Introduction
Glioblastoma (GBM) is the most common and most
devastating primary intracranial tumor (1). Despite the recommended standard
treatment of a combined strategy of maximum safe resection,
radiotherapy (RT) with concurrent and adjuvant temozolomide (TMZ)
for adult patients with GBM, the typical survival is only 12–15
months from the time of diagnosis (1–3). Thus,
there is a requirement for developing a novel therapeutic
strategy.
By contrast, malignant gliomas were highly
neovascularizated tumors with a distinct phenomenon, angiogenesis
and vasculogenesis (4). Initial
studies showed that vascular endothelial growth factor A (VEGF-A)
was overexpressed in malignant gliomas and had a pivotal role in
the processes of angiogenesis and vasculogenesis (4,5).
Therefore, VEGF became a fundamental target of antiangiogenic
therapy (6). Bevacizumab (BV), also
known as avastin, was a humanized monoclonal antibody that directly
targets the VEGF-A ligand and can inhibit vascular endothelial
cells proliferation and angiogenesis (7,8).
Additionally, preclinical evidence indicated that antiangiogenic
therapies could result in temporary vascular normalization,
potentially improving the efficacy of RT and chemotherapy (6,7). In May
2009, the Food and Drug Administration approved bevacizumab for the
first-line treatment of recurrent GBM patients (9). For recurrent GBM patients, the addition
of bevacizumab partly improved progression-free survival (PFS) and
maintenance of baseline quality of life and performance status
(3,10,11), but
no improved overall survival (OS) benefit or high-rate adverse
events could be observed in these studies. Although Khasraw et
al (12) reviewed the benefits
and side effects associated with the treatment of antiangiogenic
agents for high-grade gliomas, there is no review focusing on
bevacizumab for primary glioblastoma. Therefore, a meta-analysis
was conducted based on available eligible trials to evaluate the
efficacy of bevacizumab combination with RT/TMZ in newly diagnosed
glioblastoma patients in terms of OS, progression-free survival and
the adverse events rate.
Patients and methods
Data search and study selection
The identification of literature was performed in
two steps and the same principle was used to search each database.
Two independent review authors searched MEDLINE/PubMed, EMBASE and
Web of Science databases for studies published between January 1,
2000 and August 4, 2014. The following keywords were used for
systemic searches: Bevacizumab, avastin, maligant glioma,
glioblastoma, TMZ, RT and clinical trial. Associated studies and
their reference lists were also reviewed individually. In the
subsequent step, the following text terms were used:
Progression-free survival, OS and 6-month survival rate. All the
included studies were searched for the eligible trials by one
review author and double-checked by a second review author.
The included trials were only limited to English
language and human trials. The included trials were not limited to
randomized trials or glioblastoma [World Health Organization (WHO)
grade IV], and anaplastic glioma (WHO grade III) was also included.
Duplications of the same trials were excluded. Literature that was
not published as full studies, such as reference abstracts and
letters to editors, were excluded.
Data extraction and quality
assessment
Two authors (Peng Fu and Wei Xiang) independently
extracted the data from the trials and compared the following
results to avoid the bias in this process; all disagreements were
resolved by discussion. The following data were obtained from each
trial to compare the bevacizumb-based therapies with primary
therapy arms: The first author's name, the corresponding author,
the year of publication, the number of enrolled patients, the
duration of follow-up, the therapy region, hazard ratios (HRs) for
OS and PFS, odd ratios (ORs) for 6-month survival rate (SMSR) and
adverse events.
Outcome measures and statistical
analysis
Types of outcome measures included: i) OS defined as
the time interval from the date of diagnosis or treatment to the
date of fatality or last follow-up; ii) PFS defined as the time
interval from the date of diagnosis or treatment to date of
confirmed disease progression; and iii) adverse effects classified
according to the WHO criteria, and instances consist of anaemia,
neutropenia, leucopaenia, thrombocytopaenia, nausea and vomiting,
hypertension, fatigue, thromboembolic disease, hemorrhage, visceral
perforation and others.
HRs were used to analyze OS and PFS. If HRs were not
reported in the original publications, the HR values and their 95%
confidence intervals (CIs) in each trial would be calculated using
the methods described by Tierney et al (13). The estimated HR template that outputs
results based on the data of the Kaplan-Meier's survival
distributions in search and control groups was used from primary
trials. Heterogeneity of the included trials was calculated using
the χ2 and I2 statistic, with P<0.1 or
I2>50% considered to indicate a statistically
significant difference (14). Based
on the heterogeneity, the inverse-variance approach was implemented
using either fixed- or random-effect models.
Results
Overview and characteristics of the
studies
The search strategy is shown in Table I. All the relevant studies were
identified by the initial literature search. Following screening
using the keywords, relevant terms and full text information,
studies that were duplicated publications of the same trials and
not published as full studies and non-clinical trials were all
excluded. The included studies were all only limited to the English
language and human trials. One study was excluded (15) as it was an open-label, single-arm
clinical trial and lacked comparable data from the control group.
Eventually, three studies met the inclusion criteria and were
included in the final meta-analysis.
 | Table I.Search strategy in the review. |
Table I.
Search strategy in the review.
| Databasea | Step | Strategy |
|---|
| MEDLINE/PubMed,
EMBASE and Web of Science | 1 | Explode
‘clinical-trial’/all subheadings |
|
| 2 | Clinical near
trial |
|
| 3 | Single |
|
| 4 | Double |
|
| 5 | Single, double or
triple near blind or mask |
|
| 6 | Random |
|
| 7 | Control |
|
| 8 | #1 or 2 or 3 or 4 or
5 or 6 or 7 |
|
| 9 | EC = ‘HUMAN’ |
|
| 10 | #8 and 9 |
|
| 11 | Explode
‘brain-tumor’/all subheadings |
|
| 12 | Malignant glioma |
|
| 13 | Glioblastoma
multiform |
|
| 14 | Astrocytoma or
anaplastic astrocytoma |
|
| 15 | Avastin |
|
| 16 | Brain tumor |
|
| 17 | #11 or 12 or 13 or 14
or 15 or 16 |
|
| 18 | Bevacizumab |
|
| 19 | Temozolomide |
|
| 20 | Radiotherapy |
|
| 21 | #18 or 19 or 20 |
|
| 22 | #10 and 17 and
21 |
The main characteristics of the included studies are
presented in Table II. These three
studies represented 1,738 patients, with 848 assigned to the study
group to receive bevacizumab plus RT/TMZ and 890 derived for
comparison. One belonged to the phase II study and used results
derived from a comparable control cohort of patients treated at the
University of California (California, LA, USA) and Kaiser
Permanente Los Angeles (KPLA) and from the European Organization
for Research and Treatment of Cancer-National Cancer Institute of
Canada cohort as a comparison (16).
The other studies identified randomized, double-blind,
placebo-controlled trials (17,18). In
both trials, patients were randomly assigned to receive bevacizumab
and placebo, and therefore, data from these would provide a more
efficacious outcome of the bevacizumab combination with RT/TMZ in
GBM patients. All the patients were continued on each treatment
until disease progression or unacceptable toxic effects
developed.
 | Table II.Characteristics of the studies
included in the systematic review. |
Table II.
Characteristics of the studies
included in the systematic review.
| First author
(Refs.) | Comparison | Patients, n | Therapy region | Cycles of
therapy | Median OS,
months | HR (95% CI) for
OS | Median PFS,
months | HR (95% CI) for
PFS | SMSR, % | OR (95% CI) for
SMSR | Follow-up,
months |
|---|
| Chinot (17) | Bevacizumab plus
RT/TMZ versus control | 458 vs. 463 | 10 mg/kg bevacizumab
q2w or placebo + 2 Gy radiotherapy, 5 days/week, total 60 Gy, + 75
mg/m2 temozolomid/day, 6 weeks; maintenance 10 mg/kg
bevacizumab q2w or placebo + 150–200 mg/m2
temozolomide/day, 5 days q4w, total of 6 4-week cycle; bevacizumab
monotherapy at 15 mg/kg q3w or placebo | Until disease,
progression severe treatment-related toxicity or completion of
adjuvant therapy | 16.8 vs. 16.7 | 0.88 (0.76–1.02) | 10.6 vs. 6.2 | 0.64 (0.55–0.74) | 91.9 vs. 87.5 | 0.61 (0.40–0.95) | 12.3 vs. 8.5 |
| Gilbert (18) | Bevacizumab plus
RT/TMZ versus control | 320 vs. 317 | 2 Gy radiotherapy, 5
days/week, total 60 Gy + 75 mg/m2 temozolomide/day; 10
mg/kg bevacizumab, q2w or placebo + 150–200 mg/m2
temozolomide/day, 5 days q4w, total of 6–12 4-week cycle | Until disease
progression or unacceptable toxic effects developed | 15.7 vs. 16.1 | 1.13 (0.93–1.37) | 10.7 vs. 7.3 | 0.79 (0.66–0.94) | 84.3 vs. 82.5 | 0.88 (0.58–1.34) | 20.5 |
| Lai (16) | Bevacizumab plus
RT/TMZ versus historic control | 70 vs.
110 | 10 mg/kg
bevacizumab q2w + 2 Gy radiotherapy, 5 days/week, total 60 Gy + 75
mg/m2 temozolomide/day, 6 weeks; maintenance 10 mg/kg
bevacizumab q2w + 150–200 mg/m2 temozolomide/day, 5 days
q4w, total of 24 4-week cycle; 10 mg/kg bevacizumab monotherapy
q2w | Until disease
progression, or completion of adjuvant therapy | 19.6 vs. 21.1 | NA | 13.6 vs. 7.6 | NA | 98.6 vs. 88.2 | 0.11
(0.01–0.85) | 24.2 vs. 41.8 |
OS and 6-month survival are not
prolonged
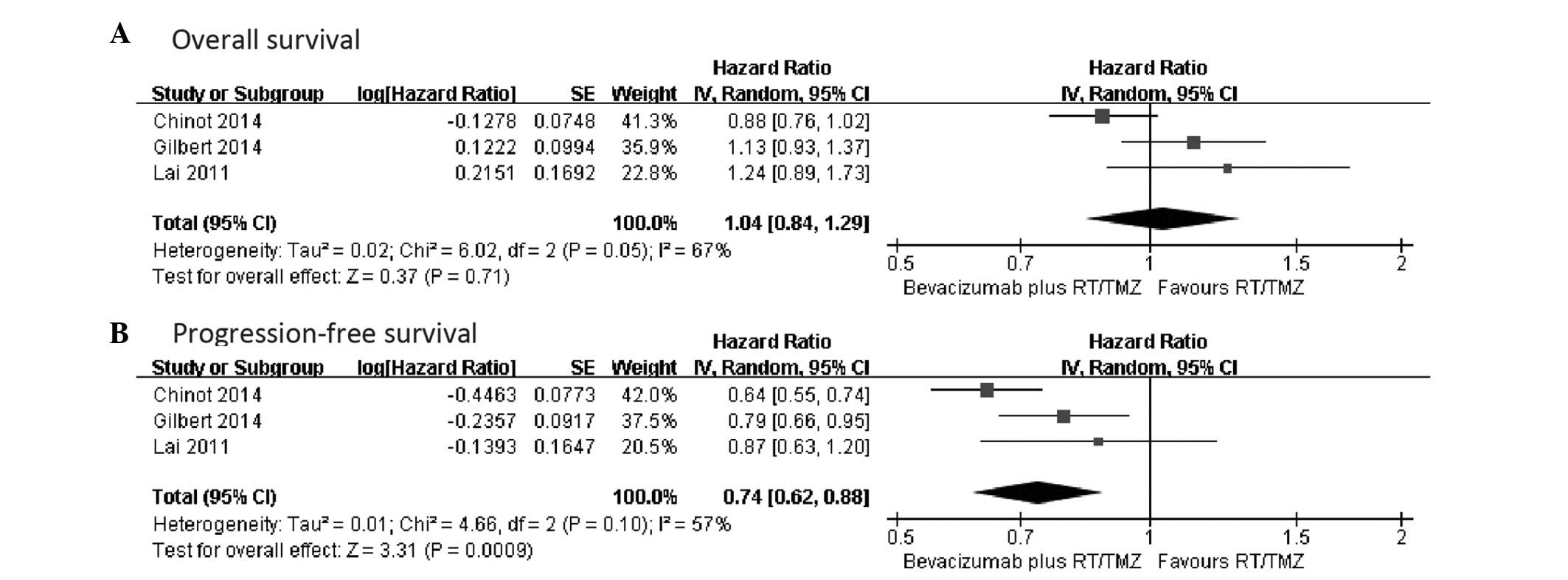
OS data were reported in all the studies (16–18).
Patients in the bevacizumab plus RT/TMZ group had a median OS
ranging from 15.7 to 19.6 months, and patients who received primary
therapy alone had a median OS ranging from 16.1 to 21.1 months.
Only two studies provided adequate data and could be included in
the pooled analysis using random-effect meta-analysis, which showed
no statistical difference in the median OS between the two groups
(HR, 0.99; 95% CI, 0.77–1.26; heterogeneity χ2=4.04,
P=0.04, I2=75%) (Fig. 1A).
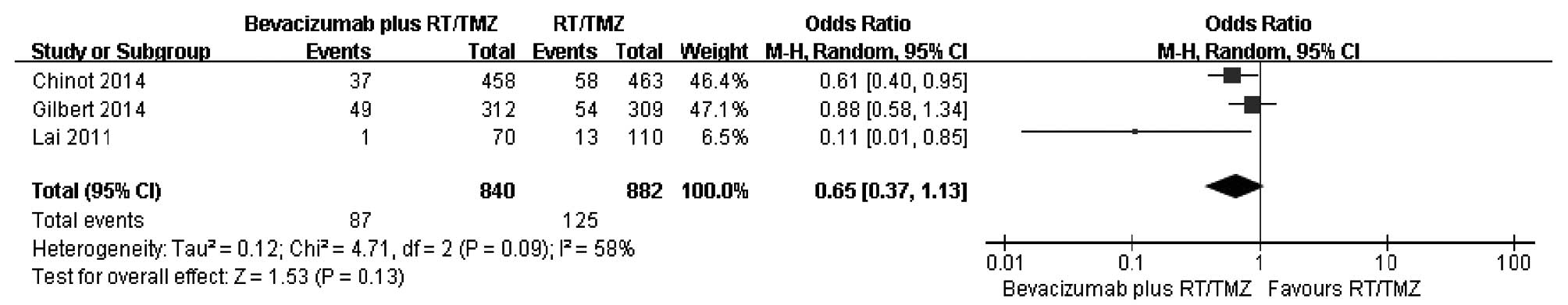
In order to elucidate the early benefit, the 6-month survival rate
was used to evaluate effectiveness of bevacizumab plus RT/TMZ.
A comparison of the 6-month survival rate was
conducted from three studies (Fig.
2). As there was large heterogeneity among the studies
subsequent to pooling the data (χ2=4.71, df=2, P=0.09;
I2=58%), a random-effects model was used for the
meta-analysis of 6-month survival. The combined OR did not reveal a
significantly higher survival among patients in the bevacizumab
plus RT/TMZ group compared to those in the primary therapy alone
group. The ORs in the three studies ranged from 0.11 to 0.88, with
a pooled estimate of 0.65 (P=0.13; 95% CI, 0.37–1.13). The number
of eligible trials providing variation-related estimates was small.
Thus, the results of subgroup analyses of OS could not be
reported.
Improvement of PFS
Three trials all presented PFS data and reported PFS
as the primary end-point (16–18). The
median PFS ranged from 10.6–13.6 months in the bevacizumab plus
RT/TMZ group and 6.2–7.6 months in the control group. By using a
random-effect model, a meta-analysis for PFS from two studies
indicated a 29% reduction in the risk of progression or fatality in
patients who received bevacizumab plus RT/TMZ (HR, 0.71; 95% CI,
0.58–0.87; P=0.001). There was statistical heterogeneity between
individual trials (heterogeneity χ2=3.08, P=0.08,
I2=68%) (Fig. 1B).
Bevacizumab-associated adverse
events
The serious adverse events (grade ≥3) associated
with bevacizumab described in the studies included in the
meta-analysis are shown in Table
III. The included studies showed different conclusions in GBM
patients for combination therapy of bevacizumab and RT/TMZ.
Although there were certain common nonhematological toxicities
(grades III and IV), including fatigue, venous thrombosis,
hypertension and proteinuria, the frequency of which was 20, 19, 11
and 11%, respectively, in one study (16), hematological toxicities were similar
in the patients treated with bevacizumab plus RT/TMZ compared to
those in the KPLA and EORTC-NCIC control group and the addition of
bevacizumab to RT/TMZ did not potentiate hematological toxicity.
Notably, the most common nonhematological toxicity, fatigue,
exhibited a similar level between the study group and the
EORTC-NCIC trial. The other two studies generally agreed with the
higher rate of serious adverse events associated with bevacizumab
compared with placebo, and increased prevalence and symptoms were
more frequent over therapy time in the former group (17,18).
Serious adverse events that were observed more frequently in the
bevacizumab group included hypertension (11.3 vs. 2.2%),
proteinuria (5.4 vs. 0%), thrombocytopenia (15 vs. 9.8%), bleeding
(3.3 vs. 1.8%) and complications of wound healing (3.3% vs. 1.6%)
in one study, while there were more common serious neutropenia (7.3
vs. 3.7%) and serious thrombocytopenia (10.2 vs. 7.7%) during
chemoradiotherapy with bevacizumab or placebo and hypertension (4.2
vs. 0.9%) and serious neutropenia (10.0 vs. 5.1%) during the
maintenance phase in the bevacizumab group compared to the placebo
group in the other. The adverse events often associated with
bevacizumab treatment mainly included hypertension, proteinuria,
thromboembolic events, complications associated with wound healing,
gastrointestinal perforation, bleeding and hematological
toxicity.
 | Table III.Adverse events (grade ≥III) in the
review. |
Table III.
Adverse events (grade ≥III) in the
review.
| First author
(Refs.) | Hypertension | Thromboembolic
disease | Hemorrhage |
Thrombocytopenia | Fatigue | Leukopenia | Neutropenia | Lymphopenia | Wound diseases | Visceral
perforation | Proteinuria |
|---|
| Chinot (17) | 52 (11.3) vs. 10
(2.2) | 58 (12.6) vs. 42
(9.3) | 15 (3.3) vs. 8
(1.8) | 69 (15) vs. 44 (9.8) | 34 (7.4) vs. 21
(4.7) | NA | NA | NA | 15 (3.3) vs. 7
(1.6) | 5 (1.1) vs. 1
(0.2) | 25 (5.4) vs. 0 |
| Gilbert (18) |
|
|
|
|
|
|
|
|
|
|
|
|
DCR | 4 (1.3) vs. 1
(0.3) | 14 (4.6) vs. 12
(4.0) | 0 vs. 1 (0.3) | 31 (10.2) vs. 23
(7.7) | 7 (2.3) vs. 8
(2.7) | 16 (5.3) vs. 7
(2.3) | 22 (7.3) vs. 11
(3.7) | 32 (10.5) vs. 27
(9.0) | 3 (1) vs. 1 (0.3) | 1 (0.3) vs. 1
(0.3) | NA |
|
DMP | 11 (4.2) vs. 2
(0.9) | 22 (7.7) vs. 11
(4.7) | 4 (1.6) vs. 2
(0.9) | 29 (11.1) vs. 27
(11.7) | 34 (13.1) vs. 21
(9.0) | 22 (8.5) vs. 14
(6.0) | 26 (10.7) vs. 12
(5.1) | 34 (13.1) vs. 31
(13.4) | 4 (1.6) vs. 2
(0.9) | 3 (1.2) vs. 1
(0.4) | NA |
| Lai (16) | 8 (11) | 13 (19) | 5 (7) | NA | 14 (20) | NA | NA | NA | 4 (6) | 2 (3) | 8 (11) |
Discussion
The present meta-analysis included two randomized
double-blind placebo-control trials (RCTs) plus one open-label
single-arm clinical trial. The results showed significantly
improved PFS in primary glioblastoma patients treated with
bevacizumab plus RT/TMZ versus those only treated with radiotherapy
with concurrent and adjuvant chemotherapeutic choices. However,
there was no significant different in OS. The pooled result from
two RCTs for PFS was 0.71 (95% CI, 0.58–0.87; P=0.0010), whereas
the pooled result from two RCTs for OS and odd ratios (ORs) for
SMSR from three studies were 0.99 (95% CI, 0.77–1.26; P=0.93) and
0.65 (95% CI, 0.37–1.13; P=0.13), respectively. Thus, despite a
combination treatment of bevacizumab and RT/TMZ that significantly
improved the PFS in patients with GBM, this treatment could not
prolong the median OS and SMSR. A possible explanation may be that
tumors have become resistant to bevacizumab or that the vasculature
may regrow rapidly following the termination of bevacizumab
treatment. In a recent meta-analysis, Khasraw et al
(12) evaluated the efficacy and
toxicity of antiangiogenic therapy in patients with high-grade
glioma and showed that bevacizumab was the antiangiogenic therapy
more likely to yield favorable results. This analysis had similar
pooled HR for PFS for three bevacizumab studies with 1,712
participants, which indicated significant improvement at 0.66 (95%
CI, 0.59–0.74; P<0.00001). This finding was also not significant
for OS (HR, 0.92; 95% CI, 0.83–1.02; P=0.12). The study by Takano
et al (19) reported the
outcomes in Japanese patients, and Japan was the only country to
approve the use of bevacizumab in combination with RT and TMZ
chemotherapy for newly diagnosed glioblastoma, and PFS and OS (12.2
and 29.2 months at median, respectively) was shown to be longer for
those treated with bevacizumab than for those not treated with the
drug.
In the present analysis, three trials were reviewed
and the heterogeneity of OS (χ2=4.04, P=0.04,
I2=75%), ORs for SMSR (χ2=4.71, P=0.09,
I2=58%) and PFS (χ2=3.08, P=0.08,
I2=68%) were calculated. As P<0.1 or
I2>50% was considered to indicate a statistically
significant difference (14), the
resources of the difference were considered, including the number
of trials, study design, treatment regimen and evaluation criteria
of outcomes. As an example using therapy, the strategy with
becacizumab was concurrently added to RT/TMZ in early stage and
subsequently adjuvant with TMZ in two studies, whereas treatment
with bevacizumab began during week 4 of RT and was continued for
cycles of maintenance chemotherapy in the trial by Gilbert et
al (18). However, despite these
differences, the present results strongly suggest that the addition
of bevacizumab could confer a PFS benefit in patients with newly
diagnosed GBM, with evidence of not prolonging OS and the 6-month
survival rate.
Bevacizumab as an antiangiogenic agent has been
approved for recurrent glioblastoma due to high response rates. A
recent comprehensive review of toxicities experienced among 3 phase
II bevacizumab trials for recurrent malignant gliomas at the
National Cancer Institute showed bevacizumab monotherapy is
well-tolerated, however, toxicity increases with combination
therapy (20). Although the three
included studies reported evidence of toxicity and serious adverse
events associated with bevacizumab, Lai et al (16) concluded toxicity attributable to
RT/TMZ was similar and additional toxicities were consistent with
those reported in other bevacizumab trials and the discrepancy of
quality-of-life findings from bevacizumab treatment was founded
between Chinot et al (17) and
Gilbert et al (18). Chinot
et al (17), whose trial was
sponsored by a pharmaceutical company, showed maintenance of
quality-of-life outcomes, whereas Gilbert et al (18), whose trial was sponsored by the
National Cancer Institute, showed decreased quality of life. The
reason of the discrepancy may be associated with the different
evaluation criteria of quality-of-life measures, distinguishing
limited health-related quality-of-life analyses based on time to
either deterioration or tumor progression from above, as well as
additional tests of symptom burden, interference and neurocognitive
function. Therefore, it is essential to acknowledge the spectrum of
bevacizumab toxicities and predisposing risk factors to weight the
risks and benefits of bevacizumab.
Several factors and limitations should be considered
when interpreting the present results. For example, a major
strength of the study is that there were only two available data
sets from RCTs, which were well performed and of high quality;
published in the New England Journal of Medicine. All the included
studies have an enlarged sample size of 1,738, and a statistical
power was enhanced to provide reliable effect estimates. Although
the present meta-analysis represents a general summary of the
efficacy of bevacizumab combined with RT/TMZ on patients with GBM,
it also worth emphasizing the potential limitations. Firstly, the
data were extracted from publications rather than individual
patient estimations, so the treatment benefits may not be defined
clearly. Secondly, the cost-effectiveness of bevacizumab treatment
was not taken into consideration as it was a type of biological
preparation, the cost of which remains relatively high, objectively
defining the cost-effectiveness as not easy. Thirdly, the small
number of studies included, heterogeneity on the basis of a
statistical design and therapy regimen of three clinical studies
may lead to somewhat bias, which makes it a challenge to draw a
significant conclusion, and additional trials are warranted to
obtain more associated data.
In conclusion, the results of the present systematic
review and meta-analysis show that the therapy of bevacizumab
combined with RT/TMZ offers a statistically significant improvement
in PFS in patients with newly diagnosed GBM, but does not benefit
OS and SMSR. Bevacizumab combined with RT/TMZ should be suggested
for first-line treatment in GBM patients as early as possible to
experience the early-stage benefits, but ongoing well-designed
clinical trials are also required until statistically significant
OS and adverse events differences are documented.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain RK, di Tomaso E, Duda DG, Loeffler
JS, Sorensen AG and Batchelor TT: Angiogenesis in brain tumours.
Nat Rev Neurosci. 8:610–622. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burrell K, Singh S, Jalali S, Hill RP and
Zadeh G: VEGF regulates region-specific localization of
perivascular bone marrow-derived cells in glioblastoma. Cancer Res.
74:3727–3739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N, Hillan KJ and Novotny W:
Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody
for cancer therapy. Biochem Biophys Res Commun. 333:328–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Irizarry Robles L, Hambardzumyan D, Nakano
I, Gladson CL and Ahluwalia MS: Therapeutic targeting of VEGF in
the treatment of glioblastoma. Expert Opin Ther Targets.
16:973–984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DR, Leeper HE and Uhm JH:
Glioblastoma survival in the United States improved after Food and
Drug Administration approval of bevacizumab: A population-based
analysis. Cancer. 119:3489–3495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahluwalia MS and Gladson CL: Progress on
antiangiogenic therapy for patients with malignant glioma. J Oncol.
2010:6890182010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narayana A, Kelly P, Golfinos J, Parker E,
Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, et
al: Antiangiogenic therapy using bevacizumab in recurrent
high-grade glioma: Impact on local control and patient survival. J
Neurosurg. 110:173–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khasraw M, Ameratunga MS, Grant R, Wheeler
H and Pavlakis N: Antiangiogenic therapy for high-grade glioma.
Cochrane Database Syst Rev. 9:CD0082182014.PubMed/NCBI
|
|
13
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narayana A, Gruber D, Kunnakkat S,
Golfinos JG, Parker E, Raza S, Zagzag D, Eagan P and Gruber ML: A
clinical trial of bevacizumab, temozolomide, and radiation for
newly diagnosed glioblastoma. J Neurosurg. 116:341–345. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai A, Tran A, Nghiemphu PL, Pope WB,
Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, et al:
Phase II study of bevacizumab plus temozolomide during and after
radiation therapy for patients with newly diagnosed glioblastoma
multiforme. J Clin Oncol. 29:142–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takano S, Ishikawa E, Nakai K, Matsuda M,
Masumoto T, Yamamoto T and Matsumura A: Bevacizumab in Japanese
patients with malignant glioma: From basic research to clinical
trial. Onco Targets Ther. 7:1551–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Odia Y, Shih JH, Kreisl TN and Fine HA:
Bevacizumab-related toxicities in the National Cancer Institute
malignant glioma trial cohort. J Neurooncol. 120:431–440. 2014.
View Article : Google Scholar : PubMed/NCBI
|