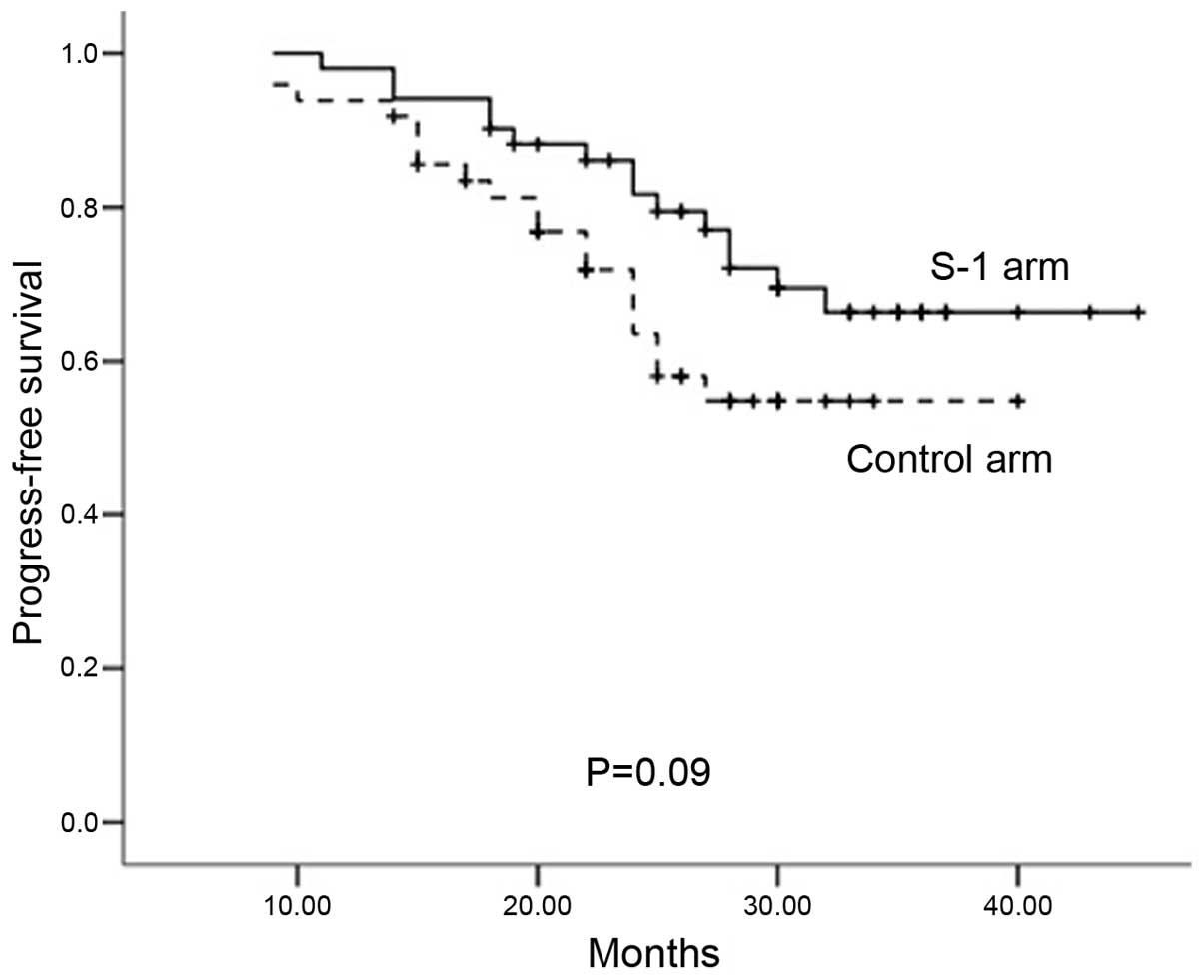
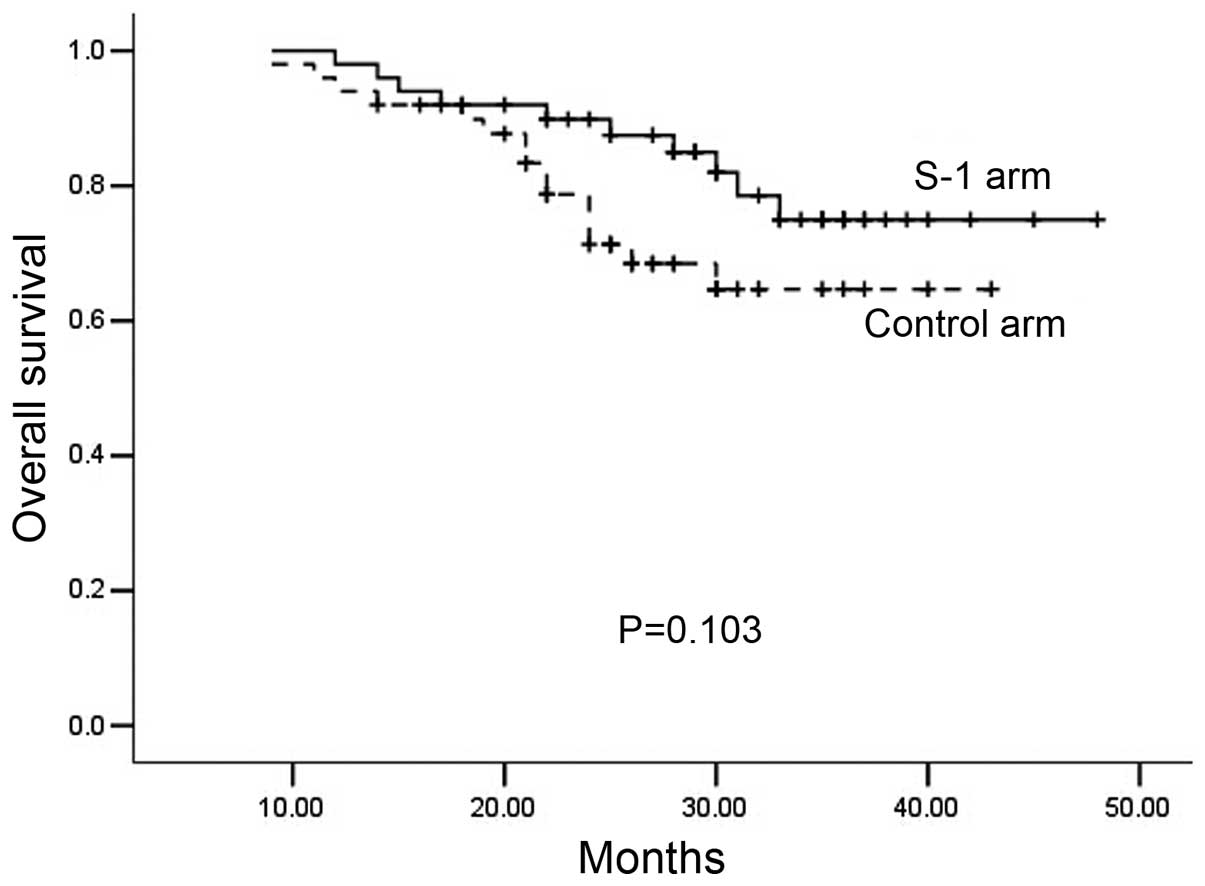
|
1
|
Cheng SH, Jian JJ, Tsai SY, Chan KY, Yen
LK, Chu NM, Tan TD, Tsou MH and Huang AT: Prognostic features and
treatment outcome in locoregionally advanced nasopharyngeal
carcinoma following concurrent chemotherapy and radiotherapy. Int J
Radiat Oncol Biol Phys. 41:755–762. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sultanem K, Shu HK, Xia P, Akazawa C,
Quivey JM, Verhey LJ and Fu KK: Three-dimensional
intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: the University of California-San Francisco experience.
Int J Radiat Oncol Biol Phys. 48:711–722. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng SH, Yen KL, Jian JJ, Tsai SY, Chu
NM, Leu SY, Chan KY, Tan TD, Cheng JC, Hsieh CY and Huang AT:
Examining prognostic factors and patterns of failure in
nasopharyngeal carcinoma following concomitant radiotherapy and
chemotherapy: impact on future clinical trials. Int J Radiat Oncol
Biol Phys. 50:717–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fareed MM, AlAmro AS, Bayoumi Y, Tunio MA,
Ismail AS, Akasha R, Mubasher M and Al Asiri M: Intensity-modulated
radiotherapy with simultaneous modulated accelerated boost
technique and chemotherapy in patients with nasopharyngeal
carcinoma. BMC Cancer. 13:3182013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan AT, Ma BB, Lo YM, Leung SF, Kwan WH,
Hui EP, Mok TS, Kam M, Chan LS, Chiu SK, Yu KH, Cheung KY, Lai K,
Lai M, Mo F, Yeo W, King A, Johnson PJ, Teo PM and Zee B: Phase II
study of neoadjuvant carboplatin and paclitaxel followed by
radiotherapy and concurrent cisplatin in patients with
locoregionally advanced nasopharyngeal carcinoma: therapeutic
monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol.
22:3053–3060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng J, Wang G, Yang GY and Wang D, Luo
X, Chen C, Zhang Z, Li Q, Xu W, Li Z and Wang D: Induction
chemotherapy with nedaplatin with 5-FU followed by
intensity-modulated radiotherapy concurrent with chemotherapy for
locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol.
40:425–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du C, Ying H, Zhou J, Hu C and Zhang Y:
Experience with combination of docetaxel, cisplatin plus
5-fluorouracil chemotherapy and intensity-modulated radiotherapy
for locoregionally advanced nasopharyngeal carcinoma. Int J Clin
Oncol. 18:464–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niu X, Hu C and Kong L: Experience with
combination of cetuximab plus intensity-modulated radiotherapy with
or without chemotherapy for locoregionally advanced nasopharyngeal
carcinoma. J Cancer Res Clin Oncol. 139:1063–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong YH, Dai J, Wang XY, Xie CH, Chen G,
Zeng L and Zhou YF: Phase II trial of neoadjuvant docetaxel and
cisplatin followed by intensity-modulated radiotherapy with
concurrent cisplatin in locally advanced nasopharyngeal carcinoma.
Cancer Chemother Pharmacol. 71:1577–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui
NJ, Lu TX and Mo HY: Results of a prospective randomized trial
comparing neoadjuvant chemotherapy plus radiotherapy with
radiotherapy alone in patients with locoregionally advanced
nasopharyngeal carcinoma. J Clin Oncol. 19:1350–1357.
2001.PubMed/NCBI
|
|
12
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: phase III randomized Intergroup
study 0099. J Clin Oncol. 16:1310–1317. 1998.PubMed/NCBI
|
|
13
|
Hong RL, Ting LL, Ko JY, Hsu MM, Sheen TS,
Lou PJ, Wang CC, Chung NN and Lui LT: Induction chemotherapy with
mitomycin, epirubicin, cisplatin, fluorouracil and leucovorin
followed by radiotherapy in the treatment of locoregionally
advanced nasopharyngeal carcinoma. J Clin Oncol. 19:4305–4313.
2001.PubMed/NCBI
|
|
14
|
Chan AT, Teo PM, Ngan RK, Leung TW, Lau
WH, Zee B, Leung SF, Cheung FY, Yeo W, Yiu HH, Yu KH, Chiu KW, Chan
DT, Mok T, Yuen KT, Mo F, Lai M, Kwan WH, Choi P and Johnson PJ:
Concurrent chemotherapy-radiotherapy compared with radiotherapy
alone in locoregionally advanced nasopharyngeal carcinoma:
progression-free survival analysis of a phase III randomized trial.
J Clin Oncol. 20:2038–2044. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang DY, Lee BH, Park HC, Song HH, Kim HJ,
Jung JY, Kim JH, Kim HY, Kwon JH, Hwang SW, Park SR, Park CH, Kim
KO, Kim MJ and Jang KM: Phase II study with oxaliplatin and S-1 for
patients with metastatic colorectal cancer. Ann Oncol. 20:892–896.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae WK, Hwang JE, Shim HJ, Cho SH, Lee KH,
Han HS, Song EK, Yun HJ, Cho IS, Lee JK, Lim SC, Chung WK and Chung
IJ: Multicenter phase II study of weekly docetaxel, cisplatin and
S-1 (TPS) induction chemotherapy for locally advanced squamous cell
cancer of the head and neck. BMC Cancer. 13:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase
M, Yamao K, Miyakawa H, Ishii H, Furuse J, Sato K, Sato T and
Okusaka T: A multicenter phase II trial of S-1 with concurrent
radiation therapy for locally advanced pancreatic cancer. Int J
Radiat Oncol Biol Phys. 85:163–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin SJ, Kim NK, Keum KC, Kim HG, Im JS,
Choi HJ, Baik SH, Choen JH, Jeung HC, Rha SY, Roh JK, Chung HC and
Ahn JB: Phase II study of preoperative chemoradiotherapy (CRT) with
irinotecan plus S-1 in locally advanced rectal cancer. Radiother
Oncol. 95:303–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui EP, Ma BB, Leung SF, King AD, Mo F,
Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R, Chan I, Ahuja AT, Zee BC and
Chan AT: Randomized phase II trial of concurrent
cisplatin-radiotherapy with or without neoadjuvant docetaxel and
cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol.
27:242–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geara FB, Glisson BS, Sanguineti G, Tucker
SL, Garden AS, Ang KK, Lippman SM, Clayman GL, Goepfert H, Peters
LJ and Hong WK: Induction chemotherapy followed by radiotherapy
versus radiotherapy alone in patients with advanced nasopharyngeal
carcinoma: results of a matched cohort study. Cancer. 79:1279–1286.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Airoldi M, Gabriele P, Gabriele AM,
Garzaro M, Raimondo L, Pedani F, Beatrice F, Pecorari G and
Giordano C: Induction chemotherapy with carboplatin and taxol
followed by radiotherapy and concurrent weekly carboplatin+taxol in
locally advanced nasopharyngeal carcinoma. Cancer Chemother
Pharmacol. 67:1027–1034. 2011. View Article : Google Scholar : PubMed/NCBI
|