|
Nishimura Y, Takiguchi S, Yoshioka K,
Nakabeppu Y and Itoh K: Silencing of SNX1 by siRNA stimulates the
ligand-induced endocytosis of EGFR and increases EGFR
phosphorylation in gefitinib-resistant human lung cancer cell
lines. Int J Oncol. 41:1520–1530. 2012.PubMed/NCBI
|
|
33
|
Worby CA and Dixon JE: Sorting out the
cellular function of sorting nexins. Nat Rev Mol Cell Biol.
3:919–931. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kurten RC, Cadena DL and Gill GN: Enhanced
degradation of EGF receptors by a sorting nexin, SNX1. Science.
272:1008–1010. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kornfeld S and Mellman I: The biogenesis
of lysosomes. Annu Rev Cell Biol. 5:483–525. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandoval IV, Arredondo JJ, Alcalde J,
Gonzalez-Noriega A, Vandekerckhove J, Jimenez MA and Rico M: The
residues Leu (Ile) 475-Ile (Leu) 476, contained in the extended
carboxyl cytoplasmic tail, are critical for targeting of the
resident lysosomal membrane protein LIMPII to lysosomes. J Biol
Chem. 269:6622–6631. 1994.PubMed/NCBI
|
|
37
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|