Introduction
Liposarcoma is one of the most common types of
sarcoma arising in adult soft tissues, accounting for 15–25% of all
types of sarcoma (1–3), and is chiefly subclassified into four
types; atypical lipomatous tumor and/or well-differentiated
liposarcoma (ALT/WDL), dedifferentiated liposarcoma, myxoid
liposarcoma, and pleomorphic liposarcoma (1,3). A
diagnosis of liposarcoma requires histological evidence of
lipoblastic differentiation, which may be easily recognizable in
ALT/WDL, but is more difficult to assess in non-lipogenic
sarcomatous regions within the dedifferentiated and pleomorphic
subtypes. Liposarcoma usually arises in the extremities,
retroperitoneum, mesenteric region, and shoulder area (1–3); its
occurrence in the pleura is rare. To the best of our knowledge,
only 30 cases of pleural liposarcoma have been previously reported
in the English literature (4–21), and
their clinicopathological features remain to be fully elucidated.
The present case report discusses a recently encountered case of
pleural dedifferentiated liposarcoma; the clinicopathological
features of this case are described to expand on current knowledge
of pleural liposarcoma.
Case report
A 45-year-old Japanese man was hospitalized in the
Japan Self-Defense Forces Central Hospital (Tokyo, Japan) for
rapidly increasing left chest pain. Computed tomography (CT)
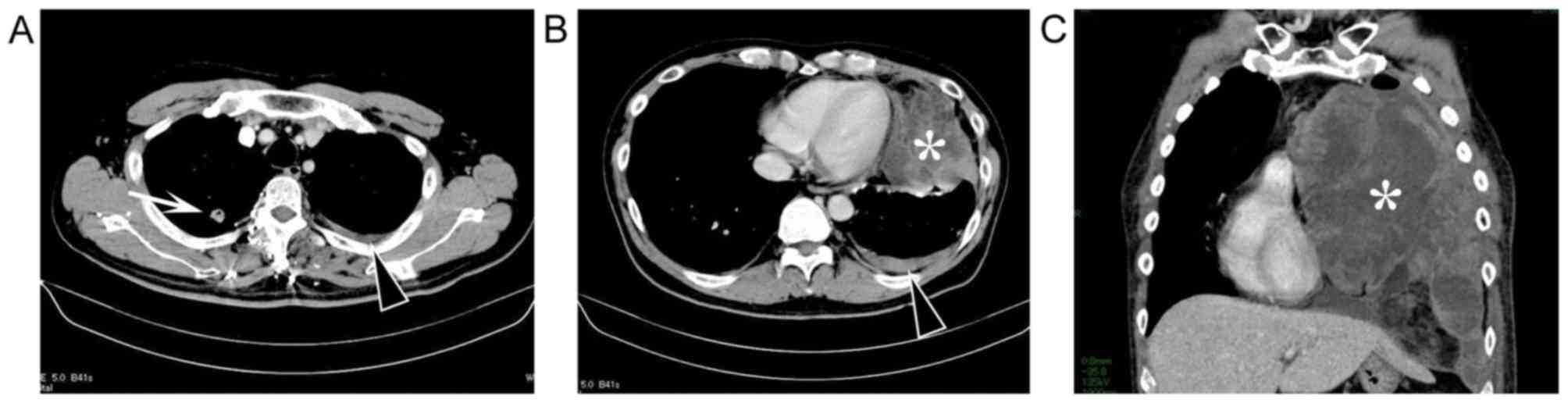
revealed a 10-cm left pleural tumor with hydrothorax and a 1.7-cm
pulmonary nodule in the right upper lobe (Fig. 1A and B).
18F-fluorodeoxyglucose positron emission tomography
(18F-FDG-PET) revealed FDG uptake in the left pleural
tumor and the right pulmonary nodule, with maximum standardized
uptake values in these lesions of 8.35–10.42 and 2.99–3.14,
respectively. Hematological analysis and bronchoscopic examination
revealed no significant findings. CT-guided percutaneous biopsy and
thoracotomy-associated incisional biopsy of the left pleural tumor
were performed. These specimens exhibited undifferentiated
sarcomatous features. Embolization of the arteries feeding the left
pleural tumor and palliative surgery were performed 1 month and 2.2
months later, respectively. Surgery consisted of partial resection
of the pleural tumor and resection of the left lower and lingua
lobes invaded by the tumor. Due to his worsened general condition,
the patient was unable to receive additional chemotherapy. The
pleural tumor regrew rapidly following surgery and the mediastinum
was shifted to the right (Fig. 1C).
The patient's condition progressively deteriorated, and he
succumbed to mortality from respiratory failure due to expansive
growth of the pleural tumor ~4.2 months following hospitalization.
Autopsy was performed.
The premortem biopsy specimens and surgically
removed specimens were histologically composed of undifferentiated
spindle-shaped and/or rounded cells with swollen irregular nuclei
proliferating in a haphazard and/or fascicular manner with myxoid
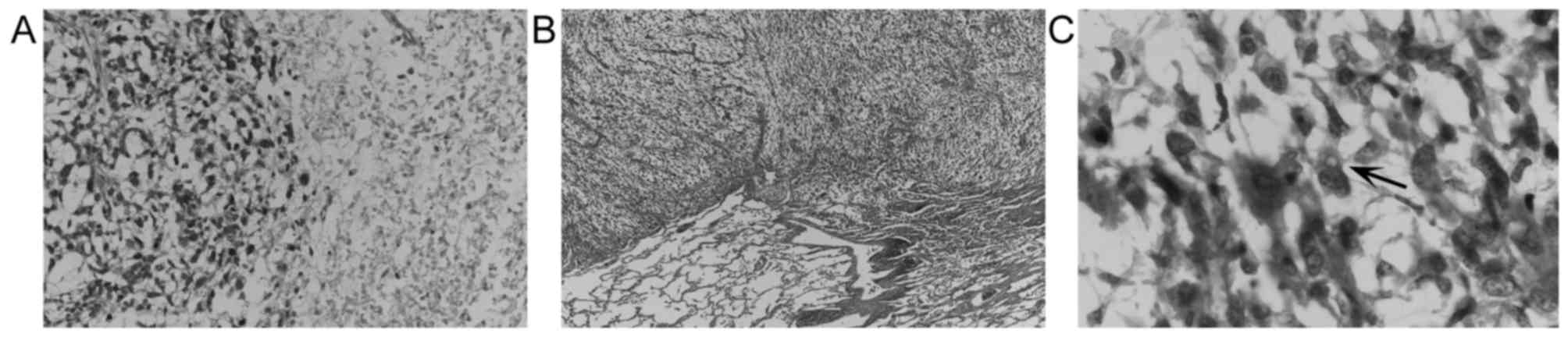
stroma and necrosis (Fig. 2). Only a
few cells with a vacuolated cytoplasm were observed (Fig. 2C); however, it was not possible to
rule out non-specific vacuolar changes. Immunohistochemically, the
tumor cells were positive for vimentin only, and were negative for
cytokeratin, S-100 protein, glial fibrillary acid protein, desmin,
α-smooth muscle actin, HMB-45, and myoglobin. Nuclear
immunoreactivity for Ki-67 was observed in 26% of the tumor cells
within the surgically removed specimens.
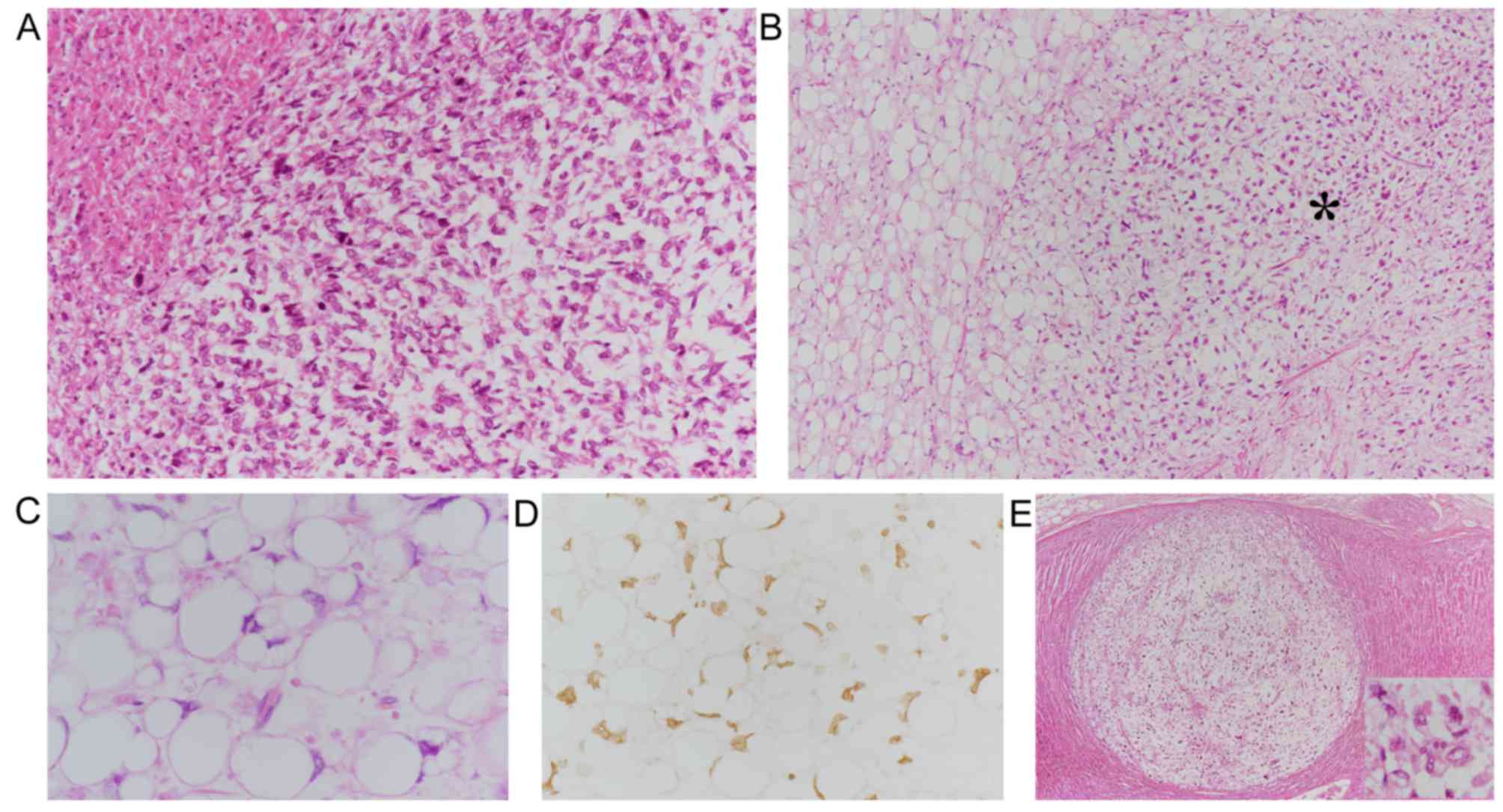
Postmortem examination revealed a 35-cm, whitish,
necrotic solid tumor occupying the left thoracic cavity (Fig. 3A), which was directly invading the
remnant left lung, chest wall, and diaphragm. Histologically, this
pleural tumor exhibited predominantly myxoid sarcomatous features
(Fig. 3A). However, in localized
areas (~5% of the tumor volume) adjacent to the diaphragm,
scattered or aggregated lipoblasts with occasionally scalloped
swollen nuclei and lipid-rich cytoplasms were observed, some of
which were intermingled with undifferentiated spindle-shaped or
rounded tumor cells (Fig. 3B and C).
The aggregated lipoblasts closely resembled those of lipoma-like
ALT/WDL. These findings indicated a diagnosis of liposarcoma. The
undifferentiated spindle-shaped/rounded tumor cells had
metastasized to both adrenal glands (Fig. 3E) and the lumbar vertebral bones. By
contrast, the 2.2-cm right pulmonary nodule was composed of
columnar tumor cells proliferating in papillary, acinar, and focal
lepidic patterns, indicating primary lung papillary-predominant
adenocarcinoma. No metastases of pulmonary adenocarcinoma were
observed. Immunohistochemically, the lipoblasts were diffusely
positive for MDM2 (Fig. 3D) and
focally and weakly positive for S-100 protein. The sarcomatous
spindle-shaped/rounded cells were focally positive for MDM2, but
were negative for S-100 protein. Autopsy also demonstrated severe
pneumonia of the collapsed remnant left lung.
Discussion
In the present case study, the left pleural tumor
had extensive involvement of non-lipogenic sarcomatous elements,
with no lipoblastic features established on premortem examination.
However, autopsy identified distinctive lipoblastic features
intermingled with undifferentiated sarcomatous elements in the
pleural tumor. The differential diagnoses included dedifferentiated
liposarcoma and pleomorphic liposarcoma. The former accounts for
18% of liposarcomas (1),
characterized by the transformation from ALT/WDL and amplification
of MDM2 (1,3). The latter, pleomorphic liposarcoma,
represents <15% of liposarcomas (1), which primarily develop de novo
without ALT/WDL-like low-grade precursor lesions and without
MDM2 amplification (1,3,22–24).
Previous studies (25,26) have reported that immunohistochemical
evaluation of the expression of MDM2 provides suitable sensitivity
for detecting MDM2 gene amplification. In the present case
study, autopsy identified ALT/WDL-like lesions within the primary
tumor, and immunohistochemical evaluation revealed MDM2-positivity
not only in the ALT/WDL-like lesions but also in sarcomatous
spindle-shaped/rounded tumor cells. These findings suggest that the
pleural tumor present was dedifferentiated liposarcoma.
Table I summarizes 31
cases of pleural liposarcoma, including 30 previously reported
cases (4–21) and the present case study. Chen et
al (20) described nine cases of
pleural liposarcoma, however, tables in this article listed 10
cases of ‘pleural’ liposarcoma. Therefore, the clinicopathological
features of one female case are unclear. The patients included 20
men and 11 women. For the 30 patients whose ages were known, the
age ranged between 19 and 80 years (mean, 49.6 years). A total of
14 tumors involved the left pleural cavity, six arose in the right
pleural cavity, and the sites of the other 11 tumors were unknown.
In the 26 tumors for which the histological subtype was reported,
myxoid or myxoid/rounded liposarcoma accounted for 12 (46%), which
is higher than the proportion of myxoid liposarcomas within all
types of liposarcoma. Other subtypes included eight ALT/WDLs (31%),
five dedifferentiated liposarcomas (19%), and one mixed type (4%).
The proportion of the dedifferentiated type liposarcomas is similar
to that reported for all liposarcomas.
 | Table I.Clinicopathological features of 31
cases of pleural liposarcoma including the present case report. |
Table I.
Clinicopathological features of 31
cases of pleural liposarcoma including the present case report.
| Author (year) | Age (years)/sex | Site of pleura | Tumor size (cm) | Histology | Therapy | Follow-up/months | Refs. |
|---|
| Ackerman and Wheeler
(1942) | 50/F | Left | NS | NSg | None (autopsy) | DOD/12 | (4) |
| Gupta and Paolini
(1967) | 51/M | Right | 21 | Por | None (autopsy) | DOD/NS | (5) |
| D'Ambrosio
(1974) | 52/M | Left | NS | NS | C-Res | ANED/66 | (6) |
| Wouters et al
(1983) | 19/M | Left | 3.5 | Myx | C-Res + Rad | Alive/55h | (7) |
| Evans et al
(1985) | 45/M | Left | NS | Myx | None (autopsy) | DOO/0.07 | (8) |
| McGregor et al
(1987) | 54/M | Right | 2-25a | WDL (>PL) | C-Res | DOU/108i | (9) |
| Munk and Müller
(1988) | 27/F | Left | NS | NS | NS | NS | (10) |
| Carroll et al
(1992) | 23/F | Left | 29, 21b | Mixed | C-Res + Rad | ANED/16 | (11) |
| Wong et al
(1994) | 38/M | Right | NS | My | C-Res + Rad | ANED/5 | (12) |
| Okby and Travis
(2000) | 45/F | NS | 16 | Myx/Ro | P/I-Res + Chem | DOD/7 | (13) |
| Okby and Travis
(2000) | 73/M | Right | NS | Myx | P/I-Res | DOD/9 | (13) |
| Okby and Travis
(2000) | 67/M | Right | 18.5 | WDL | NS | DOU/16 | (13) |
| Okby and Travis
(2000) | 80/M | Right | 20 | Myx | C- or P/I-Rec | NS | (13) |
| Minniti et
al (2005) | 50/M | Left | 13 | WDL | C-Res + Rad | ANED/12 | (14) |
| Takanami and | 59/M | Right | 12,
5.3c | Dediff | C-Res | ANED/6 | (15) |
| Imamura (2005)
Goldsmith and Papagiannopoulos (2007) | 42/M | Left | NS | Myx | C-Res + Rad |
Alive/12j | (16) |
| Goldsmith and
Papagiannopoulos (2007) | 80/F | Left | NS | Myx | P/I-Res | DOD/8 | (16) |
| Benchetritt et
al (2007) | 76/F | Left | 18, 11d | Dediff | C- or P/I-Res | DOO/0.1 | (17) |
| Peng et al
(2007) | 56/F | Left | NS | WDL | C-Res | ANED/18 | (18) |
| Alloubi et
al (2008) | 58/M | Left | NS | Myx | C-Res + Rad | ANED/10 | (19) |
| Chen et al
(2014) | 19/M | NS | NS | WDL | C-Res |
Alive/56k | (20) |
| Chen et al
(2014) | 30/F | NS | NS | WDL | C-Res | ANED/48 | (20) |
| Chen et al
(2014) | 60/M | NS | NS | WDL | C-Res | ANED/43 | (20) |
| Chen et al
(2014) | 20/F | NS | NS | Myx | C-Res | Alive/90l | (20) |
| Chen et al
(2014) | 54/M | NS | NS | Myx | C-Res | ANED/26 | (20) |
| Chen et al
(2014) | 41/M | NS | NS | Dediff | C-Res |
Died/15m | (20) |
| Chen et al
(2014) | 53/M | NS | NS | Dediff | C-Res |
Died/11n | (20) |
| Chen et al
(2014) | 61/M | NS | NS | WDL | P/I-Res | ANED/18 | (20) |
| Chen et al
(2014) | NA/Fe | NAe | NAe | NAe | NAe | NAe | (20) |
| Wang et al
(2017) | 43/F | Left | 21 | Myx | C-Res | ANED/8 | (21) |
| Present case
(2018) | 45/M | Left | 10f | Dediff | P/I-Res |
DOD/4.2o | – |
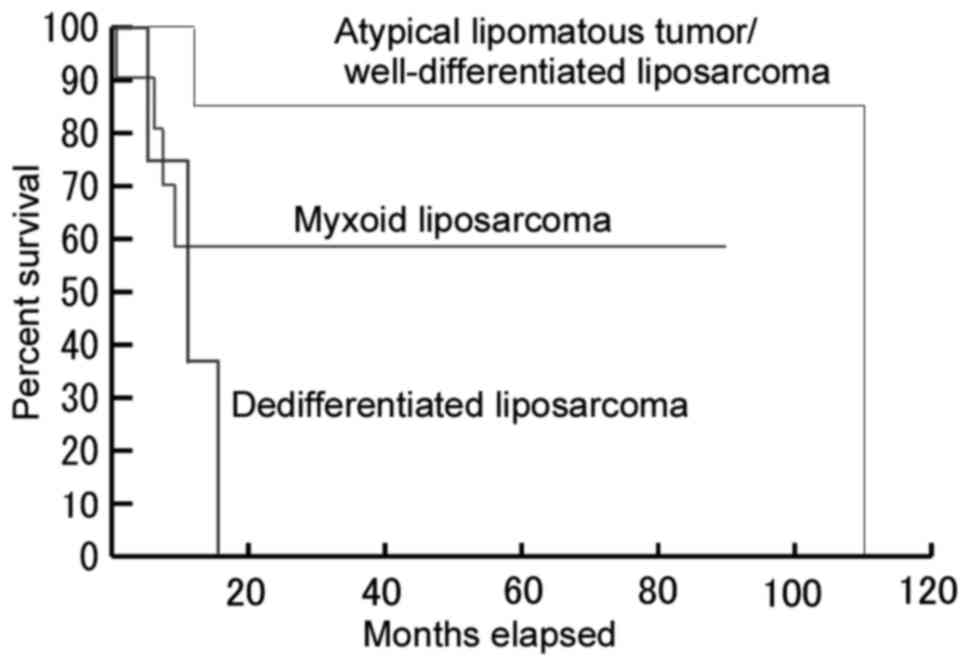
Among the 23 patients for whom follow-up data and
the histological type of the liposarcoma were available, six of
eight (75%) patients with pleural ALT/WDL were alive during months
12–56 of follow-up, whereas only one of the four (25%) patients
with pleural dedifferentiated liposarcoma remained alive without
disease during the 6 months of follow-up (Fig. 4). A log-rank test demonstrated a
significant difference in overall survival rate between patients
with ALT/WDL and dedifferentiated liposarcoma (P=0.001). Therefore,
dedifferentiated histology appears to be a negative prognostic
factor for pleural liposarcoma. No statistically significant
differences were observed between patients with ALT/WDL, vs. myxoid
liposarcoma (P=0.141) or between patients with myxoid, vs.
dedifferentiated liposarcoma (P=0.259). In the 18 reported cases of
completely resected pleural liposarcoma for which follow-up data
were available, 15 (83%) patients were alive during months 6–90 of
follow-up, with or without recurrence. These finding provides
support for the previously reported concept that radical surgery
may be a positive prognostic factor for liposarcoma (3,20),
although the remaining three (17%) patients succumbed to mortality
during months 11–108 of follow-up (9,20).
Recurrent growth following complete resection was observed in seven
(39%) of these patients (7,9,16,20),
including five cases of local recurrence, one of pulmonary
metastasis, and one recurrence at an unreported location. The case
of pulmonary metastasis developed in a patient with
dedifferentiated liposarcoma (20).
In the present case report, the initially detected pulmonary nodule
was primary lung adenocarcinoma, and not a metastasis, which may
have been responsible for the differences in maximum standardized
uptake values between these lesions in the premortem FDG-PET
examination. In addition, in the present case report, adrenal and
bone metastases of liposarcoma were observed. However, the reason
for the patient succumbing to mortality was considered to be due to
the local aggressiveness of pleural liposarcoma, rather than to
those metastases.
In conclusion, the present case report describes a
case of pleural liposarcoma diagnosed upon autopsy. Premortem
examination revealed myxoid sarcomatous features only, whereas
autopsy identified scattered or aggregated MDM-positive lipoblasts,
suggesting dedifferentiated liposarcoma. The local aggressiveness
of this tumor directly contributed to the patient's clinical
course.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
SM, YU, YK, and HT analyzed the histopathological
features. YO, KO, and TT provided clinical data, and re-analyzed
them. YU collected previously reported articles. SM drafted the
manuscript, and the remaining authors (YO, YU, KO, TT, YK, and HT)
commented on the manuscript. SM and HT revised the manuscript.
Ethics approval and consent to
participate
This retrospective study was performed according to
the Declaration of Helsinki. Although the patient succumbed to
mortality, the patient's father provided written informed consent
for autopsy examination and related further investigation.
Patient consent for publication
The patient's father provided written informed
consent for publication of the study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALT/WDL
|
atypical lipomatous tumor and/or
well-differentiated liposarcoma
|
|
CT
|
computed tomography
|
|
18F-FDG-PET
|
18F-fluorodeoxyglucose
positron emission tomography
|
References
|
1
|
Goldblum JR, Folpe AL and Weiss SW:
Enzinger and Weiss's soft tissue tumors. 6th edition. Elsevier;
Philadelphia, PA: 2014
|
|
2
|
Goldblum JR, McKenney JK, Lamps LW and
Myers JL: Rosai and Ackerman's surgical pathology. 11th edition.
Elsevier; Philadelphia, PA: 2018
|
|
3
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: WHO classification of tumours of soft tissue and
bone. 4th edition. International Agency for Research on Cancer;
Lyon: 2013
|
|
4
|
Ackerman LV and Wheeler P: Liposarcoma.
South Med J. 35:156–159. 1942. View Article : Google Scholar
|
|
5
|
Gupta RK and Paolini FA: Liposarcoma of
the pleura: Report of a case. With a review of literature and views
on histogenesis. Am Rev Respir Dis. 95:298–304. 1967.PubMed/NCBI
|
|
6
|
D'Ambrosio V: First case of liposarcoma
from the parietal pleura. J Med Soc N J. 71:17–19. 1974.PubMed/NCBI
|
|
7
|
Wouters EFM, Greve LH, Visser R and Swaen
GJ: Liposarcoma of the pleura. Neth J Surg. 35:192–193.
1983.PubMed/NCBI
|
|
8
|
Evans AR, Wolstenholme RJ, Shettar SP and
Yogish H: Primary pleural liposarcoma. Thorax. 40:554–555. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McGregor DH, Dixon AY, Moral L and Kanabe
S: Liposarcoma of pleural cavity with recurrence as malignant
fibrous histiocytoma. Ann Clin Lab Sci. 17:83–92. 1987.PubMed/NCBI
|
|
10
|
Munk PL and Müller NL: Pleural
liposarcoma: CT diagnosis. J Comput Assist Tomogr. 12:709–710.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carroll F, Kramer MD, Acinapura AJ,
Tietjen PA, Wagner I, Oiseth S and Smith F and Smith F: Pleural
liposarcoma presenting with respiratory distress and suspected
diaphragmatic hernia. Ann Thorac Surg. 54:1212–1213. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong WW, Pluth JR, Grado GL, Schild SE and
Sanderson DR: Liposarcoma of the pleura. Mayo Clin Proc.
69:882–885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okby NT and Travis WD: Liposarcoma of the
pleural cavity: Clinical and pathologic features of 4 cases with a
review of the literature. Arch Pathol Lab Med. 124:699–703.
2000.PubMed/NCBI
|
|
14
|
Minniti A, Montaundon M, Jougon J,
Hourneau M, Begueret H, Laurent F and Velly JF: Liposarcoma of the
pleural cavity. An exceptional tumour. Monaldi Arch Chest Dis.
63:170–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takanami I and Imamura T: Dedifferentiated
liposarcoma of the pleura: Report of a case. Surg Today.
35:313–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldsmith P and Papagiannopoulos K:
Pleural myxoid liposarcoma: Features of 2 cases and associated
literature review. J Cardiothorac Surg. 2:482007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benchetritt M, Hofman V, Vénissac N,
Brennetot C, Italiano A, Aurias A, Padovani B, Pedeutour F and
Hofman P: Dedifferentiated liposarcoma of the pleura mimicking a
malignant solitary fibrous tumor and associated with
dedifferentiated liposarcoma of the mediastinum: Usefulness of
cytogenetic and molecular genetic analyses. Cancer Genet Cytogenet.
179:150–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng C, Zhao X, Dong X and Jiang X:
Liposarcoma of the pleural cavity: A case report. J Thorac
Cardiovasc Surg. 133:1108–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alloubi I, Boubia S and Ridai M:
Liposarcoma of the pleural cavity. Thorac Cardiovasc Surg.
56:438–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Yang J, Zhu L, Zhou C and Zhao H:
Primary intrathoracic liposarcoma: A clinicopathologic study and
prognostic analysis of 23 cases. J Cardiothorac Surg. 9:1192014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Kiryu S, Li L, Wang Q, Li D and
Zhang L: Resectable primary pleural myxoid liposarcoma with a
pedicle: Report of a rare case and literature review. J Thorac Dis.
9:E183–E187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hornick JL, Bosenberg MW, Mentzel T,
McMenamin ME, Oliveira AM and Fletcher CDM: Pleomorphic
liposarcoma: Clinicopathologic analysis of 57 cases. Am J Surg
Pathol. 28:1257–1267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boland JM, Weiss SW, Oliveira AM,
Erickson-Johnson ML and Folpe AL: Liposarcomas with mixed
well-differentiated and pleomorphic features: A clinicopathologic
study of 12 cases. Am J Surg Pathol. 34:837–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariño-Enríquez A, Fletcher CDM, Dal Cin P
and Hornick JL: Dedifferentiated liposarcoma with ‘homologous’
lipoblastic (pleomorphic liposarcoma-like) differentiation:
Clinicopathologic and molecular analysis of a series suggesting
revised diagnostic criteria. Am J Surg Pathol. 34:1122–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Binh MBN, Sastre-Garau X, Guillou L, de
Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I and Coindre JM:
MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing
well-differentiated and dedifferentiated liposarcoma subtypes: A
comparative analysis of 559 soft tissue neoplasms with genetic
data. Am J Surg Pathol. 29:1340–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sirvent N, Coindre JM, Maire G, Hostein I,
Keslair F, Guillou L, Ranchere-Vince D, Terrier P and Pedeutour F:
Detection of MDM2-CDK4 amplification by fluorescence in situ
hybridization in 200 paraffin-embedded tumor samples: Utility in
diagnosing adipocytic lesions and comparison with
immunohistochemistry and real-time PCR. Am J Surg Pathol.
31:1476–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|