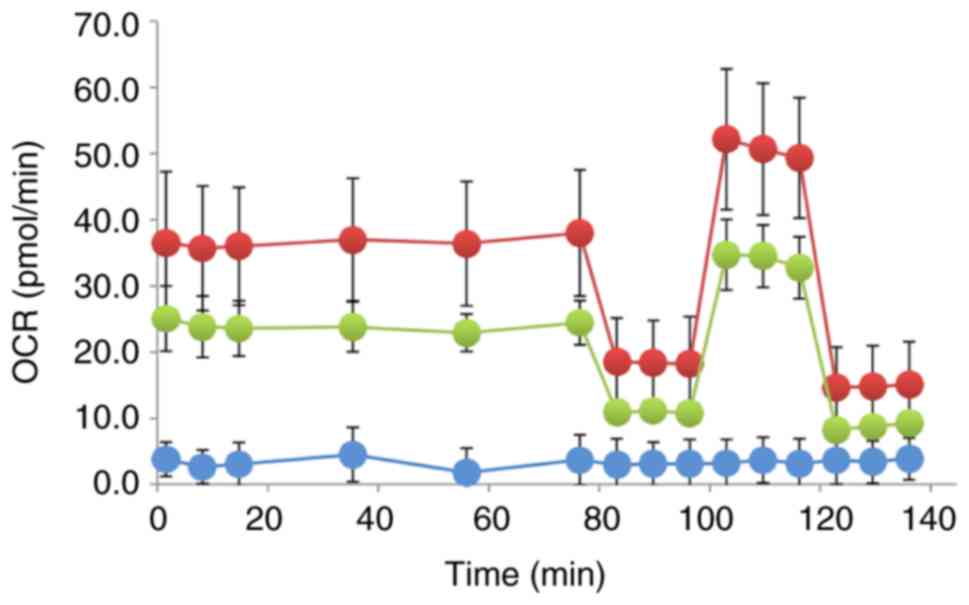
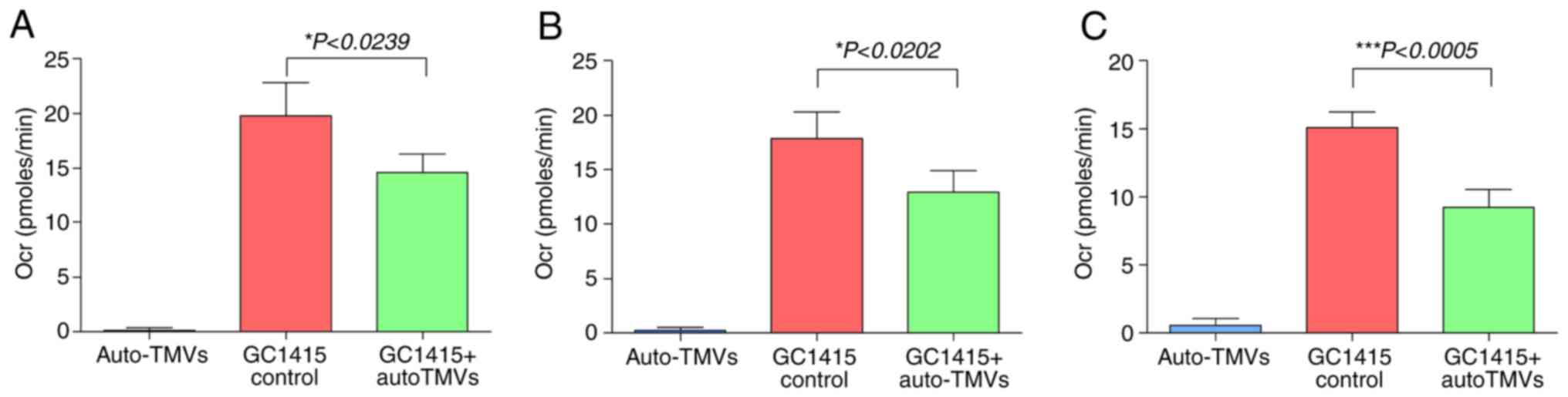
|
1
|
Yada T, Yokoi C and Uemura N: The current
state of diagnosis and treatment for early gastric cancer. Diagn
Ther Endosc. 2013:2413202013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture Eur J Cancer. 37(Suppl
8): S4–S66. 2001.
|
|
5
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Pol E, Hoekstra AG, Sturk A, Otto
C, van Leeuwen TG and Nieuwland R: Optical and non-optical methods
for detection and characterization of microparticles and exosomes.
J Thromb Haemost. 8:2596–2607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baj-Krzyworzeka M, Szatanek R, Weglarczyk
K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ and Zembala M:
Tumour-derived microvesicles carry several surface determinants and
mRNA of tumour cells and transfer some of these determinants to
monocytes. Cancer Immunol Immunother. 55:808–818. 2006. View Article : Google Scholar
|
|
10
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webber J, Steadman R, Mason MD, Tabi Z and
Clayton A: Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sidhu SS, Mengistab AT, Tauscher AN,
LaVail J and Basbaum C: The microvesicle as a vehicle for EMMPRin
in tumor-stromal interactions. Oncogene. 23:956–963. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wieckowski EU, Visus C, Szajnik M,
Szczepanski MJ, Storkus WJ and Whiteside TL: Tumor-derived
microvesicles promote regulatory T cell expansion and induce
apoptosis in tumor-reactive activated CD8+ T lymphocytes. J
Immunol. 183:3720–3730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gamperl H, Plattfaut C, Freund A, Quecke
T, Theophil F and Gieseler F: Extracellular vesicles from malignant
effusions induce tumor cell migration: Inhibitory effect of LMWH
tinzaparin. Cell Biol Int. 40:1050–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ristorcelli E, Beraud E, Mathieu S,
Lombardo D and Verine A: Essential role of Notch signaling in
apoptosis of human pancreatic tumoral cells mediated by exosomal
nanoparticles. Int J Cancer. 125:1016–1026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Nedawi K, Meehan B, Micallef J, Lhotak
V, May L, Guha A and Rak J: Intercellular transfer of the oncogenic
receptor EGFRvIII by microvesicles derived from tumour cells. Nat
Cell Biol. 10:619–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mytar B, Stec M, Szatanek R, Węglarczyk K,
Szewczyk K, Szczepanik A, Drabik G, Baran J, Siedlar M and
Baj-Krzyworzeka M: Characterization of human gastric
adeno-carcinoma cell lines established from peritoneal ascites.
Oncol Lett. 15:4849–4858. 2018.PubMed/NCBI
|
|
18
|
Stec M, Szatanek R, Baj-Krzyworzeka M,
Baran J, Zembala M, Barbasz J, Waligórska A, Dobrucki JW, Mytar B,
Szczepanik A, et al: Interactions of tumour-derived micro(nano)
vesicles with human gastric cancer cells. J Transl Med. 13:3762015.
View Article : Google Scholar
|
|
19
|
Baj-Krzyworzeka M, Mytar B, Szatanek R,
Surmiak M, Węglarczyk K, Baran J and Siedlar M: Colorectal
cancer-derived microvesicles modulate differentiation of human
monocytes to macrophages. J Transl Med. 14:362016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szatanek R, Drabik G, Baran J,
Kolodziejczyk P, Kulig J, Stachura J and Zembala M: Detection of
isolated tumour cells in the blood and bone marrow of patients with
gastric cancer by combined sorting, isolation and determination of
MAGE-1, -2 mRNA expression. Oncol Rep. 19:1055–1060.
2008.PubMed/NCBI
|
|
21
|
Rutkowska-Zapała M, Suski M, Szatanek R,
Lenart M, Węglarczyk K, Olszanecki R, Grodzicki T, Strach M,
Gąsowski J and Siedlar M: Human monocyte subsets exhibit divergent
angiotensin I-converting activity. Clin Exp Immunol. 181:126–132.
2015. View Article : Google Scholar
|
|
22
|
Théry C, Witwer KW, Aikawa E, Alcaraz MJ,
Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F,
Atkin-Smith GK, et al: Minimal information for studies of
extracellular vesicles 2018 (MISEV2018): A position statement of
the international society for extracellular vesicles and update of
the MISEV2014 guidelines. J Extracell Vesicles. 7:15357502018.
View Article : Google Scholar
|
|
23
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, et al: Sustained proliferation in
cancer: Mechanisms and novel therapeutic targets. Semin Cancer
Biol. 35(Suppl): S25–S54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McMurray RW, Ndebele K, Hardy KJ and
Jenkins JK: 17-beta-estradiol suppresses IL-2 and IL-2 receptor.
Cytokine. 14:324–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
27
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sionov RV and Haupt Y: The cellular
response to p53: The decision between life and death. Oncogene.
18:6145–6157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prives C and Hall PA: The p53 pathway. J
Pathol. 187:112–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iyer P, Shrikhande SV, Ranjan M, Joshi A,
Gardi N, Prasad R, Dharavath B, Thorat R, Salunkhe S, Sahoo B, et
al: ERBB2 and KRAS alterations mediate response to EGFR inhibitors
in early stage gallbladder cancer. Int J Cancer. 144:2008–2019.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shaw RJ: Glucose metabolism and cancer.
Curr Opin Cell Biol. 18:598–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
WARBURG O: Origin of cancer cells.
Oncologia. 9:75–83. 1956.In German. View Article : Google Scholar
|
|
36
|
Azarhoosh R, Ebneghasem R and Besharat S:
HER-2/neu gene amplification in gastric adenocarcinoma and its
relationship with clinical and pathological findings. J
Gastrointest Oncol. 8:1046–1050. 2017. View Article : Google Scholar
|
|
37
|
Tateishi M, Ishida T, Mitsudomi T, Kaneko
S and Sugimachi K: Prognostic value of c-erbB-2 protein expression
in human lung adenocarcinoma and squamous cell carcinoma. Eur J
Cancer. 27:1372–1375. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pfeiffer T, Schuster S and Bonhoeffer S:
Cooperation and competition in the evolution of ATP-producing
pathways. Science. 292:504–507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamanaka RB and Chandel NS: Targeting
glucose metabolism for cancer therapy. J Exp Med. 209:211–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iwasa S, Yanagawa T, Fan J and Katoh R:
Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric
cancer is associated with lymph node and liver metastasis.
Anticancer Res. 29:4751–4758. 2009.PubMed/NCBI
|
|
41
|
Ma DM, Luo DX and Zhang J: SDF-1/CXCR7
axis regulates the proliferation, invasion, adhesion, and
angiogenesis of gastric cancer cells. World J Surg Oncol.
14:2562016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okuma A, Hanyu A, Watanabe S and Hara E:
p16Ink4a and p21Cip1/Waf1 promote tumour
growth by enhancing myeloid-derived suppressor cells chemotaxis.
Nat Commun. 8:20502017. View Article : Google Scholar
|