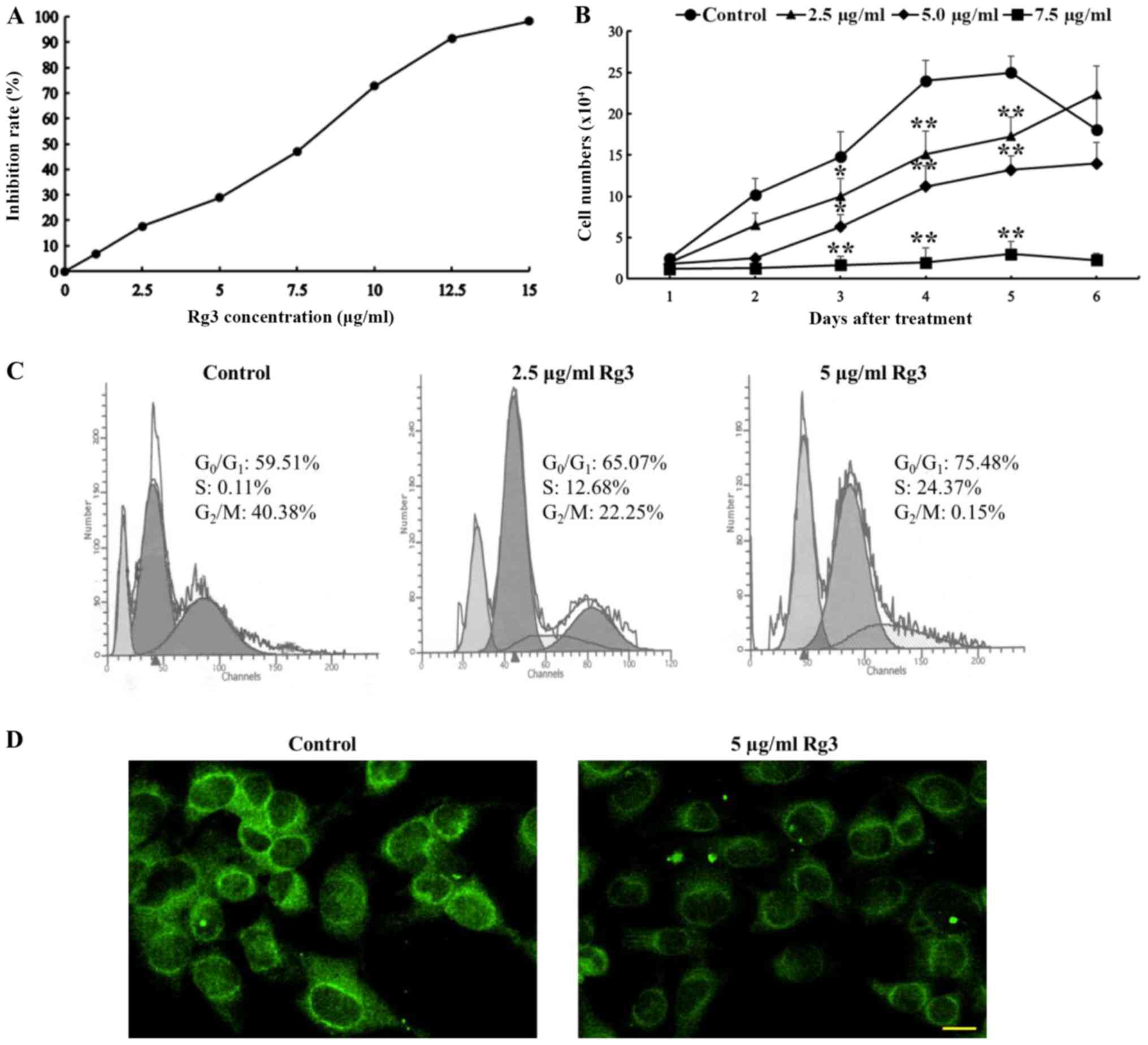
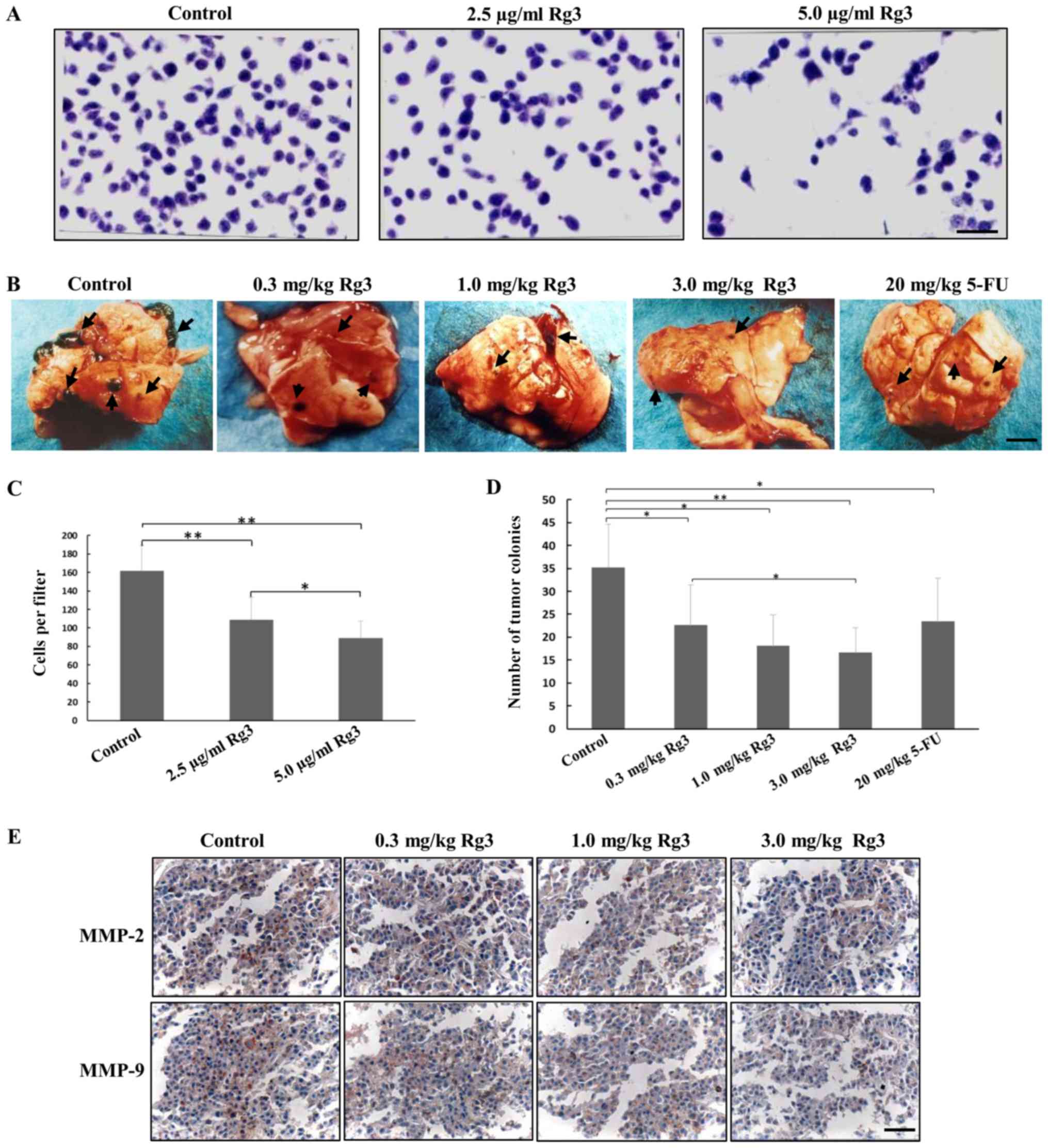
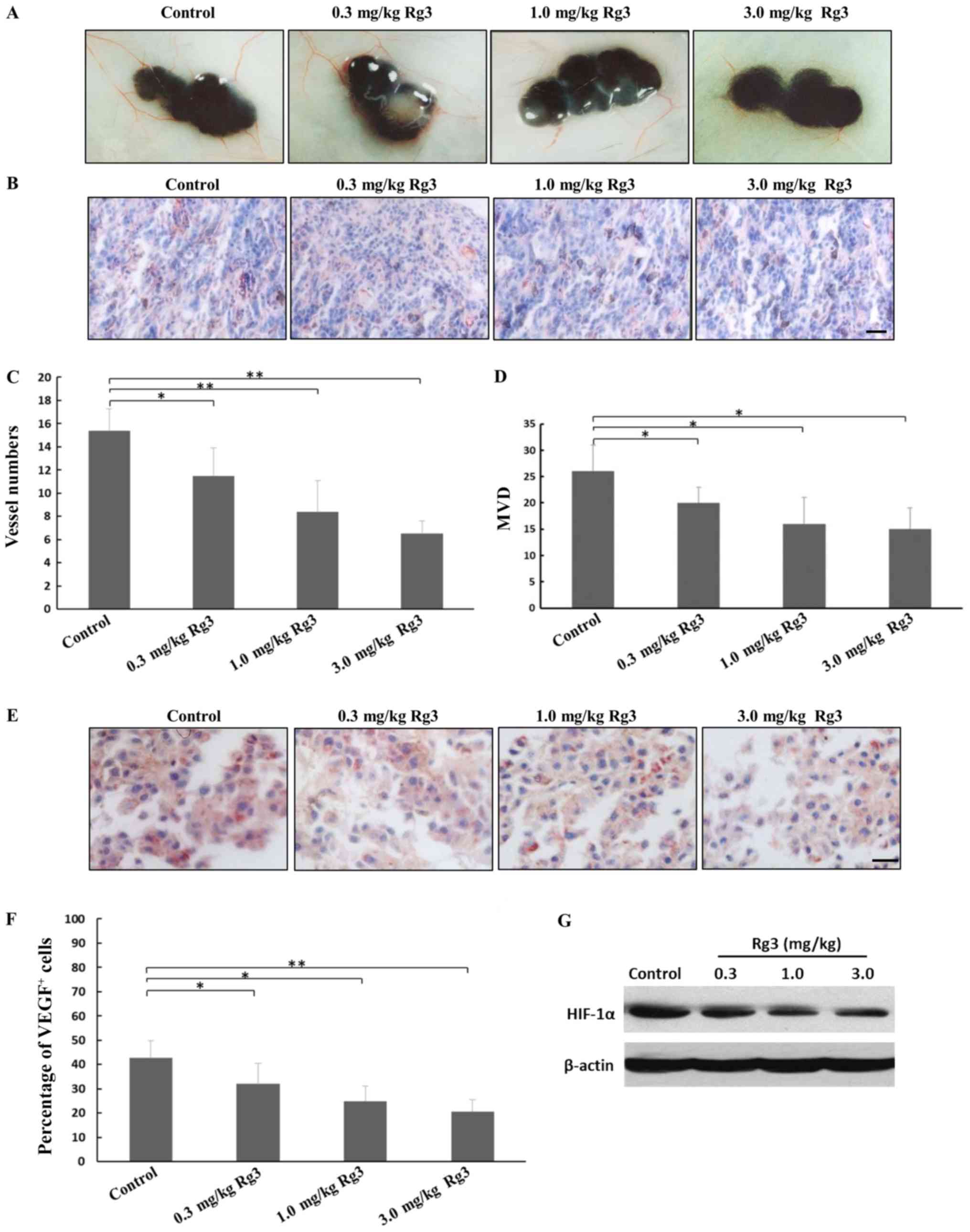
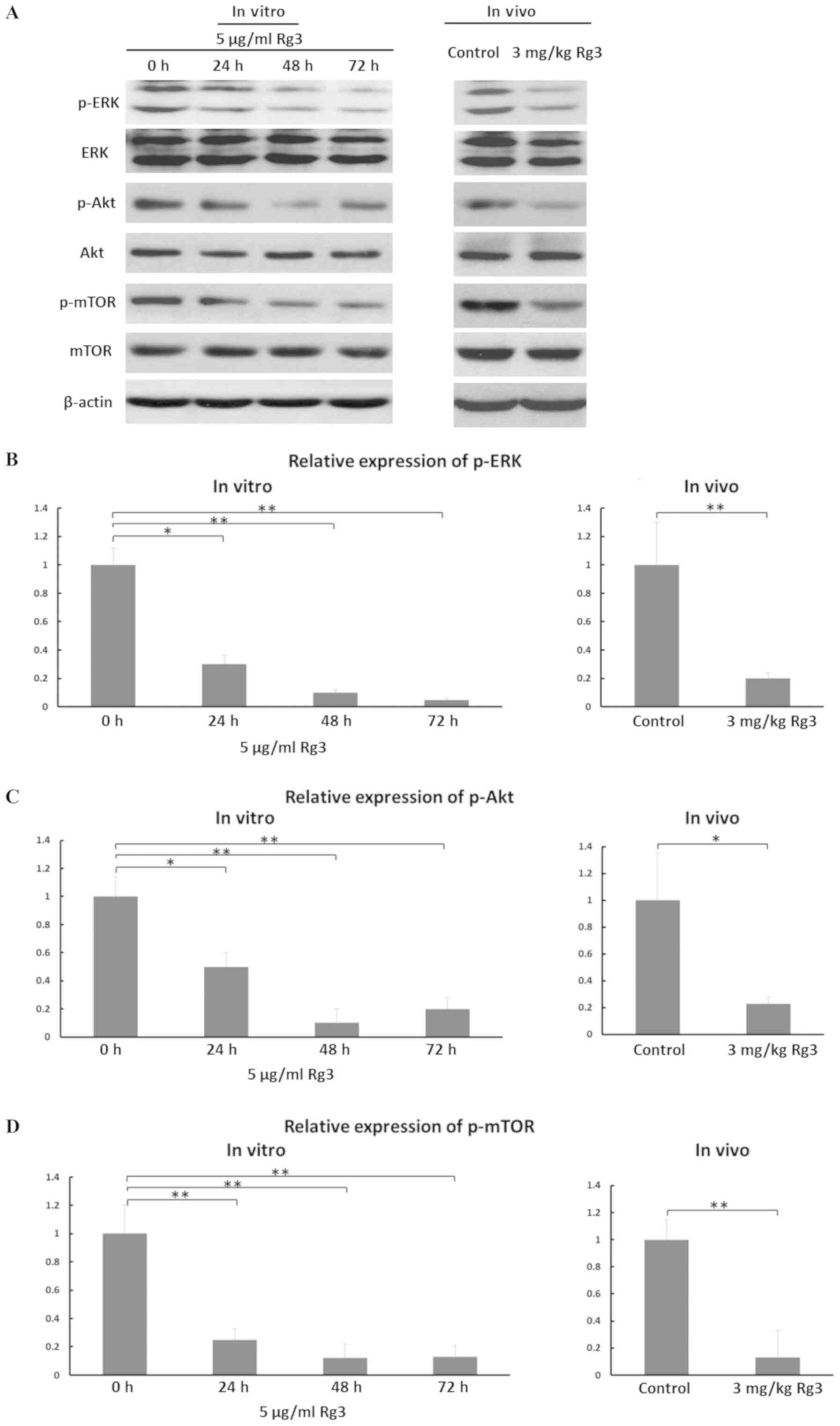
|
1
|
Clark WH Jr, Elder DE and Van Horn M: The
biologic forms of malignant melanoma. Hum Pathol. 17:443–450. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Freedman DM, Dosemeci M and McGlynn K:
Sunlight and mortality from breast, ovarian, colon, prostate, and
non-melanoma skin cancer: A composite death certificate based
case-control study. Occup Environ Med. 59:257–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burke EE and Zager JS: Pharmacokinetic
drug evaluation of tali-mogene laherparepvec for the treatment of
advanced melanoma. Expert Opin Drug Metab Toxicol. 14:469–473.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balch CM, Buzaid AC, Soong S-J, Atkins MB,
Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr,
Kirkwood JM, et al: Final version of the American Joint Committee
on Cancer staging system for cutaneous melanoma. J Clin Oncol.
19:3635–3648. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: An overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
8
|
Cerezo M, Tichet M, Abbe P, Ohanna M,
Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P,
Bertolotto C, et al: Metformin blocks melanoma invasion and
metastasis development in a AMPK/p53-dependent manner. Molecular
cancer therapeutics:. Mol Cancer Ther. 12:1605–1615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim H-S, Kim M-J, Kim EJ, Yang Y, Lee M-S
and Lim J-S: Berberine-induced AMPK activation inhibits the
metastatic potential of melanoma cells via reduction of ERK
activity and COX-2 protein expression. Biochem Pharmacol.
83:385–394. 2012. View Article : Google Scholar
|
|
10
|
Woodard J and Platanias LC: AMP-activated
kinase (AMPK)-generated signals in malignant melanoma cell growth
and survival. Biochem Biophys Res Commun. 398:135–139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meier F, Schittek B, Busch S, Garbe C,
Smalley K, Satyamoorthy K, Li G and Herlyn M: The RAS/RAF/MEK/ERK
and PI3K/AKT signaling pathways present molecular targets for the
effective treatment of advanced melanoma. Front Biosci.
10:2986–3001. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimizu T, Tolcher AW, Papadopoulos KP,
Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B,
et al: The clinical effect of the dual-targeting strategy involving
PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced
cancer. Clin Cancer Res. 18:2316–2325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zimmer L, Hillen U, Livingstone E,
Lacouture ME, Busam K, Carvajal RD, Egberts F, Hauschild A,
Kashani-Sabet M, Goldinger SM, et al: Atypical melanocytic
proliferations and new primary melanomas in patients with advanced
melanoma undergoing selective BRAF inhibition. J Clin Oncol.
30:2375–2383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vultur A, Villanueva J and Herlyn M:
Targeting BRAF in advanced melanoma: A first step toward manageable
disease. Clin Cancer Res. 17:1658–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montone KT, Elenitsas R and Elder D:
Proto-oncogene c-kit expression in malignant melanoma: protein loss
with tumor progression. Mod Pathol. 10:939–944. 1997.PubMed/NCBI
|
|
17
|
Carvajal RD, Antonescu CR, Wolchok JD,
Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ,
Chmielowski B, Lutzky J, et al: KIT as a therapeutic target in
metastatic melanoma. JAMA. 305:2327–2334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumaz N, Hayward R, Martin J, Ogilvie L,
Hedley D, Curtin JA, Bastian BC, Springer C and Marais R: In
melanoma, RAS mutations are accompanied by switching signaling from
BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res.
66:9483–9491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernández-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhomen N and Marais R: BRAF signaling and
targeted therapies in melanoma. Hematol Oncol Clin North Am.
23:529–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo J, Si L, Kong Y, Flaherty KT, Xu X,
Zhu Y, Corless CL, Li L, Li H, Sheng X, et al: Phase II,
open-label, single-arm trial of imatinib mesylate in patients with
metastatic melanoma harboring c-Kit mutation or amplification. J
Clin Oncol. 29:2904–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brose MS, Volpe P, Feldman M, Kumar M,
Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, et
al: BRAF and RAS mutations in human lung cancer and melanoma.
Cancer Res. 62:6997–7000. 2002.PubMed/NCBI
|
|
23
|
Mahoney KM, Freeman GJ and McDermott DF:
The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in
melanoma. Clin Ther. 37:764–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-p rogrammed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar
|
|
26
|
Wu R, Ru Q, Chen L, Ma B and Li C:
Stereospecificity of ginse-noside Rg3 in the promotion of cellular
immunity in hepatoma H22-bearing mice. J Food Sci. 79:H1430–H1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang LQ, Wang B, Gan H, Fu ST, Zhu XX, Wu
ZN, Zhan DW, Gu RL, Dou GF and Meng ZY: Enhanced oral
bioavailability and anti-tumour effect of paclitaxel by
20(s)-ginsenoside Rg3 in vivo. Biopharm Drug Dispos. 33:425–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ,
Hong SS, Kwon SW and Park JH: Proteomic analysis of the anti-cancer
effect of 20S-ginsenoside Rg3 in human colon cancer cell lines.
Biosci Biotechnol Biochem. 73:811–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS,
Kim Y, Han SB, Oh KW and Hong JT: Inhibition of NF-kappaB by
ginsenoside Rg3 enhances the susceptibility of colon cancer cells
to docetaxel. Arch Pharm Res. 32:755–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Y, Zhang L and Lu Q, Wang X, Yu F,
Wang X and Lu Q: NGF-induced Tyro3 and Axl function as survival
factors for differentiating PC12 cells. Biochem Biophys Res Commun.
378:371–375. 2009. View Article : Google Scholar
|
|
31
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Kang X, Yang B, Wang J and Yang
F: Antiangiogenic effect of capecitabine combined with ginsenoside
Rg3 on breast cancer in mice. Cancer Biother Radiopharm.
23:647–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J-H, Nao J-F, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar
|
|
35
|
Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X,
Xiang S, Li H, Jiang L, Tan Z, et al: 20(S)-ginsenoside Rg3
promotes senescence and apoptosis in gallbladder cancer cells via
the p53 pathway. Drug Des Devel Ther. 9:3969–3987. 2015.PubMed/NCBI
|
|
36
|
Sin S, Kim SY and Kim SS: Chronic
treatment with ginsenoside Rg3 induces Akt-dependent senescence in
human glioma cells. Int J Oncol. 41:1669–1674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu G-Y, Bu X, Yan H and Jia WW-G:
20S-protopanaxadiol-induced programmed cell death in glioma cells
through caspase-dependent and -independent pathways. J Nat Prod.
70:259–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiu X-M, Bai X, Jiang H-F, He P and Wang
J-H: 20-(s)-ginse-noside Rg3 induces apoptotic cell death in human
leukemic U937 and HL-60 cells through PI3K/Akt pathways. Anticancer
Drugs. 25:1072–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee J-Y, Jung KH, Morgan MJ, Kang YR, Lee
HS, Koo GB, Hong SS, Kwon SW and Kim YS: Sensitization of
TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated
DR5 upregulation in human hepatocellular carcinoma cells. Mol
Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
40
|
Park H-M, Kim S-J, Kim J-S and Kang H-S:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Keum Y-S, Han SS, Chun K-S, Park KK, Park
JH, Lee SK and Surh YJ: Inhibitory effects of the ginsenoside Rg3
on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB
activation and tumor promotion. Mutat Res. 523–524. 75–85.
2003.
|
|
42
|
Kang L-J, Choi Y-J and Lee S-G:
Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1
signalling by ginse-noside Rg3 attenuates hepatitis B virus
replication. Int J Biochem Cell Biol. 45:2612–2621. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu T-G, Huang Y, Cui D-D, Huang XB, Mao
SH, Ji LL, Song HB and Yi C: Inhibitory effect of ginsenoside Rg3
combined with gemcitabine on angiogenesis and growth of lung cancer
in mice. BMC Cancer. 9:2502009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim J-W, Jung S-Y, Kwon Y-H, Lee JH, Lee
YM, Lee BY and Kwon SM: Ginsenoside Rg3 attenuates tumor
angiogenesis via inhibiting bioactivities of endothelial progenitor
cells. Cancer Biol Ther. 13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yuan HD, Quan H-Y, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.
|
|
46
|
Covington KR, Brusco L, Barone I,
Tsimelzon A, Selever J, Corona-Rodriguez A, Brown P, Kumar R,
Hilsenbeck SG and Fuqua SA: Metastasis tumor-associated protein 2
enhances metastatic behavior and is associated with poor outcomes
in estrogen receptor-negative breast cancer. Breast Cancer Res
Treat. 141:375–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ji R, Tian S, Lu HJ and Lu Q, Zheng Y,
Wang X, Ding J, Li Q and Lu Q: TAM receptors affect adult brain
neurogenesis by negative regulation of microglial cell activation.
J Immunol. 191:6165–6177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wieczorek E, Jablonska E, Wasowicz W and
Reszka E: Matrix metalloproteinases and genetic mouse models in
cancer research: A mini-review. Tumour Biol. 36:163–175. 2015.
View Article : Google Scholar :
|
|
50
|
Foda HD and Zucker S: Matrix
metalloproteinases in cancer invasion, metastasis and angiogenesis.
Drug Discov Today. 6:478–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar
|
|
52
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69(Suppl 3): 4–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shan X, Fu Y-S, Aziz F, Wang X-Q, Yan Q
and Liu J-W: Ginsenoside Rg3 inhibits melanoma cell proliferation
through down-regulation of histone deacetylase 3 (HDAC3) and
increase of p53 acetylation. PLoS One. 9:e1154012014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Molinari M: Cell cycle checkpoints and
their inactivation in human cancer. Cell Prolif. 33:261–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26A:3579–3583. 2006.
|
|
59
|
Adya R, Tan BK, Punn A, Chen J and Randeva
HS: Visfatin induces human endothelial VEGF and MMP-2/9 production
via MAPK and PI3K/Akt signalling pathways: Novel insights into
visfatin-induced angiogenesis. Cardiovasc Res. 78:356–365. 2008.
View Article : Google Scholar
|
|
60
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
62
|
Kim B-M, Kim D-H, Park J-H, Surh Y-J and
Na H-K: Ginsenoside Rg3 inhibits constitutive activation of NF-κB
signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as
potential upstream targets. J Cancer Prev. 19:23–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei X, Chen J, Su F, Su X, Hu T and Hu S:
Stereospecificity of ginsenoside Rg3 in promotion of the immune
response to ovalbumin in mice. Int Immunol. 24:465–471. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Janeway CA, Travers P, Walport M and
Shlomchik MJ: Manipulation of the Immune Response. Immunobiology:
the immune system in health and disease 6th edition Garland Science
New York, NY: pp. 665–706. 2005
|
|
65
|
Ribas A, Dummer R, Puzanov I, VanderWalde
A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J,
Fernandez E, et al: Oncolytic virotherapy promotes intratumoral T
cell infiltration and improves anti-PD-1 immunotherapy. Cell.
170:1109–1119. e11102017. View Article : Google Scholar : PubMed/NCBI
|