Introduction
Liver cancer is the third leading cause of cancer
death in the world, with approximately 745 thousand deaths every
year (1). Hepatocellular carcinoma
(HCC) is the most common pathological type of primary liver cancer
in adults. It usually occurs in patients with chronic liver
disease, especially those with cirrhosis. Most patients with HCC
were frequently diagnosed late and had lost the opportunity of
resection at the time of diagnosis. Compared with placebo,
first-line sorafenib therapy prolonged overall survival (OS) of
patients with advanced HCC by 2.8 months (2). Recently, the REFLECT study showed that
lenvatinib was non-inferior to sorafenib in terms of OS. Although
this study reported serious adverse effects caused by lenvatinib,
such as liver failure, cerebral hemorrhage, and respiratory
failure, no adverse events of hepatic encephalopathy (HE) were
highlighted (3). The combination of
lenvatinib and anti-programmed cell death-1 (PD-1) monoclonal
antibody (mAb) has been explored in clinical practice. For example,
a phase III study showed that the combination of lenvatinib with
anti-PD-1 mAb led to a 17-month OS in patients with advanced HCC
(4). However, whether the combined
therapy increased the HE risk remained unclear. This study reported
a case of clinical HE occurrence in a patient with HCC receiving
the combination therapy of lenvatinib and anti-PD-1 mAb.
Case report
A 42-year-old male patient had abdominal discomfort
in September 2018, with no other symptoms such as nausea, vomiting,
melena, or back pain. Computed tomography (CT) of the abdomen
performed in a hospital in Beijing revealed a large hepatic tumor;
ascites; thrombi of the portal vein, hepatic vein, and inferior
vena cava; and varicose veins in the lower esophagus, around the
gastric fundus, and in the splenic hilum. He was diagnosed with
advanced HCC. Sorafenib 400 mg twice daily was administered for 6
months. In March 2019, his CT images examined at Taian Hospital
showed multiple nodules in the left hepatic lobe and hilar area.
The largest one was measured as about 10.7x7.2 cm2, the
right branch of the portal vein was compressed and thinned, and
some solid masses were seen in the left branch. Portal vein tumor
thrombus and moderate ascites were observed (Fig. 1). The laboratory results were as
follows: alanine aminotransferase (ALT), 29 U/l; aspartate
aminotransferase (AST), 77 U/l; total bilirubin (TBIL), 110.2
µmol/l; direct bilirubin (DBIL), 92.5 µmol/l; indirect bilirubin
(IBIL), 17.7 µmol/l; albumin (ALB), 25.7 g/l; prothrombin time
(PT), 16.8 s; and alpha-fetoprotein (AFP), 127.7 ng/ml. The patient
was defined as stage IVa (cT4N1M0), Child-Pugh grade C, and BCLC
stage D (Table I). Glutathione and
polyphosphatidylcholine were added as symptomatic treatments. On
April 12, 2019, the liver function was tested again, and the
results were as follows: ALT, 36 U/l; AST, 73 U/l; TBIL, 139
µmol/l; DBIL, 106.5 µmol/l; IBIL, 32.8 µmol/l; and ALB, 28.5 g/l.
The patient's condition got worse. On April 13, 2019, the treatment
of lenvatinib 12 mg once daily combined with anti-PD-1 mAb 240 mg
was started. On the third day after the medication, the patient
developed emotional abnormalities and mild cognitive impairment and
was not able to identify anyone. On the fourth day, the patient
developed a disorder of consciousness and slurred speech. The
patient did not cooperate with the physical examination and was
stunned, blurred, poor in spirit, and irritable with mixed aphasia.
The pupil diameter increased to 4 mm on both sides, and it was slow
to respond to light. Physical examination results were muscle
strength grade 5, normal muscle tension, bilateral palmomental
reflex (+), tendon hyperreflexia (++), Babinski sign positive, and
ataxia. A cranial CT scan showed no significant abnormalities. The
laboratory results were as follows: blood ALT, 48 U/l; AST, 98 U/l;
TBIL, 90 µmol/l; DBIL, 82.5 µmol/l; IBIL, 7.5 µmol/l; ALB, 34.4
g/l; and blood ammonia, 248 µg/dl. The patient was diagnosed as
grade 3 HE. Lenvatinib was discontinued, and the patient was
transferred to the intensive care unit for symptomatic treatment,
including oxygen inhalation, sedation, fluid replacement,
correction of electrolyte disturbances, liver protection, and
enema. The ornithine aspartate injection was also used to reduce
the blood ammonia level. On the fifth day, the patient's condition,
including consciousness, spirit, and sleep, greatly improved, and
his irritability disappeared. The blood test revealed the
following: ALT, 39 U/l; AST, 86 U/l; TBIL, 126.2 µmol/l; DBIL, 95.7
µmol/l; IBIL, 30.5 µmol/l; ALB, 28.6 g/l; and blood ammonia, 132
µg/dl. Finally, the patient was discharged due to his improved
physical status.
 | Table IGeneral characteristics of the
patient. |
Table I
General characteristics of the
patient.
| Characteristic | Value |
|---|
| Age (years) | 42 |
| Sex | Male |
| Diagnosis | Hepatocellular
carcinoma |
| Stage | IVa |
| Child-Pugh | C |
| BCLC stage | D |
| Ascites | Present |
| Tumor thrombus | |
|
Portal
vein | Present |
|
Hepatic
vein | Present |
|
Inferior
vena cava | Present |
| Varicose veins | |
|
Lower
esophagus | Present |
|
Gastric
fundus | Present |
|
Splenic
hilum | Present |
| First-line
therapy | 400 mg bid
Sorafenib |
| Second-line
therapy | 12 mg qd Lenvatinib +
240 mg qd Nivolumab |
| WBC count
(x109/l) | |
|
Before
treatment | 10.52 |
|
After
treatment | 11.33 |
|
Normal
range | 4-10 |
| HB (g/l) | |
|
Before
treatment | 107 |
|
After
treatment | 118 |
|
Normal
range | Male, 120-160;
Female, 110-150 |
| PLT
(x109/l) | |
|
Before
treatment | 239 |
|
After
treatment | 236 |
|
Normal
range | 100-300 |
| ALT (U/l) | |
|
Before
treatment | 36 |
|
After
treatment | 48 |
|
Normal
range | 0-40 |
| AST (U/l) | |
|
Before
treatment | 73 |
|
After
treatment | 98 |
|
Normal
range | 0-40 |
| TBIL (µmol/l) | |
|
Before
treatment | 139 |
|
After
treatment | 90 |
|
Normal
range | 3.7-17.1 |
| DBIL (µmol/l) | |
|
Before
treatment | 106.5 |
|
After
treatment | 82.5 |
|
Normal
range | 0.0-6.8 |
| IBIL (µmol/l) | |
|
Before
treatment | 32.8 |
|
After
treatment | 7.5 |
|
Normal
range | 1.7-10.2 |
| Alb (g/l) | |
|
Before
treatment | 28.5 |
|
After
treatment | 7.5 |
|
Normal
range | 35-51 |
| BUN (mmol/l) | |
|
Before
treatment | 3 |
|
After
treatment | 3.9 |
|
Normal
range | 2.86-7.14 |
| SCr (µmol/l) | |
|
Before
treatment | 36 |
|
After
treatment | 39 |
|
Normal
range | 44-133 |
| NH3 (µg/dl) | |
|
Before
treatment | 0 |
|
After
treatment | 248 |
|
Normal
range | 40-80 |
Discussion
The REFLECT study was an open-label, multicenter,
non-inferiority phase III clinical study to compare the efficacy
and safety of lenvatinib versus sorafenib in the first-line
treatment of patients with unresectable advanced HCC) (3). It revealed that the median survival
time of lenvatinib was 13.6 months, which was non-inferior to that
of sorafenib (12.3 months). The study showed that the most common
any-grade adverse events associated with the use of lenvatinib
included hypertension (42%), diarrhea (39%), decreased appetite
(34%), and weight loss (31%). Fatal adverse events occurred in 11
(2%) patients, including liver failure (3 patients), cerebral
hemorrhage (3 patients), and respiratory failure (2 patients). The
adverse events associated with the use of sorafenib included
hand-foot syndrome, diarrhea, hypertension, and decreased appetite.
No adverse events of HE were reported in the REFLECT study.
HE is a serious complication of severe liver
disease. It is mainly manifested as a spectrum of neuropsychiatric
abnormalities in patients with liver dysfunction, after exclusion
of brain disease, and characterized by personality changes,
intellectual impairment, and a depressed level of consciousness.
The inducing factors mainly included severe liver disease,
extensive portosystemic shunt, infection, upper gastrointestinal
hemorrhage, and massive drainage of ascites. HE caused by anti-PD-1
mAb or a combination of anti-PD-1 mAb and lenvatinib in HCC has not
been reported so far.
Namba et al, (5) reported a case of lenvatinib
monotherapy-induced HE in HCC. The patient was diagnosed with HCC
in 2016 and received hepatic lobectomy. His CT showed a
portosystemic shunt between the superior mesenteric vein and the
right testicular vein. In July 2018, the reexamination of CT and
laboratory parameters showed multiple lung metastases and lymph
node metastases, with the blood ammonia level of 39 µmol/l; the
patient was reviewed as Child-Pugh A grade. The patient was treated
with lenvatinib after progression. Five days after taking
lenvatinib (12 mg/day), the patient developed grade 3 HE (type B).
Lenvatinib was discontinued after the eighth day of medication due
to no significant improvement in the condition after the
corresponding therapy, and then the symptoms were relieved. On the
fourth day of discontinuation, lenvatinib was restarted with a
reduced dose (8 mg/day). Grade 2 HE and elevated blood ammonia
level (145 µmol/l) appeared again, and lenvatinib was discontinued
again. A surgery was performed to block the collateral circulation
of the portal system, and then lenvatinib was re-administered (12
mg/day) successfully without the recurrence of HE. It was concluded
that lenvatinib might induce HE by increasing the portal collateral
circulation of the hepatic portal vein.
In addition, a multicenter, open-label phase I
clinical trial on the use of lenvatinib in ttreating advanced HCC
enrolled 20 patients. Two patients had grade 3 HE: one with
Child-Pugh A and the other with Child-Pugh B. They took lenvatinib
at doses of 16 and 12 mg/day, respectively. It was presumed that
high doses of lenvatinib might induce HE (6).
This novel study reported a case of HE induced by
lenvatinib combined with anti-PD-1 mAb in treating advanced HCC
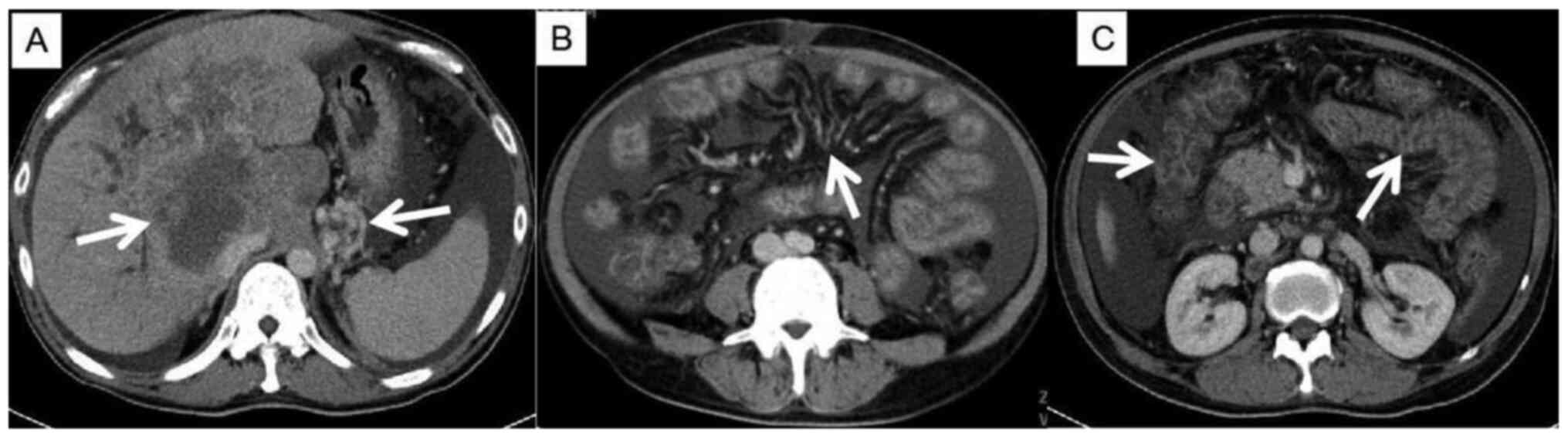
with Child-Pugh C. This patient had advanced HCC with cirrhosis,
and the CT image showed a collateral circulation of the portal
vein, causing significant varicose veins in the gastric fundus
(Fig. 1A), mesenteric varices
(Fig. 1B), and edema of colon and
small intestine (Fig. 1C). On the
third day of the combination treatment, the patient developed grade
1 HE and rapidly progressed to grade 3 HE. After lenvatinib was
discontinued, the blood ammonia level decreased and symptoms
relieved. In this case, HE occurred after the treatment of
lenvatinib combined with anti-PD-1. This patient presented
Child-Pugh C and had a very high bilirubin level, which were common
manifestations in advanced HCC and had a trend to present with HE.
However, the patient did not display key clinical characteristics
for the diagnosis of HE, including abnormal blood ammonia level,
before the combined treatment of lenvatinib with anti-PD-1 mAb.
After a few days of this combination treatment, he again presented
with HE. Which drug induced HE in this patient with Child-Pugh C
was not known. Based on the aforementioned findings, it was
believed that HE was mainly induced by lenvatinib in this case. HE
has many causative factors, such as the portal-systemic venous
shunt. This case illustrated that the formation of collateral
circulation in the portal vein might be the basis of
lenvatinib-induced HE. Therefore, when treating patients with HCC
having a poor liver function, such as cirrhosis and portal vein
collateral circulation, whether to use lenvatinib, what dose to
use, and under what conditions to choose anti-PD-1 monoclonal
antibody combination therapy need to be addressed in the
future.
In conclusion, the patient with HCC showing a portal
vein collateral circulation on CT/magnetic resonance imaging images
might have a high risk of developing HE after treatment with the
combination therapy of lenvatinib and anti-PD-1.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the Shandong
Medical and Health Science and Technology Development Plan Project
(grant no. 2017WS266) and the Scientific Research Project of the
Shandong Academy of Medical Sciences (grant nos. 201730, 201808 and
201838).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL and SZ carried out the case report, collected the
data and drafted the manuscript. ZL, WX, ZF and SZ performed
statistical analysis and participated the study design. ZL, HW, LS,
YM and XX participated in the acquisition, analysis and
interpretation of data, and drafted the manuscript. All authors
read and approved the final manuscript. ZL and SZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The patient provided written informed consent.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Can. 136:E359–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamashita T, Kudo M, Ikeda K, Izumi N,
Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, et
al: REFLECT-a phase 3 trial comparing efficacy and safety of
lenvatinib to sorafenib for the treatment of unresectable
hepatocellular carcinoma: An analysis of Japanese subset. J
Gastroenterol. 55:113–122. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Namba M, Kawaoka T, Aikata H, Kodama K,
Uchikawa S, Ohya K, Morio K, Fujino H, Nakahara T, Murakami E, et
al: Percutaneous transvenous shunt occlusion for portosystemic
encephalopathy due to lenvatinib administration to a patient with
hepatocellular carcinoma and portosystemic shunt. Clin J
Gastroenterol. 12:341–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ikeda M, Okusaka T, Mitsunaga S, Ueno H,
Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K and Kumada H:
Safety and pharmacokinetics of lenvatinib in patients with advanced
hepatocellular carcinoma. Clin Cancer Res. 22:1385–1394.
2016.PubMed/NCBI View Article : Google Scholar
|