Introduction
Concurrent chemoradiotherapy has been established as
one of the standard therapies for patients with locally advanced,
unresectable esophageal carcinoma based on the results of the
Radiation Therapy Oncology Group (RTOG) 85–01 and 95–04 trials,
which demonstrated a significant survival advantage of concurrent
chemoradiation over radiation alone (1,2).
However, a standard and effective chemotherapeutic regimen for
combining with radiotherapy has not yet been established.
Although the standard chemotherapeutic agents for
esophageal carcinoma have not yet been determined, various types of
chemotherapy regimens have been investigated in an attempt to
prolong survival and improve quality of life. The most frequently
used chemotherapeutic agents in esophageal cancer treatment are
combined cisplatin and 5-fluorouracil (5-FU) (1–3). A phase
II study (3) by the Japan Clinical
Oncology Group reported that the complete response rate with
cisplatin/5-FU (PF regimen) and radiotherapy for stage II–III
esophageal squamous cell carcinoma (SCC) achieved a response rate
of 62.2% (46/74); the median survival time was 29 months, with 3-
and 5-year survival rates of 44.7 and 36.8%, respectively. However,
half the cases in these series of patients included potentially
resectable carcinomas. A better prognosis with chemoradiotherapy in
esophageal SCC was reported by Zhao et al (4), with a median survival time of 30.8
months and a 5-year survival rate of 40% for stage I–III patients
treated with the PF regimen combined with late-course accelerated
hyperfractionated radiotherapy (LCAHRT).
In order to increase the therapeutic ratio over that
of standard PF-based chemoradiotherapy, attempts have been made in
a phase I/II study to incorporate next-generation cytotoxic
chemotherapeutic agents, such as docetaxel (5,6).
However, survival remains disappointing and did not improve with
the standard PF regimen. Treatment-related toxicities may
compromise clinical efficacy (6).
Therefore, new drugs and combinations with a better therapeutic
index are required.
More recently, pemetrexed was introduced in phase I
trials for esophageal SCC and the preliminary results are promising
(7). As a novel antimetabolite,
pemetrexed acts as a multitargeted antifolate by inhibiting several
key enzymes involved in nucleotide synthesis (8). Pemetrexed, as a single agent or
combined with platinum, has also demonstrated broad antitumor
activity in a wide variety of solid tumors (9). In a phase I trial (7), pemetrexed was evaluated in combination
with cisplatin and concurrent selective lymph node LCAHRT for
patients with locally advanced esophageal SCC; that study
demonstrated that the maximum tolerated dose of pemetrexed was 500
mg/m2 and the recommended dose was 400 mg/m2.
Although toxicities were common, the protocol was overall safe,
well-tolerated, and achieved an encouraging outcome. One phase II
study (10) investigated 500
mg/m2 neoadjuvant pemetrexed and carboplatin in
conjunction with concomitant radiation of 50.4 Gy followed by
surgery for locally advanced esophageal cancer and gastroesophageal
junction tumors. This phase II study reported a 23% (6/26)
pathological complete response and 22 patients underwent complete
cancer resection, with a median survival time of 17.8 months [95%
confidence interval (CI): 12.2–30.7 months]. However, 22 patients
had at least one grade ≥3 adverse event, and 3 deaths were reported
postoperatively.
To the best of our knowledge, until recently there
were no published studies focusing on the efficacy and safety of
pemetrexed/cisplatin (PP regimen) compared with the PF regimen in
concomitant chemoradiotherapy. Therefore, the objective of the
present study was to evaluate the combination of pemetrexed and
cisplatin in patients with locally advanced, unresectable
esophageal SCC.
Patients and methods
Design
A retrospective study was conducted to determine the
efficacy and safety of the PP vs. the PF regimen in patients with
locally advanced, unresectable esophageal SCC treated with
concomitant chemoradiotherapy. The primary objective was to assess
tumor response and overall survival, and the secondary objective
was to assess treatment-related toxicity. The Institutional Review
Board of the Shandong Tumor Hospital (Jinan, China) approved the
protocol of this retrospective study and all the patients provided
written informed consent.
Patient population and
eligibility
Between January, 2004 and November, 2011, 72
eligible patients underwent concomitant LCAHRT and cisplatin-based
chemotherapy with a curative intent for the treatment of locally
advanced, unresectable esophageal SCC at the Shandong Tumor
Hospital. The eligibility criteria for this study were as follows:
i) Karnofsky performance status score ≥70; ii) patients aged ≤75
years; iii) histologically confirmed SCC, previously untreated; and
iv) clinical stage T1-T4, N0/1, M0/1a according to the American
Joint Committee staging system (2002) (11). The exclusion criteria included
distant organ metastases, evidence of esophageal perforation and
other serious underlying medical conditions.
Treatment evaluation and details
The pretreatment evaluation generally included
complete history and physical examination, complete blood cell
count and serum chemistry profile, endoscopy with biopsy, upper
gastrointestinal, chest and abdominal computed tomography (CT)
scans and bone scan with single photon emission CT. In order to
exclude patients with distant organ metastases, a magnetic
resonance imaging scan of the brain and neck and a whole-body
18F-fluorodeoxyglucose positron emission tomography scan
were performed as part of routine evaluation.
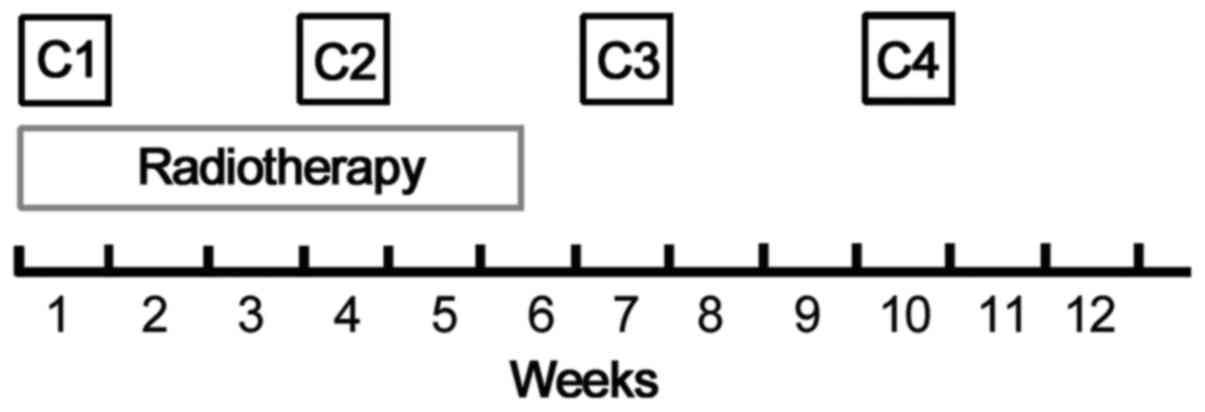
The treatment scheme is summarized in Fig. 1. All the patients were scheduled to
receive two cycles of concurrent cisplatin-based chemotherapy and
radiation [LCAHRT; 59.6 Gy/34 fractions (fx)], followed by an
additional two cycles of consolidation chemotherapy.
Chemotherapy
Patients in arm A were treated with the PF regimen
(intravenous infusion of cisplatin 25 mg/m2/day on days
1–3 and continuous intravenous infusion of 5-fluorouracil 800
mg/m2/24 h on days 1–5, every 21 days). Patients in arm
B were treated with the PP regimen (intravenous infusion of
cisplatin 25 mg/m2/day on days 1–3 and pemetrexed 500
mg/m2 on day 1, every 21 days). All the patients treated
with the PP regimen received folic acid, vitamin B12 and steroid
prophylaxis. Appropriate antiemetics were prescribed, and human
granulocyte colony-stimulating factor was permitted during
treatment.
Radiotherapy
The radiation dose was the same in both arms. The
radiation was delivered by 6-MV X-rays using a two-course
irradiation schedule: The first course of radiation covered the
primary tumors, metastatic regional lymph node(s) and high-risk
nodal regions (7,12), administered at 2 Gy per fx, 5
fx/week, to a total dose of 40 Gy in 20 fx; the second course of
radiation was delivered to the boost volume for an additional dose
of 19.6 Gy twice a day in 14 fx within 7 days at 1.4 Gy/fx, with a
6-h minimal interval between fractions. The total dose administered
to the clinical tumor was 59.6 Gy/34 fx/35 days.
Treatment assessments
Tumor response was assessed according to the
Response Evaluation Criteria in Solid Tumors (RECIST) guidelines
(13). The response criteria for the
target lesions are as follows: Complete response (CR),
disappearance of all target lesions; partial response (PR), ≥30%
decrease; stable disease (SD), neither PR nor PD criteria met; and
progressive disease (PD), ≥20% increase or appearance of new
lesion(s). The overall survival was calculated from the date of
radiotherapy initiation until death or the last follow-up
evaluation.
Treatment-related toxicity and
follow-up
Treatment-related toxicity assessment was performed
at least weekly during treatment, 4 weeks after completion of
therapy, every 3 months for 2 years and every 6 months thereafter,
using the National Cancer Institute Common Toxicity Criteria,
version 3.0 (14). A full history
and physical examination, as well as repeat blood work, were
conducted at these visits. Spiral CT scans of the chest and upper
gastrointestinal tract were obtained at every follow-up examination
to evaluate the status of the disease.
Statistical analysis
The statistical analysis was performed using SPSS
software, version 10.0 (SPSS Inc., Chicago, IL, USA). The survival
analysis was performed using the actuarial Kaplan-Meier method, and
differences between the curves were analyzed using the log-rank
test. The constituent ratio was analyzed using the Chi-squared
test. All statistical comparisons were performed with two-tailed
tests on an intent-to-treat basis. A P-value of <0.05 was
considered statistically significant.
Results
Patient characteristics
Between January, 2004 and November, 2011, 29
patients were treated with the PF regimen (arm A) and 31 patients
with the PP regimen (arm B). All the patients completed the
treatment schedules and were assessable for treatment efficacy and
toxicity.
The two arms were similar for baseline
characteristics (Table I). Although
more patients with early-stage disease (IIa+IIb) were included in
arm B (29.0%) compared with arm A (13.8%), the difference was not
statistically significant (P=0.266). The majority of the patients
had stage III and IVa disease located in the thoracic
esophagus.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| Characteristics | PF (arm A; n=29) | PP (arm B; n=31) | P-value |
|---|
| Age (years) |
|
| 0.722 |
| Median
(range) | 62 (42–75) | 62 (40–75) |
| Gender |
|
| 0.389 |
|
Male:female | 24:5 | 28:3 |
| Karnofsky performance
status |
|
| 0.898 |
| Median
(range) | 80 (70–100) | 80 (70–100) |
| Location |
|
| 0.787 |
|
Cervical:upper:middle:lower | 4:11:12:2 | 3:12:12:4 |
| Stage |
|
| 0.266 |
|
IIa:IIb:III:IVa | 4:0:16:9 | 6:3:12:10 |
| Tumor length
(cm) |
|
| 0.576 |
| Median (range) | 6.5 (2.0–11.5) | 5.0 (3.5–10.0) |
Treatment response
According to RECIST, a response was reported in 26
of the 29 patients (89.7%) in arm A and in 29 of the 31 patients
(93.5%) in arm B (Table II). The
difference was not statistically significant (P=0.304).
 | Table II.Treatment response according to
RECIST. |
Table II.
Treatment response according to
RECIST.
| Type of response, n
(%) | PF (arm A; n=29) | PP (arm B; n=31) | Overall (n=60) |
|---|
| CR | 16 (55.2) | 13 (41.9) | 29 (48.3%) |
| PR | 10 (34.5) | 16 (51.6) | 26 (43.3%) |
| RR (CR+PR) | 26 (89.7) | 29 (93.5) | 55 (91.7%) |
| SD | 3 (10.3) | 1 (3.2) | 4 (6.7%) |
| PD | 0 (0.0) | 1 (3.2) | 1 (1.7%) |
Survival
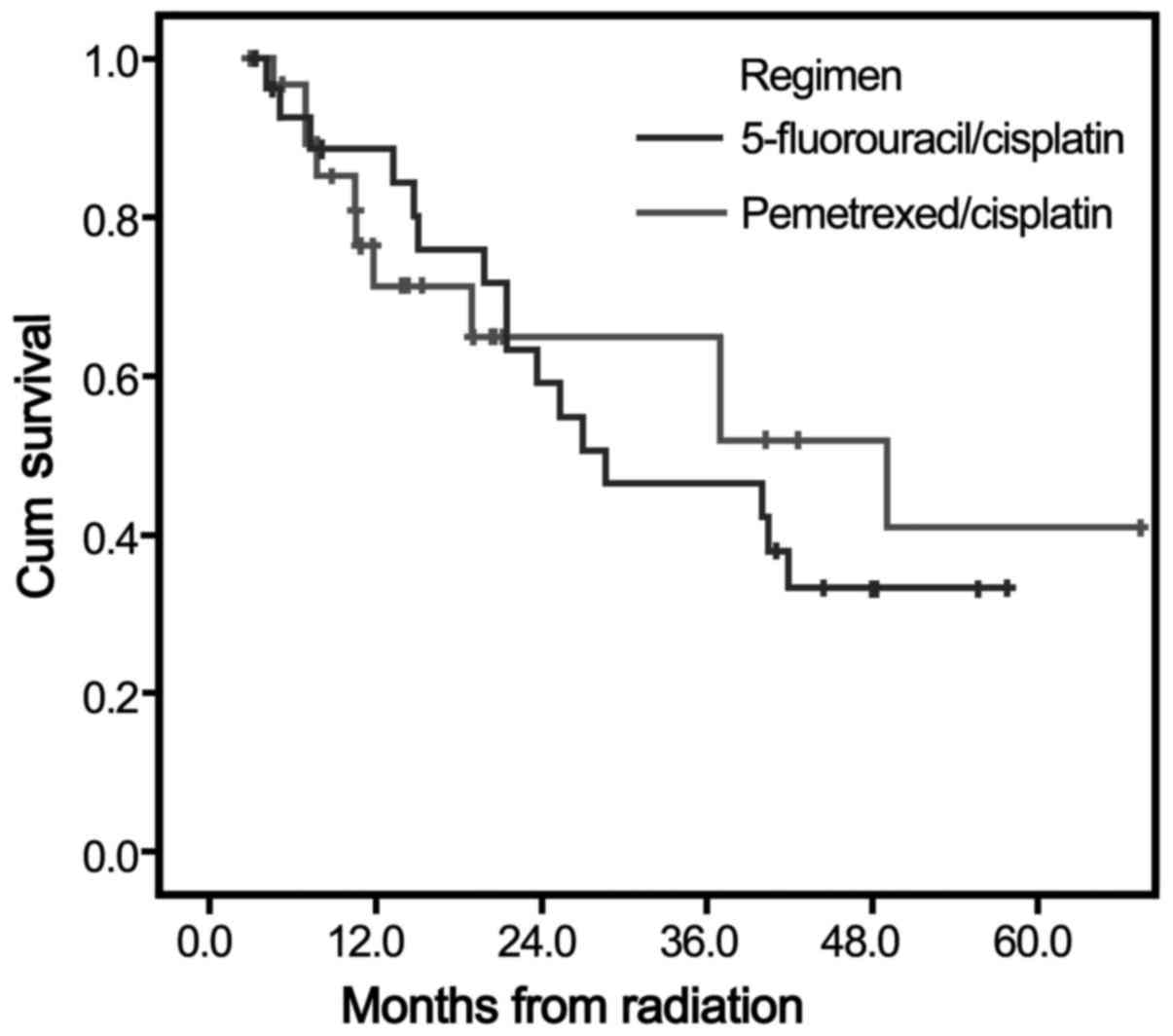
The median follow-up was 25.1 months in both arms.
The median survival time was 26.1 months (95% CI: 15.3–36.8 months)
in arm A and 28.7 months (95% CI: 9.4–48.0 months) in arm B. At 1,
3 and 5 years, the overall survival rate was 84.4, 42.2 and 33.2%
in arm A, and 71.3, 51.9 and 40.9% in arm B. However, there were no
significant difference between arm A and arm B in terms of
long-term survival (Chi-squared=0.034, P=0.853) (Fig. 2).
Toxicity
As demonstrated in Table III, the most frequently reported
severe (grade ≥3) adverse effects were hematological toxicity and
esophagitis. Treatment-related severe leukopenia and
thrombocytopenia occurred in 4 (13.8%) and 1 (3.4%) patients,
respectively, in arm A, vs. 12 (38.7%) and 6 (19.4%) patients,
respectively, in arm B; the differences were statistically
significant (P=0.029 and 0.041, respectively). Severe anaemia was
reported in 3.4% (1 patient developed grade 4 anaemia) of the
patients in arm A, vs. 12.9% in arm B; however, the difference was
not statistically significant (P=0.059). A total of 9 patients
(31.0%) in arm A and 4 patients (12.9%) in arm B developed grade 3
radiation esophagitis and the difference was statistically
significant (P=0.036). Severe gastrointestinal reactions were
infrequent in both arms. No patients developed grade ≥3 radiation
pneumonitis.
 | Table III.Treatment-related hematological and
non-hematological toxicity. |
Table III.
Treatment-related hematological and
non-hematological toxicity.
| Toxicity | PF (arm A; n=29) | PP (arm B; n=31) | P-value |
|---|
| Leukopenia |
|
| 0.029 |
| Grade
0–1:2:3:4:5 | 12:13:4:0:0 | 4:15:11:1:0 |
| Anaemia |
|
| 0.059 |
| Grade
0–1:2:3:4:5 | 28:0:0:1:0 | 25:2:4:0:0 |
| Thrombocytopenia |
|
| 0.041 |
| Grade
0–1:2:3:4:5 | 23:5:1:0:0 | 19:6:4:2:0 |
| Vomiting |
|
| 0.051 |
| Grade
0–1:2:3:4:5 | 25:4:0:0:0 | 20:10:1:0:0 |
| Pneumonitis |
|
| 0.170 |
| Grade
0–1:2:3:4:5 | 29:0:0:0:0 | 30:1:0:0:0 |
| Esophagitis |
|
| 0.036 |
| Grade
0–1:2:3:4:5 | 2:18:9:0:0 | 3:24:4:0:0 |
Six deaths were considered to be possibly related to
the treatment regimens: 4 in arm A (upper gastrointestinal
hemorrhage occurred in 1 patient and esophagotracheal fistula in 3
patients), and 2 in arm B (1 case each of upper gastrointestinal
hemorrhage and esophagotracheal fistula); all patients were male
and had stage T4 disease.
Discussion
On the basis of previous clinical phase I study, 400
mg/m2 pemetrexed administered on day 1 once every 21
days was the recommended regimen for locally advanced esophageal
SCC (7) in combination with
cisplatin-based chemoradiotherapy. Although limited by the small
number of patients, our data suggest that the administration of
pemetrexed may be feasible and well-tolerated in combination with
radiotherapy; furthermore, this PP-based chemoradiotherapy achieved
a tumor response rate of 93.5%, with acceptable toxicity and only 2
possibly treatment-related deaths. The control arm with the PF
regimen exhibited a mildly inferior response rate (89.7%), whereas
there were 4 reported toxicity-related deaths. The present study
demonstrated that the incidence of hematological toxicities was
higher with the PP compared with the PF regimen, which should be
taken into consideration. However, the incidence of esophagitis
with the PP regimen was lower compared with that with the PF
regimen. The median survival in the PF arm was 26.1 months (95% CI:
5.3–36.8 months) and was superior to those reported by the RTOG
85-01 and RTOG 94-05 trials (14.1 and 18.1 months, respectively)
(1,2). With the PP regimen, the median survival
in our trial was 28.7 months (95% CI: 9.4–48.0 months), which was
considered to be satisfactory, as it was longer by 2.6 months
compared with the PF regimen. Of note, this rather good median
survival was obtained while M1a-stage patients were included in
this study, contrary to the RTOG 94-05 study.
It is considered that this survival benefit may be
acquired by using an accelerated radiation scheme. In China, Shi
et al (15) initiated a study
on LCAHRT for esophageal SCC treatment and yielded very encouraging
results. Compared with conventional fractionation, the 5-year
survival (34 vs. 15%) and local control (55 vs. 21%) rates were
markedly improved with the LCAHRT regimen. Recently, three
independent meta-analyses added to the evidence of LCAHRT being
therapeutically beneficial for esophageal carcinoma (16–18).
However, the optimal combination of chemotherapy regimens and
accelerated radiation to maximize long-term survival remains to be
determined. Zhao et al (4)
reported the results of a phase III clinical trial on LCAHFR
combined with PF, and the 1, 3 and 5-year survival rates were 67,
44 and 40%, respectively, in the combination group, and 77, 39 and
28%, respectively, in the radiotherapy alone group (P=0.310); in
addition, the incidence of grade ≥3 toxicities were 42 and 25%,
respectively (P=0.05). Liu et al (17) reported a meta-analysis on LCAHRT in
esophageal carcinoma, including 21 randomized controlled trials,
and the results indicated that LCAHRT combined with the PF regimen
may improve the 5-year overall survival and 3-year local control in
esophageal cancer compared with LCAHRT alone, with a significantly
increased incidence of acute toxicities.
Pemetrexed was recently approved in combination with
cisplatin as first-line treatment for advanced non-squamous-cell
lung cancer and pleural mesothelioma. Pemetrexed combined with
platinum compounds was also recommended for locally advanced head
and neck SCC as an induction regimen (19,20).
However, there are very few data in the literature focusing on the
treatment of esophageal SCC. To date, only one phase I study by Li
et al (7) was conducted to
evaluate the efficacy and safety of pemetrexed combined with
cisplatin for locally advanced esophageal SCC. That study included
12 patients with T3-4N0-1M0-1a thoracic esophageal SCC. The total
radiation dose administered was 59.6 Gy in 34 fx in 5.4 weeks, and
concurrent chemotherapy regimens were prescribed with cisplatin 10
mg/m2 on days 1–5 and pemetrexed 400–500
mg/m2 once every 21 days. The tumor response was as high
as 100%, with CR in 66.7% (8/12) and PR in 33.3% (4/12) of the
patients. Furthermore, no patient experienced cancer progression,
with a median follow-up of 9 months (range, 3–22 months). Another
phase II study investigated neoadjuvant pemetrexed/carboplatin
combined with concomitant radiation for locally advanced esophageal
cancer and gastroesophageal junction tumors (10). That phase II trial demonstrated
antineoplastic activity, but did not achieve a complete
pathological response (pCR). According to the results reported by
Berger et al (21), overall
survival was correlated with pCR and the 5-year survival of
patients who achieved pCR following preoperative chemoradiotherapy
was ~50%.
In the present study, although supportive treatment
with oral folic acid and intramuscular vitamin B12 was routinely
administered, the incidence of leukopenia and thrombocytopenia was
higher with pemetrexed at a dose of 500 mg/m2 on day 1
once every 21 days, compared with that with 5-FU (Table III). Generally, these toxicities
were tolerable; in only 3 patients the consolidation chemotherapy
was delayed due to grade 4 hematological toxicity (2 patients
developed thrombocytopenia and 1 developed leukopenia), and only 2
deaths were considered possibly related to this treatment regimen
(1 patient developed upper gastrointestinal hemorrhage and 1
patient developed esophagotracheal fistula). As regards overall
survival, the present study demonstrated that the PP regimen was
marginally superior to the PF regimen for locally advanced
esophageal SCC (5-year survival rate, 40.9 vs. 33.2%,
respectively), although more patients with early-stage disease
(IIa+IIb) were included in the PP group compared with the PF group
(29.0 vs. 13.8%, respectively). As demonstrated in Fig. 2, a trend toward better survival among
patients who received the PP regimen was observed, but the
difference did not reach statistical significance for this limited
patient population.
Several strengths and limitations should be noted.
This was only a retrospective study with a relatively small sample
size, which may limit the generalizability of our findings. This
study cohort consisted of an inhomogeneous patient population
including patients with stage II, III and IVa disease, who had
different prognoses following treatment.
In conclusion, the present study demonstrated that
chemoradiotherapy with pemetrexed/cisplatin was similar with
cisplatin/5-FU; however, the incidence of hematological toxicity
was higher, whereas that of esophagitis was lower. These results
should be validated in a large prospective cohort of patients.
References
|
1
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato K, Muro K, Minashi K, Ohtsu A,
Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y and Fukuda H:
Gastrointestinal Oncology Study Group of the Japan Clinical
Oncology Group (JCOG): Phase II study of chemoradiotherapy with
5-fluorouracil and cisplatin for Stage II–III esophageal squamous
cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol
Phys. 81:684–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao KL, Shi XH, Jiang GL, Yao WQ, Guo XM,
Wu GD and Zhu LX: Late course accelerated hyperfractionated
radiotherapy plus concurrent chemotherapy for squamous cell
carcinoma of the esophagus: A phase III randomized study. Int J
Radiat Oncol Biol Phys. 62:1014–1020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi K, Koizumi W, Tanabe S, Sasaki T,
Katada C, Ishiyama H and Hayakawa K: A phase I trial of definitive
chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil
(DCF-R) for advanced esophageal carcinoma: Kitasato digestive
disease & oncology group trial (KDOG 0501). Radiother Oncol.
87:398–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiarion-Sileni V, Corti L, Ruol A,
Innocente R, Boso C, Del Bianco P, Pigozzo J, Mazzarotto R, Tomassi
O and Ancona E: Phase II trial of docetaxel, cisplatin and
fluorouracil followed by carboplatin and radiotherapy in locally
advanced oesophageal cancer. Br J Cancer. 96:432–438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li BS, Gong HY, Huang W, Yi Y, Zhang ZC,
Li HS, Wang ZT and Yu JM: Phase I study of concurrent selective
lymph node late course accelerated hyper-fractionated radiotherapy
and pemetrexed, cisplatin for locally advanced esophageal squamous
cell carcinoma. Dis Esophagus. 24:251–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seiwert TY, Connell PP, Mauer AM, Hoffman
PC, George CM, Szeto L, Salgia R, Posther KE, Nguyen B, Haraf DJ
and Vokes EE: A phase I study of pemetrexed, carboplatin, and
concurrent radiotherapy in patients with locally advanced or
metastatic non-small cell lung or esophageal cancer. Clin Cancer
Res. 13:515–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adjei AA: Pemetrexed (Alimta): A novel
multitargeted antifolate agent. Expert Rev Anticancer Ther.
3:145–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jatoi A, Soori G, Foster NR, Hiatt BK,
Knost JA, Fitch TR, Callister MD, Nichols FC III, Husted TM and
Alberts SR: Phase II study of preoperative pemetrexed, carboplatin,
and radiation followed by surgery for locally advanced esophageal
cancer and gastroesophageal junction tumors. J Thorac Oncol.
5:1994–1998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green FL, Page DL, Fleming ID, Fritzet AG,
Balch CM, Haller DG and Morrow M: AJCC cancer staging manual, 6th
edition. Ann Oncol. 14:345–346. 2003. View Article : Google Scholar
|
|
12
|
Li BS, Zhou T, Wang ZT, Li HS, Sun HF,
Zhang ZC, Lin HQ, Wei YM, Gong HY, Huang W, et al: Phase I study of
concurrent selective lymph node late course accelerated
hyper-fractionated radiotherapy and Capecitabine, Cisplatin for
locally advanced esophageal squamous cell carcinoma. Radiother
Oncol. 93:458–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Therasse P, Eisenhauer EA and Verweij J:
RECIST revisited: A review of validation studies on tumour
assessment. Eur J Cancer. 42:1031–1039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi XH, Yao W and Liu T: Late course
accelerated fractionation in radiotherapy of esophageal carcinoma.
Radiother Oncol. 51:21–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YW, Chen L, Bai Y and Zheng X:
Long-term outcomes of late course accelerated hyper-fractionated
radiotherapy for localized esophageal carcinoma in Mainland China:
A meta-analysis. Dis Esophagus. 24:495–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CX, Li XY and Gao XS: Meta-analysis of
late course accelerated hyperfractionated radiotherapy combined
with FP chemotherapy for esophageal carcinoma. Chin J Cancer.
29:889–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou ZG, Gao XS, Qiao XY and Zhang P:
Literature analysis of radiotherapy for esophageal cancer in China.
Chin J Cancer. 29:873–881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gilbert J, Murphy B, Dietrich MS, Henry E,
Jordan R, Counsell A, Wirth P, Yarbrough WG, Slebos RJ and Chung
CH: Phase 2 trial of oxaliplatin and pemetrexed as an induction
regimen in locally advanced head and neck cancer. Cancer.
118:1007–1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villaflor VM, Haraf D, Salama JK,
Kocherginsky M, Langerman A, Gomez-Abuin G, Beniwal P, Blair EA,
Stenson KM, Portugal L, et al: Phase II trial of pemetrexed-based
induction chemotherapy followed by concomitant chemoradiotherapy in
previously irradiated patients with squamous cell carcinoma of the
head and neck. Ann Oncol. 22:2501–2507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berger AC, Farma J, Scott WJ, Freedman G,
Weiner L, Cheng JD, Wang H and Goldberg M: Complete response to
neoadjuvant chemoradiotherapy in esophageal carcinoma is associated
with significantly improved survival. J Clin Oncol. 23:4330–4337.
2005. View Article : Google Scholar : PubMed/NCBI
|