Introduction
Gastric cancer is the third most commonly diagnosed
cancer type worldwide and one of the leading causes of
cancer-associated mortality (1). In
patients with recurrent, metastatic, or advanced gastric cancer,
chemotherapy can prolong survival and improve quality of life
compared with the best supportive care.
Human epidermal growth factor receptor (HER)2 is a
member of a family of receptors that is associated with tumor cell
proliferation, apoptosis, adhesion, migration and differentiation.
It is overexpressed in 15–20% of patients with primary gastric and
gastroesophageal junction cancers (2,3).
Trastuzumab, a monoclonal antibody that targets HER2, induces
antibody-dependent cellular cytotoxicity and inhibits HER2-mediated
signaling by binding to the extracellular domain of HER2 (4). The trastuzumab for gastric cancer (ToGA)
trial recently validated the additive effects of trastuzumab for
HER2-positive, unresectable advanced or recurrent gastric cancer.
This trial revealed a significant increase in the overall survival
and progression-free survival when trastuzumab was used in
combination with chemotherapy, thus supporting the use of
trastuzumab in individualized, biomarker-based treatment (5). Additionally, it has been shown that
second-line and later lines of chemotherapy can significantly
improve patient outcomes, and that the additive effect of molecular
targeted drug therapy is promising (4,6–10).
In the present study, the clinicopathological
features and outcomes of patients with HER2-positive gastric cancer
who were treated with trastuzumab and cytotoxic anti-cancer agents
were analyzed.
Patients and methods
Study design
The present study performed a retrospective review
of patients with unresectable advanced or recurrent gastric cancer
who were treated with systemic chemotherapy.
Patients
Patients with unresectable advanced or recurrent
gastric cancer who were treated with chemotherapy at Kochi Medical
School (Kochi, Japan) between January 2007 and December 2013 were
identified from a medical information database. Gastric cancer
diagnoses were determined by esophagogastroduodenoscopy, biopsy
specimen analysis, computed tomography, magnetic resonance imaging,
ultrasonography of the abdomen and positron emission
tomography.
HER2 testing and treatment
In 86/213 patients, HER2 status was examined by
immunohistochemical (IHC) staining using tumor specimens obtained
by endoscopic biopsy or obtained from resected primary lesions,
according to the IHC scoring criteria for gastric cacner (11). If the IHC score was 2+, fluorescence
in-situ hybridization (FISH) analyses were performed using DNA
probes (PathVysion HER2 DNA Probe kit; Abbott, Tokyo, Japan). The
histological type for each tumor was categorized as intestinal type
(well differentiated, moderately differentiated and papillary
adenocarcinoma) or diffuse type (poorly differentiated, mucinous
adenocarcinoma and signet ring cell carcinoma), according to Lauren
(12) classification. If a patient
tumor sample scored 3+ on IHC or scored 2+ on IHC and was FISH
positive, the patient was treated with trastuzumab plus
chemotherapy.
In the trastuzumab plus chemotherapy regimen,
chemotherapy was administered every 3 weeks until disease
progression, unacceptable toxicity, or withdrawal of consent.
Capecitabine (1,000 mg/m2) was administered orally twice
a day for 14 days, and this was followed by a 1 week rest period.
Cisplatin (80 mg/m2) was administered by intravenous
infusion on day 1. Trastuzumab (8 mg/kg) was administered by
intravenous infusion on day 1 of the first cycle of chemotherapy,
followed by a dose of 6 mg/kg every 3 weeks.
Safety and efficacy evaluation
Toxicity was evaluated according to National Cancer
Institute Common Terminology Criteria for Adverse Events version
4.0. The clinical tumor response was assessed following two or
three courses of chemotherapy, according to criteria in the
Response Evaluation Criteria In Solid Tumors (RECIST) guideline
version 1.1 (13). Responses were
defined as complete response, partial response, stable disease or
progressive disease. The overall survival time was defined as the
interval between the date of chemotherapy initiation and the date
of mortality or last contact. Surviving patients were censored at
the last follow-up date.
Statistical analysis
Differences between the mean values for the two
groups of patients were assessed for significance using the
Mann-Whitney U test for continuous variables and the Pearson's
χ2 test for categorical variables. The Kaplan-Meier
method was used to generate cumulative survival rates and these
were compared using the log-rank test to evaluate statistically
significant differences. Statistical analyses were performed using
SPSS for Windows, version 13.0 (SPSS Inc, Chicago, IL, USA).
Results
Patients characteristics
A total of 213 patients with unresectable advanced
or recurrent gastric cancer were identified. Patient clinical
characteristics are summarized in Table
I. A total of 15 patients (13 men/2 women) received trastuzumab
plus chemotherapy. The median age of this group was 66-years-old
(range, 49–82 years). Of these 15 patients, 13 had an intestinal
type tumor and 2 had a diffuse type tumor, 12 were classified as
having metastatic cancer at the time of diagnosis, and 3 were
classified as having recurrent cancer following curative resection
of gastric cancer.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| No. patients |
|
|---|
|
|
|
|
|---|
| Characteristic | Trastuzumab plus
chemotherapy (n=15) | Chemotherapy alone
(n=198) | P-value |
|---|
| Median age, years
(range) | 66 (49–82) | 70 (19–89) | 0.574 |
| Gender (%) |
|
|
|
| Male | 13 | 124 | 0.111 |
|
Female | 2 | 74 |
|
| Histology |
|
|
|
|
Intestinal type | 13 | 66 | <0.001 |
| Diffuse
type | 2 | 132 |
|
| Disease status |
|
|
|
| Initially
metastatic | 12 | 140 | 0.722 |
|
Recurrence after curative
resection | 3 | 54 |
|
| Metastatic site |
|
|
|
|
Liver | 9 | 55 |
|
|
Peritoneum | 3 | 96 |
|
| Lymph
node | 3 | 37 | 0.218 |
| Lung | 1 |
8 |
|
| Bone | 0 | 11 |
|
The prevalence of intestinal type tumors was
significantly higher in the trastuzumab plus chemotherapy group
compared with in the chemotherapy alone group (86.7 vs. 33.3%;
P<0.001). No significant differences in age, sex or disease
status were clear at the time of diagnosis, and metastatic site
between the trastuzumab plus chemotherapy and chemotherapy alone
groups. Of the 15 patients in the trastuzumab plus chemotherapy
group, 14 exhibited tumor specimens that scored IHC 3+ and the
other exhibited a tumor specimen that scored IHC 2+ and was FISH
positive.
Clinicopathological characteristics of
HER2-positive patients
HER2 testing of tumor specimens was undertaken for a
total of 86 patients. The IHC scores of 0, 1+, 2+ and 3+ were
revealed for 37, 21, 14 and 14 of these patients, respectively. Of
those with an IHC score of 2+, one was FISH positive, and the rate
of the strong positive HER2 expression was 17.4% (15/86).
Intestinal type tumors had a significantly higher rate of strong
positive HER2 expression compared with diffuse type tumors [23.6%
(13/55) vs. 6.5% (2/31); P=0.044].
Safety of the trastuzumab plus
chemotherapy regimen
The main hematological and non-hematological adverse
events experienced by patients in the trastuzumab plus chemotherapy
group during all cycles of treatment are shown in Table II. Among the hematological adverse
events, the proportions of patients who had grade 3–4 neutropenia
and anemia were 26.7 and 13.3%, respectively. Creatinine levels
were elevated in 40% of patients. The most common non-hematological
adverse event was fatigue (all grades, 46.7%; grades 3–4, 13.3%).
With the exception of fatigue, no grade 3 or 4 non-hematological
adverse events occurred. Only 1 patient (6.7%) exhibited a grade 2
infusion-associated reaction, which was alleviated by symptomatic
therapy, and it was possible to restart trastuzumab administration
for this patient with diclofenac sodium premedication and an
adjusted trastuzumab dosage rate. No patients suffered from heart
failure and no treatment-associated mortalities occurred.
 | Table II.Adverse events experienced by the 15
patients in the trastuzumab plus chemotherapy group during all
cycles of treatment. |
Table II.
Adverse events experienced by the 15
patients in the trastuzumab plus chemotherapy group during all
cycles of treatment.
|
| Number of patients
(%) |
|---|
|
|
|
|---|
| Adverse event | All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
|
Non-hematological |
|
|
Fatigue | 7 (46.7) | 2 (13.3) | 3 (20.0) | 2 (13.3) | 0 |
|
Anorexia | 5 (33.3) | 3 (20.0) | 2 (13.3) | 0 | 0 |
|
Nausea | 5 (33.3) | 3 (20.0) | 2 (13.3) | 0 | 0 |
|
Diarrhea | 2 (13.3) | 1 (6.7) | 1 (6.7) | 0 | 0 |
|
Constipation | 1 (6.7) | 1 (6.7) | 0 | 0 | 0 |
|
Stomatitis | 2 (13.3) | 1 (6.7) | 1 (6.7) | 0 | 0 |
|
Hand-foot syndrome | 4 (26.7) | 3 (20.0) | 1 (6.7) | 0 | 0 |
|
Infusion-related reaction | 1 (6.7) | 0 | 1 (6.7) | 0 | 0 |
| Hematological |
|
|
Leukopenia | 10 (66.7) | 3 (20.0) | 4 (26.7) | 3 (20.0) |
|
|
Neutropenia | 11 (73.3) | 3 (20.0) | 4 (26.7) | 3 (20.0) | 1 (6.7) |
|
Thrombocytopenia | 5 (33.3) | 5 (33.3) | 0 | 0 | 0 |
|
Anemia | 8 (53.3) | 3 (20.0) | 3 (20.0) | 2 (13.3) |
|
|
Creatinine elevation | 6 (40.0) | 5 (33.3) | 1 (6.7) | 0 | 0 |
Tumor response and patient
survival
Objective tumor responses of patients treated with
trastuzumab plus chemotherapy, based on RECIST criteria, are shown
in Table III. The overall objective
response rate and disease control rate were 46.7 (7/15) and 86.7%
(13/15). Of these patients, 1 had a complete response, 6 had a
partial response, 6 had stable disease and 2 had progressive
disease. For the trastuzumab plus chemotherapy group, the median
observation period at the time of the analysis was 28.5 months, and
the median duration of treatment with trastuzumab was 13
months.
 | Table III.Objective tumor responses for 15
patients treated with trastuzumab plus chemotherapy, based on
Response Evaluation Criteria In Solid Tumors criteria. |
Table III.
Objective tumor responses for 15
patients treated with trastuzumab plus chemotherapy, based on
Response Evaluation Criteria In Solid Tumors criteria.
| Best overall
response | Number of patients
(%) |
|---|
| Complete
response | 1 (6.7) |
| Partial
response | 6 (40.0) |
| Stable disease | 6 (40.0) |
| Progressive
disease | 2 (13.3) |
| Objective response
rate | 46.7% |
| Disease control
rate | 86.7% |
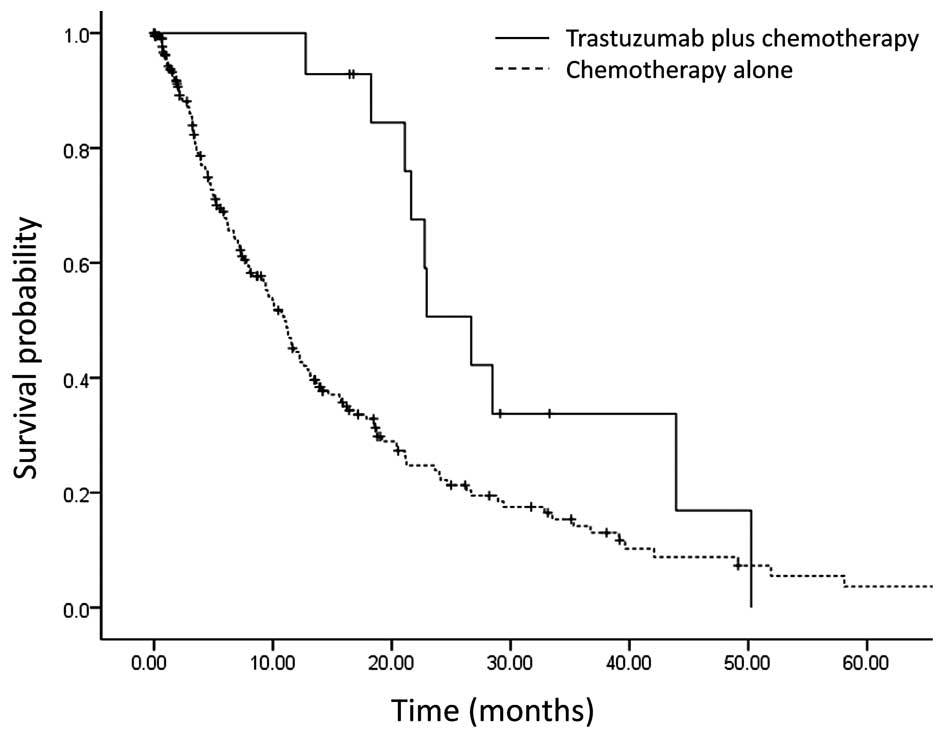
The median overall survival time for the trastuzumab
plus chemotherapy group was 22.9 months, which was significantly
longer compared with that for the chemotherapy alone group (11.6
months; P=0.014; Fig. 1). Second-line
therapy after disease progression was administered to all patients
in the trastuzumab plus chemotherapy group. Trastuzumab
administration during second and later lines of chemotherapy was
continued beyond progression for 7 patients (46.7%).
Discussion
The present results revealed that trastuzumab plus
chemotherapy is an effective and feasible treatment option for
patients with HER2-positive metastatic gastric cancer. Trastuzumab
is a potent targeted drug that exhibits cytostatic action; it
brings about antiproliferative effects on tumor cells via
inhibition of HER2 signaling and antibody-dependent cellular
cytotoxicity. The ToGA trial revealed that patients with
HER2-positive advanced gastric cancer, who were treated trastuzumab
in combination with cisplatin plus capecitabine or fluorouracil,
had a median overall survival of 13.8 months, compared with 11.1
months for patients in the control group who received chemotherapy
alone. Furthermore, it demonstrated that patients with higher
levels of HER2 expression had a median overall survival of 16.0
months (4). Following publication of
the results of the ToGA study, trastuzumab was recommended for use
with chemotherapy as a first-line treatment for patients with
HER2-positive unresectable advanced or recurrent gastric cancer.
The present results are similar to those of the ToGA study. The
median overall survival time for patients treated with trastuzumab
was significantly longer compared with patients in the chemotherapy
alone group (22.9 vs. 11.6 months).
High efficacy of trastuzumab has been confirmed
prior to and following chemotherapy in patients with HER2-positive
breast cancer, and expanded indications in gastric cancer are
anticipated (14,15). Previous studies that have assessed
chemotherapy plus trastuzumab treatment regimens for patients with
HER2-positive gastric cancer have revealed good tolerance and high
efficacy with cytotoxic chemotherapy including S-1 plus cisplatin,
capecitabine plus oxaliplatin (or docetaxel), and cisplatin plus
S-1, however, not capecitabine plus cisplatin (16–21).
Therefore, trastuzumab with cytotoxic chemotherapy is considered a
standard treatment regimen for patients with HER2-positive gastric
cancer. However, the potential benefits of using trastuzumab in
combination with second-line chemotherapy following disease
progression and following the use of trastuzumab in combination
with first-line therapy remain to be determined.
Other previous studies have shown that second-line
therapy can significantly improve survival of patients with
unresectable advanced or recurrent gastric cancer who are treated
with first-line chemotherapy that includes fluoropyrimidines and
platinum (5–7). Furthermore, ramucirumab, a human
immunoglobulin G1 monoclonal antibody vascular endothelial growth
factor receptor 2 antagonist, with or without paclitaxel,
significantly increases the overall survival compared with the
placebo in patients with unresectable advanced or recurrent gastric
cancer that has progressed following first-line chemotherapy
(8,9).
Trastuzumab therapy may contribute to improved treatment outcomes
following disease progression; however, further investigations,
including prospective randomized controlled trials, are required to
confirm this.
In the present study, certain patients in the
trastuzumab plus chemotherapy group developed neutropenia and
thrombocytopenia, likely induced by chemotherapy. These were
mitigated by reducing the dose of capecitabine or cisplatin. In
addition, no cases of cardiac failure or mortality due to cardiac
toxicity were observed. Similarly, previous reports have shown that
adding trastuzumab to chemotherapy does not increase the toxic
effects associated with standard fluoropyrimidine and platinum
chemotherapy (4,16–19). The
toxicity profile of trastuzumab plus chemotherapy has been shown to
be comparable to that for chemotherapy alone in phase II and III
trials (4,20,21).
In the present study, the rate of strong positive
HER2 expression in patients with gastric cancer was 17.4%, which is
consistent with results from previous studies (3,4,22). Gastric cancer differs from breast
cancer in that gastric cancer tumors are highly heterogeneous, plus
HER2 expression patterns are different in gastric cancer compared
with breast cancer. These differences present a range of challenges
for HER2 testing (22,23). It is critical to optimize factors that
affect the accuracy of HER2 testing, including the amount of time
between specimen extraction and specimen fixation, and the
concentration of fixing agent. In the present study and previous
research, HER2 overexpression was more common in intestinal type
tumors, which are generally associated with a better prognosis
compared with tumors with diffuse histology (24). While overexpression of HER2 is a
factor that is associated with poor prognosis in patients with
breast cancer, its association with prognosis in patients with
gastric cancer is widely debated (25). For this reason, improvements in the
accuracy of HER2 testing and further research on this topic are
required (26,27). Nonetheless, the survival advantage
provided by trastuzumab plus chemotherapy in patients with
HER2-positive gastric cancer has been confirmed, with data showing
that this regimen is efficacious and safe.
The present study does have certain limitations.
Firstly, it included the errors and biases inherent in a
retrospective, single-center study, and potential confounding
factors may not have been counterbalanced completely. Secondly,
pathological characteristics differed between HER2-positive and
HER2-negative tumor types, as HER2 positivity was significantly
associated with histological grade. Thirdly, a variety of
chemotherapy regimens were included in the present study, which may
have contributed to selection bias. Further studies with adequate
statistical power and a larger number of patient subgroups are
required to reliably and accurately assess the efficacy of
trastuzumab continuation beyond disease progression after
first-line therapy for advanced gastric cancer.
In conclusion, although this was not a prospective
study, the present results suggested that trastuzumab can be safely
administered in combination with chemotherapy in clinical practice
by managing adverse events, and that trastuzumab may extend
survival when used in combination with second and later lines of
chemotherapy in patients with HER2-positive unresectable advanced
or recurrent gastric cancer. A well-designed prospective study is
required to confirm the utility of trastuzumab continuation beyond
disease progression.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Begnami MD, Fukuda E, Fregnani JH,
Nonogaki S, Montagnini AL, da Costa WL Jr and Soares FA: Prognostic
implications of altered human epidermal growth factor receptors
(HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor
outcome. J Clin Oncol. 29:3030–3036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurokawa Y, Matsuura N, Kimura Y, Adachi
S, Fujita J, Imamura H, Kobayashi K, Yokoyama Y, Shaker MN,
Takiguchi S, et al: Multicenter large-scale study of prognostic
impact of HER2 expression in patients with resectable gastric
cancer. Gastric Cancer. 18:691–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang JH, Lee SI, Lim Do H, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thuss-Patience PC, Kretzschmar A, Bichev
D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer-a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chrom P, Stec R and Szczylik C:
Second-line treatment of advanced gastric cancer: Current options
and future perspectives. Anticancer Res. 35:4575–4583.
2015.PubMed/NCBI
|
|
11
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gomez-Martín C, Lopez-Rios F, Aparicio J,
Barriuso J, García-Carbonero R, Pazo R, Rivera F, Salgado M, Salud
A, Vázquez-Sequeiros E and Lordick F: A critical review of
HER2-positive gastric cancer evaluation and treatment: From
trastuzumab, and beyond. Cancer Lett. 351:30–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boku N: HER2-positive gastric cancer.
Gastric Cancer. 17:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS,
Park SR, Han HS, Chung IJ, Song EK, Lee KH, et al: Multicenter
phase II study of trastuzumab in combination with capecitabine and
oxaliplatin for advanced gastric cancer. Eur J Cancer. 51:482–488.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsui Y, Sato Y, Miyamoto H, Fujino Y,
Takaoka T, Miyoshi J, Kagawa M, Ohnuma H, Hirakawa M, Kubo T, et
al: Trastuzumab in combination with docetaxel/cisplatin/S-1 (DCS)
for patients with HER2-positive metastatic gastric cancer:
Feasibility and preliminary efficacy. Cancer Chemother Pharmacol.
76:375–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang YK, Rha SY, Tassone P, Barriuso J, Yu
R, Szado T, Garg A and Bang YJ: A phase IIa dose-finding and safety
study of first-line pertuzumab in combination with trastuzumab,
capecitabine and cisplatin in patients with HER2-positive advanced
gastric cancer. Br J Cancer. 111:660–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M,
Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, et
al: Phase II study of trastuzumab in combination with S-1 plus
cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer.
110:1163–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chua C, Tan IB, Yamada Y, Rha SY, Yong WP,
Ong WS, Tham CK, Ng M, Tai DW, Iwasa S, et al: Phase II study of
trastuzumab in combination with S-1 and cisplatin in the first-line
treatment of human epidermal growth factor receptor HER2-positive
advanced gastric cancer. Cancer Chemother Pharmacol. 76:397–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Honma Y, Shimada Y, Takashima A, Iwasa S,
Kato K, Hamaguchi T, Yamada Y, Taniguchi H, Sekine S and Kushima R:
Efficacy of S-1 plus cisplatin combination chemotherapy in patients
with HER2-positive advanced gastric cancer. Int J Clin Oncol.
19:863–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from ToGA: Targeting HER2 in gastric and
gastroesophageal junction cancer. Gastric Cancer. 18:476–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ock CY, Lee KW, Kim JW, Kim JS, Kim TY,
Lee KH, Han SW, Im SA, Kim TY, Kim WH, et al: Optimal patient
selection for trastuzumab treatment in HER2-positive advanced
gastric cancer. Clin Cancer Res. 21:2520–2529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nashimoto A, Akazawa K, Isobe Y, Miyashiro
I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et
al: Gastric cancer treated in 2002 in Japan: 2009 annual report of
the JGCA nationwide registry. Gastric Cancer. 16:1–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu MZ, Li Q, Wang ZQ, Liu TS, Liu Q, Wei
XL, Jin Y, Wang DS, Ren C, Bai L, et al: HER2-positive patients
receiving trastuzumab treatment have a comparable prognosis with
HER2-negative advanced gastric cancer patients: A prospective
cohort observation. Int J Cancer. 134:2468–2477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shitara K, Yatabe Y, Matsuo K, Sugano M,
Kondo C, Takahari D, Ura T, Tajika M, Ito S and Muro K: Prognosis
of patients with advanced gastric cancer by HER2 status and
trastuzumab treatment. Gastric Cancer. 16:261–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Namikawa T, Shiga M, Ichikawa K, Kitagawa
H, Kobayashi M and Hanazaki K: Metachronous liver and bone
metastasis from small early gastric carcinoma without lymph node
involvement: A case report. Mol Clin Oncol. 1:249–252.
2013.PubMed/NCBI
|