Introduction
The incidence of cancer diagnosis during pregnancy
is estimated to be 1 in every 1,000 pregnant women. Breast cancer,
melanoma and cervical cancer are the most commonly diagnosed types
of cancer during pregnancy (1).
Large-cell neuroendocrine carcinoma (LCNEC) is an uncommon
histological subtype (0.5–3%) of cervical cancer associated with
poor survival, whereas the occurrence of LCNEC during pregnancy is
particularly rare. Although recent data support the use of
perioperative chemotherapy, in particular platinum, with or without
etoposide, to improve the survival of patients with LCNEC (2), treatment during pregnancy remains
completely experimental. To the best of our knowledge, the present
report describes the first case of LCNEC in the literature to date
that was successfully managed with a pregnancy-preserving
approach.
Case report
A 34-year-old woman, gravida 3, para 3, was referred
to Hospital Clinic de Barcelona (Barcelona, Spain) in March, 2015
with a biopsy result of NEC of the cervix at 21 weeks of gestation.
The patient had no history of medical conditions or previous
surgery. Gynecological examination revealed a 3-cm exophytic
cervical tumor, without parametrial invasion, but with involvement
of the anterior vaginal fornix. Legal interruption of the pregnancy
was offered, but the patient declined due to her religious
beliefs.
Ultrasonographic examination revealed a cervical
tumor sized 40×19×35 mm, with involvement of the anterior fornix of
the vagina, without evidence of parametrial invasion, and a
normally growing fetus with normal hemodynamic parameters. Magnetic
resonance imaging (MRI) revealed no evidence of lymph node
involvement or metastatic disease (Fig.
1). The tumor was classified as clinical stage IIA1 according
to the guidelines of the International Federation of Gynecology and
Obstetrics.
On histological examination of biopsy material, the
mass was diagnosed as high-grade cervical LCNEC, and
immunohistochemistry revealed positive staining for chromogranin,
synaptophysin, CD56, cytokeratin (CK) 7 and K-i67 (>80%), and
negative for p63. The levels of carcinoembryonic antigen and
squamous cell carcinoma antigen were within the normal range. Human
papillomavirus (HPV) 18 was identified by polymerase chain reaction
analysis.
The patient received neoadjuvant chemotherapy (NACT)
with 3 cycles of cisplatin (CDDP; 50 mg/m2) and
etoposide (VP-16; 100 mg/m2) every 3 weeks, without
associated toxicity and with good fetal development. The patient
was clinically followed every 2 weeks with ultrasonographic and
gynecological examinations. Fetal control was regularly performed
with ultrasound and Doppler examination. The tumor remained stable
during that period.
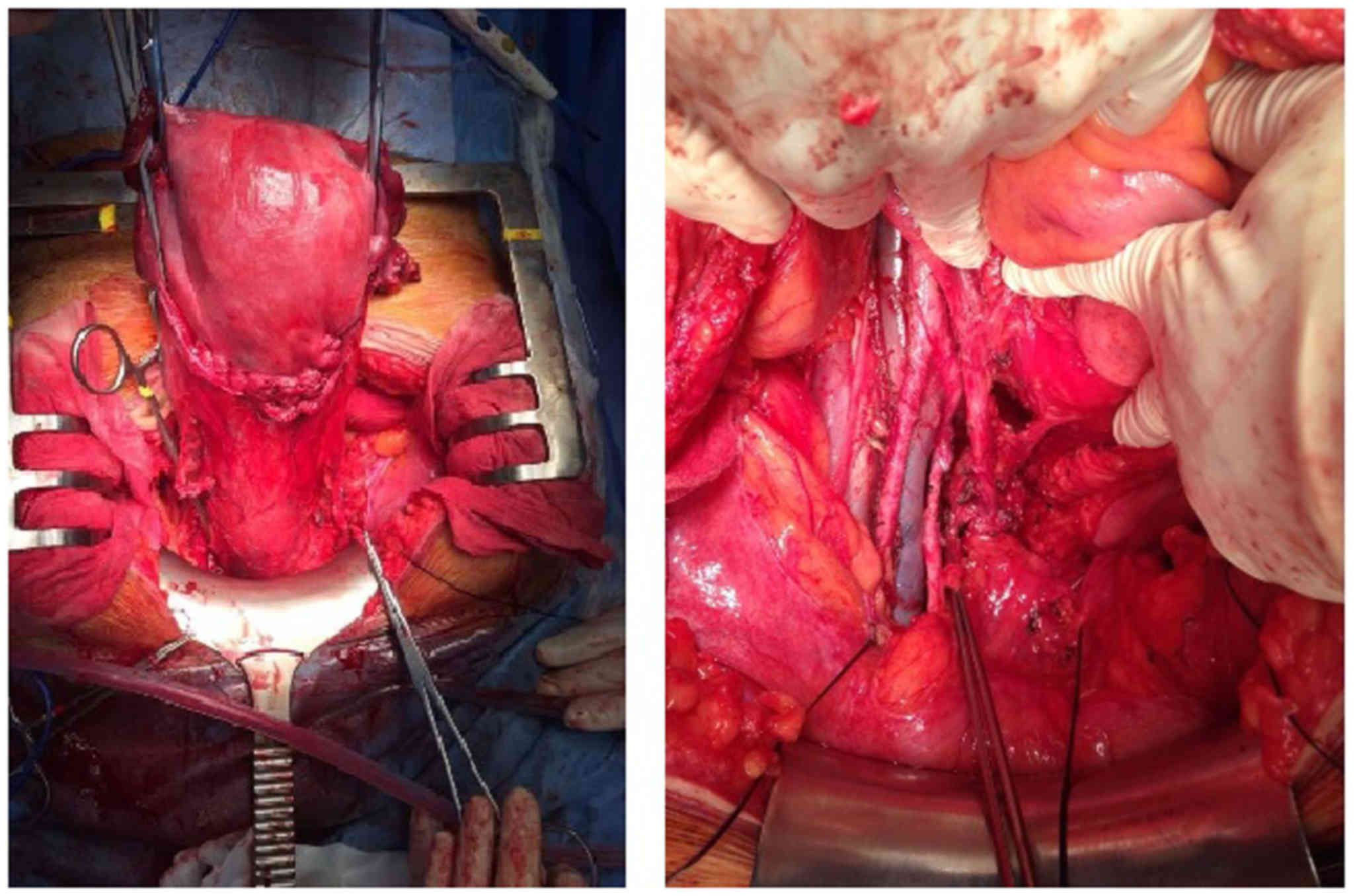
At 31.4 weeks, the patient underwent radical
hysterectomy with nerve sparing (type C1), along with bilateral
adnexectomy and pelvic and inframesenteric para-aortic
lymphadenectomy (Fig. 2). The fetus
was delivered with caesarian section, without complications or
morphological malformations. There were no intraoperative or
postoperative complications. Within the first 2 days the patient
had post-void residual urine volume <100 ml. The hospital stay
was 6 days.
The surgical specimen displayed a high-grade
cervical LCNEC sized 40×16 mm, with positive lymphovascular space
invasion, but without parametrial, vaginal, placental or lymph node
involvement (pelvic nodes 0/22, para-aortic nodes 0/18). The
surgical margins were negative (Fig.
3).
The patient completed treatment with chemoradiation
(intensity-modulated radiotherapy at 45 Gy/15 fr and 5 cycles of
concomitant weekly CDDP at 40 mg/m2); she was unable to
receive the sixth cycle due to the development of G3 asthenia after
the fifth cycle.
During follow-up, physical examination, cytology,
measurement of tumor marker levels and computed tomographic
examination revealed no evidence of recurrence or metastasis at 38
months postoperatively. The baby has also been followed up, without
any signs of neurodevelopmental disorders in July 2018.
Discussion
Over the past decades, there has been a rising trend
of pregnancy at more advanced ages; therefore, cancer during
gestation is an increasing problem in clinical practice. Cervical
cancer is the second most commonly diagnosed cancer during
pregnancy, following breast cancer. NEC of the cervix appears to
occur in women who are younger compared with those with the more
common HPV-associated cervical cancers; therefore, pregnancy may be
a more serious concern for such patients (3).
A growing body of evidence indicates that gestation
does not adversely affect the prognosis of cervical cancer. In
2009, Stensheim et al (4),
using data from the Cancer Registry and the Medical Birth Registry
of Norway, investigated whether cervical cancer diagnosed during
pregnancy or lactation was associated with increased risk of
cause-specific mortality. No increase in the risk of cause-specific
mortality (hazard ratio=0.89; 95% confidence interval: 0.52–1.53)
in patients diagnosed during pregnancy was observed in the
multivariate analyses adjusted for age at diagnosis, extent of
disease and diagnostic period, with a median follow-up of 10.8
years (4). Pettersson et al
(5) in 2010, using the records at
the Radiumhemmet (Solna, Sweden) between 1914 and 2004, compared
the survival of 41 patients diagnosed with cervical cancer while
pregnant or within 6 months postpartum with 82 non-pregnant women
matched for age, stage and histopathology. No significant
difference in 10-year survival rates was observed between the two
groups.
Based on these findings, and taking into
consideration the patient's wishes and our extensive experience
with treating breast cancer diagnosed during pregnancy, we decided
to proceed with treatment without pregnancy interruption. A
multidisciplinary approach between Obstetrics and Gynecological
Oncology groups in such cases is crucial for optimizing maternal
treatment and fetal protection (6–8).
The European Society of Medical Oncology and
European Society of Gynecological Oncology (ESGO) have published
guidelines on gynecological cancer in pregnancy, in which treatment
of cervical cancer during gestation is indicated, albeit
experimental. It is important to note that patients who wish to
preserve their pregnancy should be informed that the treatment will
be individualized out of the standard process. In these guidelines,
when cervical cancer stage is higher than IB2 and the nodes are
negative, NACT is considered as the only method for preserving
pregnancy until fetal maturity.
The therapeutic value of pelvic staging
lymphadenectomy prior to the initiation of NACT remains unknown.
The ESGO guidelines indicate that it may be safely performed
between the 13th and 22nd weeks of gestation to assess pelvic nodal
status (1,9). However, it was decided against in the
present case, as MRI during pregnancy has also demonstrated a good
positive predictive value for nodal metastasis (10).
The Society of Gynecological Oncology guidelines
published in 2011 recommend multimodal management for all stages of
NEC of the cervix (11), and the
majority of patients receive some combination of surgery, radiation
and chemotherapy. For women with bulky lesions, NACT followed by
radical hysterectomy with lymphadenectomy plus adjuvant
chemotherapy ± radiotherapy is a viable option (12).
Exposure to chemotherapy during the first trimester
of pregnancy increased the risk of fetal defects compared with
administration during the second and third trimesters, which has
not been associated with significant adverse effects in the fetus
in the short or long term; however, not all chemotherapeutic agents
are safe (1).
No standard treatment for LCNEC has yet been
established (13), and the regimen
with CDDP and VP-16 was selected based on the management of
small-cell lung cancer (2,14).
In the NTP Monograph on Cancer Chemotherapy during
pregnancy, published by the U.S. Department of Health and Human
Services in May 2013, CDDP is a widely accepted treatment during
pregnancy at doses ranging from 20 mg/m2 for 5 days up
to 75 mg/m2 every 3 weeks (15). The rate of malformations following
exposure to CDDP in the first trimester was 20%; however, in the
second or third trimester of pregnancy, that percentage decreased
to 1%. The most frequent minor malformations were blepharophimosis,
microcephaly and hydrocephalus. CDDP exposure is associated with
decreased intrauterine growth by 13%. Data on the use of VP-16
during pregnancy are limited: A 3% rate of major malformations
following administration in the second and third trimesters and 24%
fetal growth restriction have been reported. The most frequent
major malformation was cerebral atrophy and ventriculomegaly
(9,16).
In the present case, based on the advice of the
multidisciplinary team, the patient received three cycles of NACT
with CDDP 50 mg/m2 and VP-16 100 mg/m2 every
3 weeks.
Due to the aggressive nature of cervical NECs,
surveillance with tumor assessment was performed every 2 weeks,
including physical examination and vaginal ultrasound, always
performed by the same Gynecological Oncology specialist
sonographer. No more MRI scans were indicated, as the tumor was
stable. Fetal well-being was evaluated by the High Pregnancy Risk
Unit, with fetal growth assessment every 2 weeks. During
surveillance, NACT appeared to stabilize the tumor, preventing
dissemination without harming the fetus.
Although it remains unclear when is the best time to
deliver the fetus, the general trend is after 28 weeks of gestation
or after 3 cycles of chemotherapy. Regardless, a 3-week period
should be allowed between the last chemotherapy dose and the
expected date of delivery, ~33.2 weeks on average. We decided to
deliver after the third cycle of NACT, at 31 weeks of gestation,
and radical surgery was performed concomitantly with caesarean
section (1). Nerve-sparing radical
hysterectomy type C1 with complete tumor resection was performed,
in order to decrease the risk of bladder, rectal and sexual
dysfunction. The identification and preservation of hypogastric and
pelvic splanchnic nerves and the pelvic plexus after caesarean
section was feasible without increasing the volume of blood
loss.
In a recent study by Stecklein et al
investigating survival in cervical NEC, 80% of the patients
progressed and 70% succumbed to the disease during a median
follow-up of 21.5 months [5-year progression free survival (PFS)
rate of 20%, and 5-year overall survival (OS) rate of 27%]
(17). The patient in the present
case remained disease-free after 32 months of follow-up. In that
same study, patients with cervical LCNEC had significantly better
median PFS (median not reached vs. 10.0 months, P=0.02) and
exhibited a trend toward better median OS (153 vs. 21 months,
P=0.08) compared with patients with other histological types
(17).
It may be concluded that NACT appears to be a viable
option for preserving pregnancy in advanced cases, while
stabilizing the disease and preventing tumor dissemination before
reaching fetal maturity (sufficient gestational age and stage of
fetal development). The present case demonstrated that NACT
followed by radical hysterectomy concomitantly with caesarean
section may be considered as a pregnancy-sparing treatment option.
In several countries with specialized multidisciplinary teams, this
may be a feasible alternative to pregnancy termination.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
BGI reviewed the literature, conducted data
collection, surgery and prepared the manuscript. PR reviewed the
literature, conducted data collection. ELL reviewed the literature,
performed fetal monitoring during pregnancy. LFM reviewed the
literature, conducted medical oncology analysis. AG performed
pathological examinations and critically revised the manuscript for
intellectual important content. BDF reviewed the literature,
conducted data collection, and surgery, and critically revised the
manuscript for intellectual important content. All authors approval
of the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient agreed with the publication of the case
and has named her daughter Milagros (miracle).
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Peccatori FA, Azim HA Jr, Orecchia R,
Hoekstra HJ, Pavlidis N, Kesic V and Pentheroudakis G; ESMO
Guidelines Working Group, : Cancer, pregnancy and fertility: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi160–vi170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Embry JR, Kelly MG, Post MD and Spillman
MA: Large cell neuroendocrine carcinoma of the cervix: Prognostic
factors and survival advantage with platinum chemotherapy. Gynecol
Oncol. 120:444–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frumovitz M: Small- and large-cell
neuroendocrine cervical cancer. Oncology (Williston Park).
30:70–77, 78, 93. 2016.PubMed/NCBI
|
|
4
|
Stensheim H, Møller B, van Dijk T and
Fosså SD: Cause-specific survival for women diagnosed with cancer
during pregnancy or lactation: A registry-based cohort study. J
Clin Oncol. 27:45–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pettersson BF, Andersson S, Hellman K and
Hellström AC: Invasive carcinoma of the uterine cervix associated
with pregnancy: 90 years of experience. Cancer. 116:2343–2349.
2010.PubMed/NCBI
|
|
6
|
Córdoba O, Llurba E, Cortés J, Sabadell
MD, Lirola JL, Ferrer Q and Xercavins J: Complete pathological
remission in a patient with hormone-receptor positive and c-erbB-2
expression-negative breast cancer treated with FAC chemotherapy
during pregnancy. Tumori. 96:629–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Córdoba O, Bellet M, Vidal X, Cortés J,
Llurba E, Rubio IT and Xercavins J: Pregnancy after treatment of
breast cancer in young women does not adversely affect the
prognosis. Breast. 21:272–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Córdoba O, Llurba E, Saura C, Rubio I,
Ferrer Q, Cortés J and Xercavins J: Multidisciplinary approach to
breast cancer diagnosed during pregnancy: Maternal and neonatal
outcomes. Breast. 22:515–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amant F, Halaska MJ, Fumagalli M,
Steffensen Dahl K, Lok C, Van Calsteren K, Han SN, Mir O, Fruscio
R, Uzan C, et al: Gynecologic cancers in pregnancy: Guidelines of a
second international consensus meeting. Int J Gynecol Cancer.
24:394–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balleyguier C, Fournet C, Ben Hassen W,
Zareski E, Morice P, Haie-Meder C, Uzan C, Gouy S, Duvillard P and
Lhommé C: Management of cervical cancer detected during pregnancy:
Role of magnetic resonance imaging. Clin Imaging. 37:70–76. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gardner GJ, Reidy-Lagunes D and Gehrig PA:
Neuroendocrine tumors of the gynecologic tract: A Society of
Gynecologic Oncology (SGO) clinical document. Gynecol Oncol.
122:190–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plante M: Bulky early-stage cervical
cancer (2-4 cm lesions): Upfront radical trachelectomy or
neoadjuvant chemotherapy followed by fertility-preserving surgery:
Which is the best option? Int J Gynecol Cancer. 25:722–728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delaloge S, Pautier P, Kerbrat P,
Castaigne D, Haie-Meder C, Duvillard P, Guivarch C, Goupil A, Borel
C and Lhommé C: Neuroendocrine small cell carcinoma of the uterine
cervix: What disease? What treatment? Report of ten cases and a
review of the literature. Clin Oncol (R Coll Radiol). 12:357–362.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zivanovic O, Leitao MM Jr, Park KJ, Zhao
H, Diaz JP, Konner J, Alektiar K, Chi DS, Abu-Rustum NR and
Aghajanian C: Small cell neuroendocrine carcinoma of the cervix:
Analysis of outcome, recurrence pattern and the impact of
platinum-based combination chemotherapy. Gynecol Oncol.
112:590–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Toxicology Program, . NTP
Monograph: Developmental Effects and Pregnancy Outcomes Associated
With Cancer Chemotherapy Use During Pregnancy. https://ntp.niehs.nih.gov/pubhealth/hat/noms/chemo/index.htmlMay
13–2013
|
|
16
|
Correa A, Cragan JD, Kucik JE, Alverson
CJ, Gilboa SM, Balakrishnan R, Strickland MJ, Duke CW, O'Leary LA,
Riehle-Colarusso T, et al: Reporting birth defects surveillance
data 1968–2003. Birth Defects Res A Clin Mol Teratol. 79:65–186.
2007.PubMed/NCBI
|
|
17
|
Stecklein SR, Jhingran A, Burzawa J,
Ramalingam P, Klopp AH, Eifel PJ and Frumovitz M: Patterns of
recurrence and survival in neuroendocrine cervical cancer. Gynecol
Oncol. 143:552–557. 2016. View Article : Google Scholar : PubMed/NCBI
|