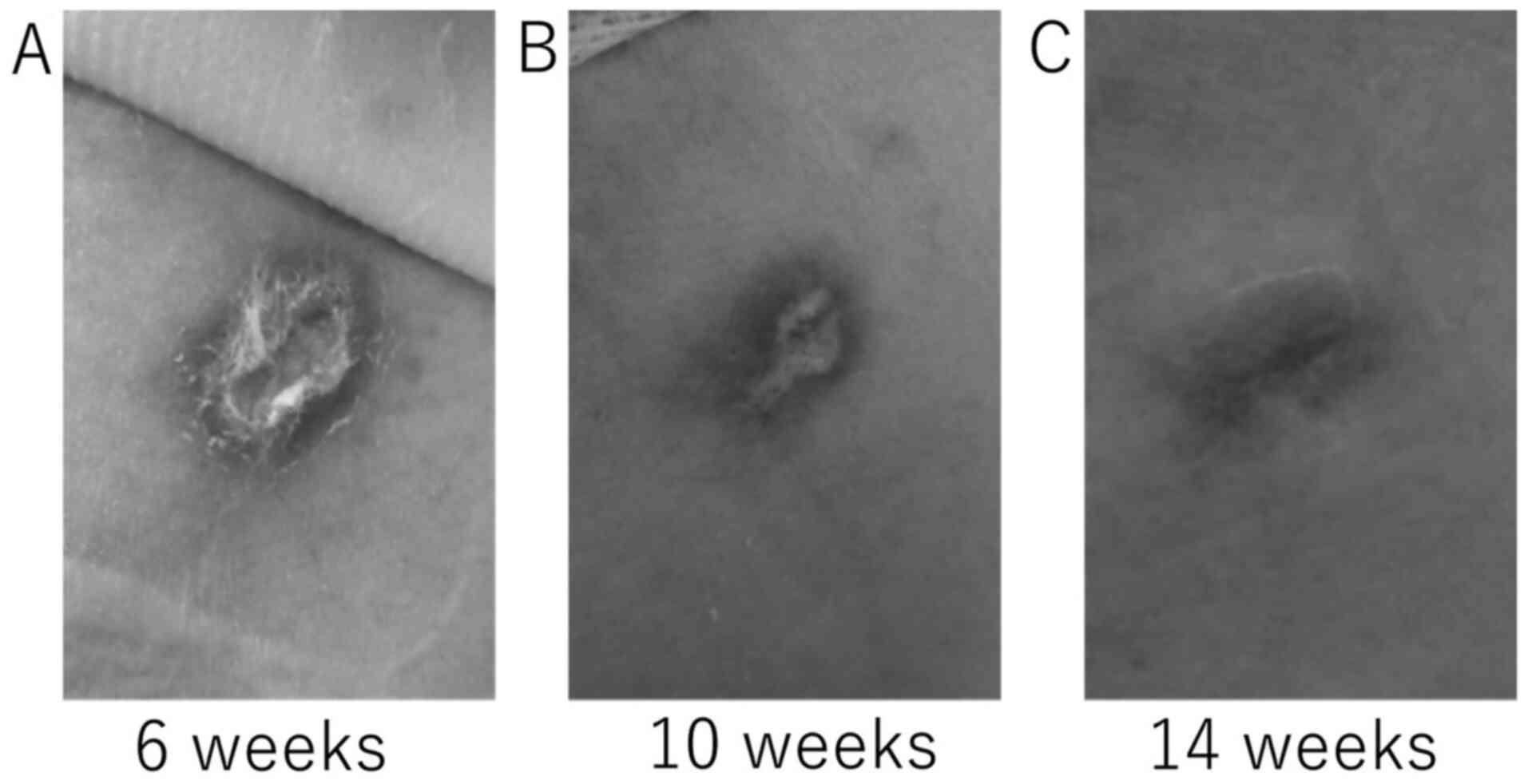
|
1
|
Matsui J, Funahashi Y, Uenaka T, Watanabe
T, Tsuruoka A and Asada M: Multi-kinase inhibitor E7080 suppresses
lymph node and lung metastases of human mammary breast tumor
MDA-MB-231 via inhibition of vascular endothelial growth
factor-receptor (VEGF-R) 2 and VEGF- R3 kinase. Clin Cancer Res.
14:5459–5465. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Matsui J, Yamamoto Y, Funahashi Y,
Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T and Asada M: E7080,
a novel inhibitor that targets multiple kinases, has potent
antitumor activities against stem cell factor producing human small
cell lung cancer H146, based on angiogenesis inhibition. Int J
Cancer. 122:664–671. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brose MS, Worden FP, Newbold KL, Guo M and
Hurria A: Effect of age on the efficacy and safety of lenvatinib in
radioiodine-refractory differentiated thyroid cancer in the Phase
III SELECT Trial. J Clin Oncol. 35:2692–2699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iwasaki H, Toda S, Suganuma N, Murayama D,
Nakayama H and Masudo K: Lenvatinib vs. palliative therapy for
stage IVC anaplastic thyroid cancer. Mol Clin Oncol. 12:138–143.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Locati LD, Piovesan A, Durante C, Bregni
M, Castagna MG, Zovato S, Giusti M, Ibrahim T, Puxeddu E, Fedele G,
et al: Real-world efficacy and safety of lenvatinib: data from a
compassionate use in the treatment of radioactive iodine-refractory
differentiated thyroid cancer patients in Italy. Eur J Cancer.
118:35–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berdelou A, Borget I, Godbert Y, Nguyen T,
Garcia ME, Chougnet CN, Ferru A, Buffet C, Chabre O, Huillard O, et
al: Lenvatinib for the treatment of radioiodine-refractory thyroid
cancer in real-life practice. Thyroid. 28:72–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Blevins DP, Dadu R, Hu M, Baik C,
Balachandran D, Ross W, Gunn B and Cabanillas ME: Aerodigestive
fistula formation as a rare side effect of antiangiogenic tyrosine
kinase inhibitor therapy for thyroid cancer. Thyroid. 24:918–922.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Allegra CJ, Yothers G, O'Connell MJ,
Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins
JN, Seay TE, et al: Initial safety report of NSABP C-08: A
randomized phase III study of modified FOLFOX6 with or without
bevacizumab for the adjuvant treatment of patients with stage II or
III colon cancer. J Clin Oncol. 27:3385–3390. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Scappaticci FA, Fehrenbacher L, Cartwright
T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar
S and Hurwitz H: Surgical wound healing complications in metastatic
colorectal cancer patients treated with bevacizumab. J Surg Oncol.
91:173–180. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.PubMed/NCBI(In Japanese).
|
|
13
|
Iwasaki H, Toda S, Ito H, Nemoto D,
Murayama D, Okubo Y, Hayashi H and Yokose T: A case of unresectable
papillary thyroid carcinoma treated with lenvatinib as neoadjuvant
chemotherapy. Case Rep Endocrinol. 2020(6438352)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Roman CD, Choy H, Nanney L, Riordan C,
Parman K, Johnson D and Beauchamp RD: Vascular endothelial growth
factor-mediated angiogenesis inhibition and postoperative wound
healing in rats. J Surg Res. 105:43–47. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cornacoff JB, Howk K, Pikounis B,
Mendenhall V and Martin P: Development of a method for the
evaluation of wound tensile strength in cynomolgus macaques. J
Pharmacol Toxicol Methods. 57:74–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu JF, Bruno R, Eppler S, Novotny W, Lum B
and Gaudreault J: Clinical pharmacokinetics of bevacizumab in
patients with solid tumors. Cancer Chemother Pharmacol. 62:779–786.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamaguchi K, Fujitani K, Nagashima F,
Omuro Y, Machida N, Nishina T, Koue T, Tsujimoto M, Maeda K and
Satoh T: Ramucirumab for the treatment of metastatic gastric or
gastroesophageal junction adenocarcinoma following disease
progression on first-line platinum- or fluoropyrimidine-containing
combination therapy in Japanese patients: A phase 2, open-label
study. Gastric Cancer. 21:1041–1049. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoshino T, Yamazaki K, Yamaguchi K, Doi T,
Boku N, Machida N, Onozawa Y, Asayama M, Fujino T and Ohtsu A: A
phase I study of intravenous aflibercept with FOLFIRI in Japanese
patients with previously treated metastatic colorectal cancer.
Invest New Drug;. 31:910–917. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strumberg D, Richly H, Hilger RA,
Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D,
Haase CG, et al: Phase I clinical and pharmacokinetic study of the
Novel Raf kinase and vascular endothelial growth factor receptor
inhibitor BAY 43-9006 in patients with advanced refractory solid
tumors. J Clin Oncol. 23:965–972. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dubbelman AC, Rosing H, Nijenhuis C,
Huitema ADR, Mergui-Roelvink M, Gupta A, Verbel D, Thompson G,
Shumaker R, Schellens JH and Beijnen JH: Pharmacokinetics and
excretion of (14) C-lenvatinib in patients with advanced solid
tumors or lymphomas. Invest New Drug. 33:233–240. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Faivre S, Delbaldo C, Vera K, Robert C,
Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et
al: Safety, pharmacokinetic, and antitumor activity of SU11248, a
novel oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 24:25–35. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cowey CL, Amin C, Pruthi RS, Wallen EM,
Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, et
al: Neoadjuvant clinical trial with sorafenib for patients with
stage II or higher renal cell carcinoma. J Clin Oncol.
28:1502–1507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Li Y, Deng J, Ji Z, Yu H and Li
H: Sorafenib neoadjuvant therapy in the treatment of high risk
renal cell carcinoma. PLoS One. 10(E0115896)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Resteghini C, Locati LD, Bossi P,
Bergamini C, Guzzo M and Licitra L: Do not throw the baby out with
the bathwater: SELECT a personalized, de-escalated lenvatinib
schedule allows response in locally advanced DTC while controlling
major drug-related bleeding. Ann Oncol. 28:2321–2322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mancuso MR, Davis R, Norberg SM, O'Brien
S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et
al: Rapid vascular regrowth in tumors after reversal of VEGF
inhibition. J Clin Invest. 116:2610–2621. 2006.PubMed/NCBI View
Article : Google Scholar
|