Introduction
Over the last decade, the treatments and outcomes of
diffuse large B-cell lymphoma (DLBCL) have significantly improved
owing to recent therapeutic advances (such as rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone
treatments) (1). However, ~5% of
patients still suffer from the undesirable devastating complication
of central nervous system (CNS) relapse (2,3). CNS
relapse usually occurs 12 months after completion of systemic
chemotherapy (4-11)
and is associated with poor outcomes and a median survival time of
6 months after relapse. El-Galaly et al (12) conducted an international cohort
study to evaluate the prognostic factors and treatment-related
differences in the outcome of CNS involvement in patients with
DLBCL, and found that those patients treated with first-line
immune-chemotherapy had poor outcomes following CNS involvement,
but that a moderate proportion of patients with isolated CNS
involvement, who received intensive therapies, achieved durable
remission. Therefore, additional CNS prophylaxis is recommended for
patients with high-risk DLBCL. However, owing to the relatively low
rate of CNS relapse, administering CNS prophylaxis to all patients
might expose a number of them to unnecessary toxicities, as only
high-risk patients might benefit from this prophylaxis (3).
The definition of high-risk DLBCL has been poorly
described in the literature. Commonly reported risk factors for CNS
involvement are a high IPI score, increased serum lactate
dehydrogenase (LDH) levels, an advanced stage of lymphoma, >1
site of extranodal involvement, and involvement of specific
anatomical sites such as the kidneys, testes, uterus and breasts
(13-15).
The CNS-International Prognostic Index (CNS-IPI) score is commonly
used for risk stratification of patients with DLBCL into risk
groups of <1% (low-risk group) and >10% (high-risk group).
This prognostic model was validated using combined data from the
Germen High-Grade Lymphoma Study Group and the British Columbia
Cancer Agency, and became the most widely used model (15).
For CNS prophylaxis, intrathecal chemotherapy was
first proposed, but was found to be ineffective, since CNS
involvement in DLBCL affects the brain parenchyma in 60-80% of
patients (16,17). Unlike intrathecal prophylaxis,
systematic prophylaxis with high-dose intravenous methotrexate
(HDMTX) has the advantage of reaching the brain parenchyma owing to
its ability to cross the blood-brain barrier at high
concentrations. Although the addition of CNS prophylaxis with HDMTX
to the standard rituximab plus cyclophosphamide, doxorubicin,
vincristine and prednisone or prednisolone (R-CHOP) chemotherapy
has been advocated in the literature, evidence on the clinical
benefits of this prophylaxis is unclear owing to limited and
conflicting data (16).
Abramson et al (18) retrospectively evaluated the
outcomes of 65 patients with DLBCL and different CNS risk factors
who received intravenous MTX as CNS prophylaxis in addition to the
standard R-CHOP chemotherapy. The study found that the addition of
CNS prophylaxis with intravenous MTX was safe and was associated
with a low risk of CNS relapse in high-risk patients. In a
multicenter phase II trial of patients with breast DLBCL,
first-line immunochemotherapy and intrathecal MTX led to meaningful
survival outcomes, but were not optimal for CNS prophylaxis
(19). Another study by Garwood
et al (20) showed that
among 205 patients with DLBCL, of whom 28 were selected for two
doses of HDMTX, no significant differences in CNS relapse rate or
CNS-IPI distribution were identified in the propensity-matched
analysis.
As there is no consensus on the methods and criteria
for CNS prophylaxis among patients with DLBCL, the clinical
practice in these patients varies across different locations.
Therefore, the present observational retrospective study was
conducted to determine the outcomes of HDMTX CNS prophylaxis in
patients with intermediate and high-risk DLBCL using real-world
data from a single center.
Patients and methods
Ethics
The present study followed the Strengthening the
Reporting of Observational Studies in Epidemiology statement
guidelines as the standard reporting guidelines for observational
cohort studies (21). The study
was approved by the Ethics Committee of the King Abdullah
International Medical Research Center (KAIMRC) (approval no.
#SP19/196/J).
Study design, setting, and
duration
This observational retrospective cohort study was
conducted at the Princess Noorah Oncology Center (King Abdulaziz
Medical City, Jeddah, Saudi Arabia; under the jurisdiction of
KAIMRC) between January 2010 and December 2020.
Study participants and variables
Patients who met the following inclusion criteria
were included in the study: i) A diagnosis of DLBCL; ii) age >15
years; iii) biopsy-proven DLBCL; and iv) available CNS evaluation
data. CNS evaluation included clinical examination, brain imaging,
cerebral spinal fluid (CSF) flow cytometry or biopsy if required.
Patients with were excluded if they had double/triple-hit lymphoma
or Burkitt's lymphoma.
Baseline characteristics, including IPI, number and
type of extranodal sites, frontline chemotherapy and type of CNS
prophylaxis, were recorded for all patients. Clinical data and
follow-up outcomes were retrospectively retrieved from the hospital
records.
CNS prophylaxis
For patients in the high-risk group (those with
testicular lymphoma, epidural disease, sinus involvement, bone
marrow involvement, or renal and adrenal involvement), intravenous
HDMTX (3.5 g/m2) was administered on days 10-15
post-R-CHOP or following the completion of chemotherapy for 4-6
cycles. R-CHOP chemotherapy alternating with intravenous HDMTX (8
g/m2) was administered as the frontline regimen for
patients with synchronous CNS involvement. Occasionally,
intrathecal MTX (12 mg) was administered for synchronous and early
CNS relapses at the physician's discretion.
Sampling method and sample size
calculation
A convenience sampling method was employed to
collect the data. Sample size was calculated using the
Raosoft® software using its associated website
(www.raosoft.com/samplesize.html). The total number of
patients having CNS involvement among patients with DLBCL between
January 2010 and December 2020 was 358. The required sample size
was estimated at the 95% confidence level with an estimated 5%
prevalence of CNS involvement in the DLBCL among intermediate- and
high-risk patients, and a margin of error of ±5%. The required
minimum sample size was determined to be 61. As the population was
small, all the population in the specified time was included to
make the results representative.
Statistical analysis
Categorical data are described as frequency and
percentage, while continuous data are described as mean and
standard deviation. The association between categorical variables
was analyzed using the χ2 test and an unpaired Student's
t-test. An α level <0.05 was considered for statistical
significance. The overall survival (OS) time was calculated from
the date of confirmed diagnosis to the date of the last follow-up
date or death. Similarly, the progression-free survival (PFS) time
was calculated from the date of confirmed diagnosis to the date of
disease progression, death or the last follow-up. The Kaplan-Meier
survival method was used to calculate OS and PFS rates, with the
lo-rank test applied for the analysis. A stepwise forward Cox
proportional hazard's model, including all the significant factors
from the univariate analysis, was used to determine the risk
factors of CNS relapse. Factors with a two-sided P-value of ≤0.1
were then studied in the multivariate analysis. P≤0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS version 26 (IBM Corp.).
Results
Characteristics of the study
population
A total of 358 patients were included in the present
study. The overall median age of the study participants was 58.5±16
years (range, 15-93 years). The male-to-female ratio was 1.37. Most
of the patients had an advanced stage of cancer (75%). The cell of
origin was a non-germinal center in 37% of patients, a germinal
center in 35% of patients and not reported in 28% of patients. In
total, 67% of patients had at least one site of extranodal
involvement, 61% had an IPI of 2-3 and 39% had a CNS-IPI of 4-6.
Overall, 74% of patients had an elevated LDH level. The median
follow-up time was 35 months for the CNS prophylaxis group and 49
months for the non-CNS prophylaxis group. The baseline
characteristics of all 358 patients are shown in Table I.
 | Table IDemographic and clinical
characteristics of the study population. |
Table I
Demographic and clinical
characteristics of the study population.
| Characteristic | CNS prophylaxis group
(n=32) | Non-CNS prophylaxis
group (n=326) | P-valuea |
|---|
| Mean age (SD),
years | 48.1 (17.3) | 57.5 (16.5) | 0.003 |
| Age group, n
(%) | | | |
|
<60
years | 25 (78.1) | 162 (49.7) | 0.002 |
| Sex, n (%) | | | |
|
Male | 22 (68.8) | 185 (56.7) | 0.190 |
|
Female | 10 (31.3) | 141 (43.3) | |
| Origin of cell, n
(%) | | | |
|
GCB | 15 (46.9) | 110 (33.7) | 0.188 |
|
Non-GCB | 12 (37.5) | 121 (37.1) | |
|
Not
specified | 5 (15.6) | 95 (29.1) | |
| Extranodal sites, n
(%) | | | |
|
≥1 | 30 (93.8) | 209 (64.1) | 0.001 |
| Stage of cancer, n
(%) | | | |
|
1-2 | 3 (9.4) | 88 (27.0) | 0.029 |
|
3-4 | 29 (90.6) | 238 (73.0) | |
| Abnormal high
serum | 29 (90.6) | 235 (72.1) | 0.018 |
| LDH level, n
(%) | | | |
| CNS-IPI, n (%) | | | |
|
2-3 | 16 (50.0) | 201 (61.7) | 0.198 |
|
4-6 | 16 (50.0) | 125 (38.3) | |
CNS prophylaxis vs. no CNS
prophylaxis
In total, 32 patients (9%) received prophylactic
HDMTX. The main differences between the CNS prophylaxis and the
non-CNS prophylaxis groups were the following characteristics:
Younger age (P=0.003), extranodal site involvement (P=0.001),
advanced stage (P=0.029) and a high LDH level at presentation
(P=0.018). There were no significant differences in the cell of
origin or the CNS-IPI between the patients receiving prophylaxis or
not. The differences between the CNS prophylaxis and the non-CNS
prophylaxis groups are shown in Table
I.
CNS relapse rates, characteristics and
risk factors
A total of 10 CNS relapses were detected in the
study population (4/32 patients in the CNS prophylaxis group vs.
6/326 patients in the non-CNS prophylaxis group). The difference in
the relapse rate between the two groups was statistically
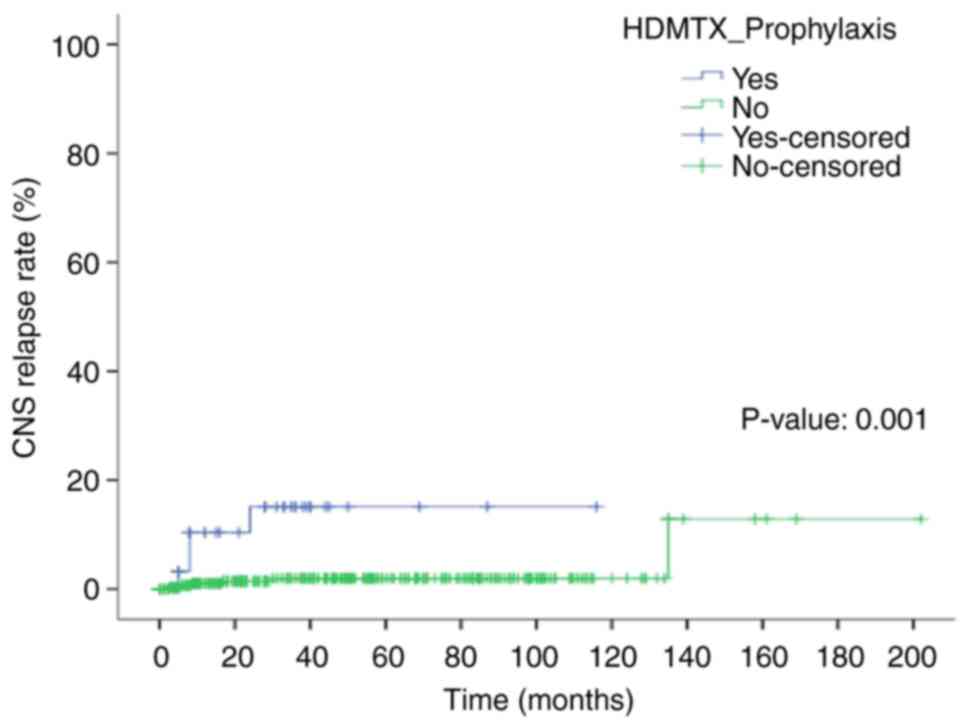
significant (12.5% vs. 1.8%; P=0.008). The 5-year CNS relapse rates
were 15 and 2% in the CNS prophylaxis and non-CNS prophylaxis
groups, respectively (P<0.0001) (Fig. 1).
Out of the 10 patients, 9 had isolated CNS relapses.
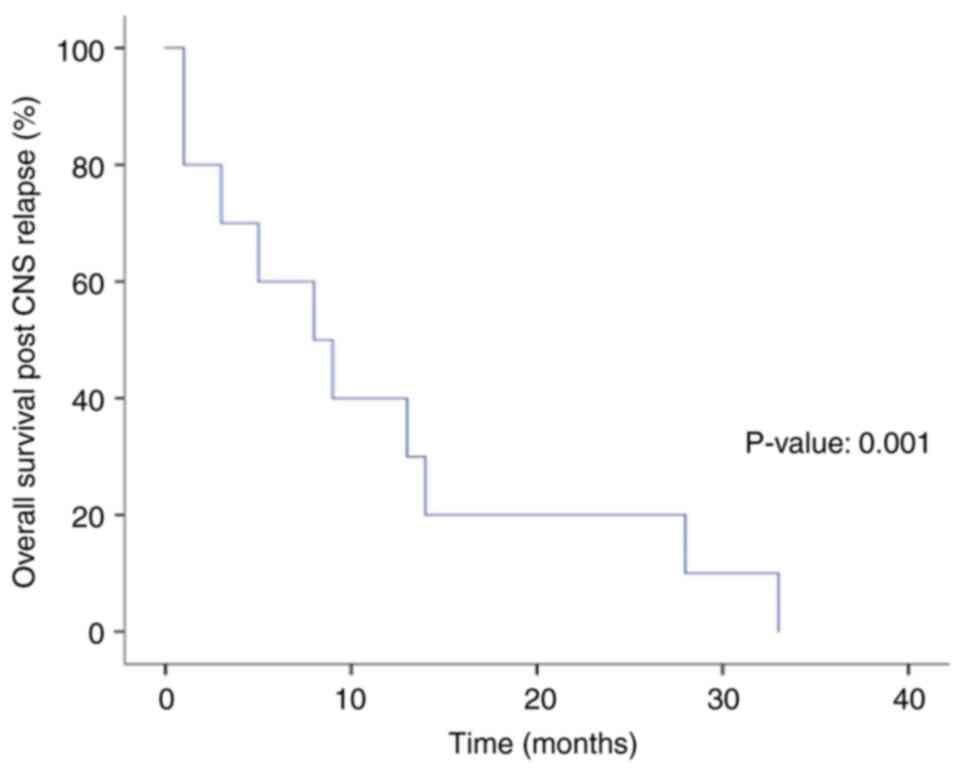
For the CNS prophylaxis group, the median follow-up time was 35
months, the median time to CNS relapse was 8.5 months, the median
overall survival after CNS relapse was 8 months, and the 2-year and
5-year OS rates were 20 and 0%, respectively (Fig. 2).
Clinical characteristics of CNS
relapses
The predominant clinical characteristics of patients
with CNS relapse were advanced disease at initial presentation, a
non-germinal center phenotype and high CNS-IPI. The frequencies and
percentages of these characteristics are shown in Table II.
 | Table IIClinical characteristics of patients
with CNS relapse (n=10). |
Table II
Clinical characteristics of patients
with CNS relapse (n=10).
| Variable | Prevalence ratio
(among patients with CNS relapse) | Percentage |
|---|
| Advanced disease at
initial presentation | 10/10 | 100 |
| Non-GC
phenotype | 7/10 | 70 |
| High CNS-IPI | 7/10 | 70 |
| Extranodal
involvement | 6/10 | 60 |
| Leptomeningeal
involvement | 6/10 | 60 |
| Currently,
alive | 5/10 | 50 |
| Currently, with a
progressive disease | 2/10 | 20 |
| Testicular
lymphoma | 1/10 | 10 |
Risk factors of CNS relapses
Results of the regression analysis showed that
CNS-IPI, CNS-relapse, relapsed disease, IPI (all P<0.001) and
stage of DLBCL (P=0.006) were significantly associated with the
risk of death. In the multivariate analysis, only relapsed disease
retained significance as an independent variable (P<0.001)
(Table III).
 | Table IIIUnivariate and multivariate analyses
of the risk factors for death. |
Table III
Univariate and multivariate analyses
of the risk factors for death.
| | Univariate | Multivariate |
|---|
| Variable | HR | CI | P-value | HR | CI | P-value |
|---|
| Sex | 1.181 | 0.70-1.98 | 0.522 | | | |
| Cell of origin | 0.810 | 0.58-1.12 | 0.204 | | | |
| HDMTX | 1.138 | 0.41-3.15 | 0.804 | | | |
| Extranodal
involvement | 0.730 | 0.39-1.25 | 0.231 | | | |
| CNS-IPI | 2.910 | 1.71-4.95 |
<0.001a | 1.837 | 0.83-5.28 | 0.259 |
| CNS-relapse | 6.280 | 6.28-13.90 |
<0.001a | 0.738 | 0.32-2.23 | 0.738 |
| Relapsed
disease | 0.220 | 0.13-0.36 |
<0.001a | 0.239 | 0.13-0.41 |
<0.001a |
| IPI | 2.065 | 1.41-2.29 |
<0.001a | 1.241 | 0.67-2.29 | 0.491 |
| Stage of DLBCL | 3.300 | 1.41-7.68 | 0.006a | 1.303 | 0.46-3.65 | 0.608 |
Systemic relapse, survival rates and
factors associated with death
In total, 59 patients (16%) experienced a systemic
relapse. The median time to the systemic relapse was 18 months. The
median survival time after the relapse was 12 months, and the
5-year OS rate for patients with systemic relapses was 10%. The
median OS time was 27 months (95% CI, 13.7-40.2) for the CNS
relapsed group, but was not reached for the non-CNS relapsed
patients (P=0.004). The 5-year OS rate for the non-CNS relapsed
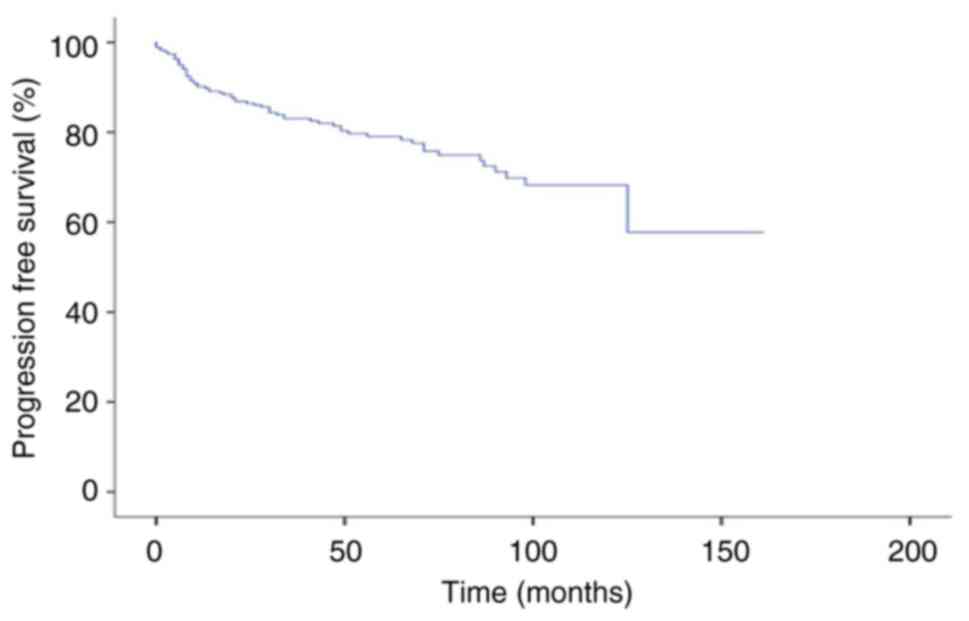
group was 84% (Fig. 3). The median
PFS time was not reached and the 5-year PFS rate was 79% for the
entire population (Fig. 4).
Discussion
The present study aimed to evaluate the outcomes of
CNS prophylaxis with HDMTX among patients with intermediate and
high CNS-IPI DLBCL. This study reflects real-world data from
experience in a single center and consisted of 358 patients who
were treated either with or without HDMTX for CNS prophylaxis. The
study expands the literature by providing information about the
clinical outcomes of HDMTX CNS prophylaxis in patients with
intermediate and high CNS-IPI DLBCL. CNS prophylaxis using HDMTX is
a prophylactic treatment strategy that has been debated in the
literature for a long time due to the limited evidence, conflicting
data from observational studies and the lack of clear class
evidence on its benefits or futility of use in this population.
The present results showed that among patients who
received CNS prophylaxis, the proportions of patients with an age
of <60 years, ≥1 extranodal site, an advanced stage of cancer
(3,12) and abnormally high serum LDH values
were significantly higher than those in the non-CNS prophylaxis
group. Furthermore, patients with HDMTX CNS prophylaxis had a
higher CNS relapse rate and a lower survival rate, in comparison
with the non-CNS prophylaxis group (12.5% vs. 1.8%; P=0.008). These
results were consistent with the data from the UK National Cancer
Research Institute (NCRI) R-CHOP-14 vs. 21 trial where the non-CNS
group had a 1.9% CNS relapse rate in contrast to the CNS
prophylaxis group which had a higher CNS relapse rate of 2.8%
(17); however, the rate was much
lower than the 12.5% reported in the present study population. In a
single-center cohort study by Lee et al (22), 130 patients were evaluated, and it
was reported that the 64 patients receiving HDMTX had a higher risk
of CNS relapse in comparison to the other 66 patients not receiving
prophylaxis (8.1% vs. 6.9%).
By contrast, in a retrospective analysis of 95
high-risk DLBCL patients treated with R-CHOP with (n=57) or without
(n=38) CNS prophylaxis using systemic HDMTX, Kuitunen et al
(23) reported that the 5-year
isolated CNS relapse rate was 5% in the CNS-prophylaxis group and
26% in the non-CNS prophylaxis group, which suggested that HDMTX
decreased the risk of CNS failure.
The timing of systemic HDMTX was assessed in the
study by Wilson et al (24), which found no differences in the
survival or CNS relapse rates between intercalated HDMTX (between
R-CHOP-21) and end of treatment HDMTX (at the end of R-CHOP-21).
Furthermore, intercalated HDMTX was associated with increased
toxicity and delays of the R-CHOP (24). Therefore, individualizing the
timing of HDMTX CNS prophylaxis was recommended, as well as
scheduling the intercalated HDMTX before day 10 of the R-CHOP
cycles to avoid increased toxicity.
Intrathecal MTX has also been studied extensively in
the literature, but is considered to be ineffective due to its
inability to cross the blood-brain barrier. Intrathecal
administration of MTX has become the standard of care for patients
with leptomeningeal involvement. MTX penetrates the BBB poorly when
used in low doses. However, high doses of intravenous (IV) MTX
reach the CNS and are effective against leptomeningeal metastasis.
There are several possible reasons why patients with CNS
involvement who are treated with high doses of IV MTX experience
better outcomes than those treated with intrathecal therapy. Two
important ones are i) the inability of intrathecal MTX to get
absorbed beyond the subarachnoid space and ii) the fact that most
CNS relapses frequently involve the brain parenchyma (14,18,23-26).
Intrathecal therapy must diffuse into the tumor from
the interface between the CSF and the tumor. Tumor cells in areas
of bulk disease, common in non-leukemic meningeal malignancies, and
cells that have spread deep into the cerebral sulci, are not likely
to be fully exposed to drugs administered into the CSF (27). The international phase II trial
(International Extranodal Lymphoma Study Group 10) showed that
combined treatment with R-CHOP21, intrathecal MTX and testicular
radiotherapy was associated with a good outcome in patients with
primary testicular lymphoma, but did not prevent CNS relapses. It
was concluded that further research into CNS prophylaxis is still
needed (28).
Eyre et al (29) analyzed data on 690 patients aged
≥70 years with DLBCL who were consecutively treated with R-CHOP
across 8 UK centers (2009-2018). It was found that stand-alone
intrathecal CNS prophylaxis was not associated with any benefits in
terms of CNS relapse rates. In a multicentre phase II clinical
trial, 33 patients with primary breast DLBCL were treated with an
R-CHOP regimen and four doses of intrathecal MTX (12 mg) (10). The study found that intrathecal MTX
was not optimal for CNS prophylaxis, since CNS relapses occurred in
4 patients, with a 2-year cumulative incidence rate of CNS relapse
of 12.5%. In another single-center retrospective study of 21
patients with DLBCL who received CNS prophylaxis by intrathecal and
intravenous MTX, half of the patients had CNS relapses, with a poor
prognosis and a median survival time of 54 days (30).
In terms of the risk factors for CNS relapses, the
present study showed that CNS prophylaxis, stage of DLBCL and IPI
were significantly associated with CNS relapse were significantly
associated with CNS relapses, while factors associated with the
risk of death were CNS relapse and systemic relapse. Similar risk
factors were reported by the UK NCRI R-CHOP-14 vs. 21 trial, where
a performance status of 2, elevated lactate dehydrogenase level,
IPI, >1 extranodal site of disease and the presence of a
‘high-risk’ extranodal site were significant risk factors for CNS
relapses (17). In the study by
Nazir et al (30), an
advanced stage, high LDH level and extranodal involvement at the
first presentation were significant risk factors for CNS
relapse.
In the study, the 5-year OS rate was 84 and 83% for
the CNS prophylaxis and non-CNS prophylaxis groups, respectively,
while the 5-year PFS rate was 84 and 79% for the CNS prophylaxis
and non-CNS prophylaxis groups, respectively. In the study by Lee
et al (12), the PFS and OS
rates were 66.3 and 77.5%, respectively, for the CNS prophylaxis
group, and 67.4 and 71.4%, respectively, for the non-CNS
prophylaxis group. Data from the phase II Nordic Lymphoma Group
study showed that among the 156 patients treated with R-CHOP with
the addition of etoposide followed by a course of high-dose
cytarabine and a course of HDMTX, the 3-year OS and failure-free
survival rates were 81 and 65%, respectively (31).
It is evident that the literature data on the
clinical benefits or futility of using HDMTX CNS prophylaxis are
conflicting and controversial. However, it should not escape notice
that most of these studies have observational designs. Therefore,
these studies are susceptible to confounding bias, which means that
patients with several CNS relapse risk factors are more likely to
receive CNS prophylaxis in the clinical setting than those with a
low risk of CNS relapses. When studying this population in an
observational design, the unbalanced CNS relapse risk factors in
the study groups likely influence the relapse rates. Therefore, the
differences in the relapse rates between the CNS prophylaxis and
non-CNS prophylaxis groups cannot be attributed to CNS prophylaxis
alone. However, one study used propensity score matching analysis
to compare the study groups, and found no significant difference in
the CNS relapse rates between HDMTX CNS prophylaxis patients and
non-CNS prophylaxis patients (10). Siegal and Goldschmidt (32) proposed that CNS relapse in some
patients with DLBCL might be related to occult lymphoma cells that
are present in the CNS at the time of diagnosis, while in others,
it is due to a later penetration of the CNS by some malignant
clones; therefore, as long as evidence on occult CNS involvement
does not exist, there will be no strong indication that any
strategy of CNS prophylaxis will be beneficial.
The present study has several strong points,
including i) the relatively large sample size of the study (n=358
patients); and ii) the inclusion of patients with intermediate and
high CNS-IPI in the study population. The limitation of the study
is that it was an observational study; therefore, it will be
difficult to establish a causative relationship between patient
outcomes and CNS prophylaxis due to the lack of control over risk
assignment and the lack of random allocation of the study
participants to the treatment groups. Another limitation is that
CNS assessment was not uniformly performed in all patients, thus
occult CNS involvement in some patients would have a bias in the
overall results. Additionally, the small number of CNS prophylaxis
patients in the study limits the strength of the study.
Furthermore, this study did not involve age-, stage- or
extranodal-matched patients in both groups. In summary, the study
is susceptible to confounding bias, and the results should be
confirmed by conducting large randomized controlled trials to
establish whether CNS prophylaxis provides benefits to patients
with intermediate and high CNS-IPI DLBCL. Additional biomarkers and
a larger sample size from a variety of centres should be assessed
in future studies to establish the association more
conclusively.
In conclusion, the present results highlight the
fact that among patients with intermediate and high CNS-IPI DLBCL,
those who received HDMTX CNS prophylaxis had worse survival rates
compared with those without CNS prophylaxis. CNS relapse was found
to be associated with other key parameters such as stage of DLBCL
and IPI. The study also demonstrated that not all intermediate and
high-risk patients require prophylaxis and highlighted the
importance of extranodal involvement. Moreover, in the present
study, the risk of CNS relapse was higher in high CNS-IPI patients
despite the use of CNS prophylaxis. The study data help fill the
gap that exists in the literature from the Middle East and North
Africa region. Future studies with a larger cohort and multiple
centres are needed to evaluate the efficacy of HDMTX CNS
prophylaxis in patients with DLBCL in the context of randomized
controlled trials to mitigate the limitation of a small sample of
CNS prophylaxis patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have critically reviewed and approved
the final draft, and are responsible for the content and similarity
index of the manuscript. MAM conceptualized and designed the study,
and wrote the initial draft of the manuscript. AA, RAM, MA, IEH,
SA, SE and AA contributed in the data collection and analysis. SSA
conceptualized the study, analyzed the data, and edited and revised
the manuscript in the final form. MAK statistically analyzed the
collected data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of King Abdullah International Medical Research Centre
(KAIMRC), a research wing of King Saud Bin Abdulaziz University for
Health Sciences (Jeddah, Kingdom of Saudi Arabia). Every
participant provided informed consent during the execution of the
end-of-course evaluation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Linch D: Developments over the last 60
years in diffuse large B-cell lymphomas. Br J Haematol.
191:552–557. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harrysson S, Eloranta S, Ekberg S, Enblad
G, Jerkeman M, Wahlin BE, Andersson PO and Smedby KE: Incidence of
relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including
CNS relapse in a population-based cohort of 4243 patients in
Sweden. Blood Cancer J. 11(9)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zahid MF, Khan N, Hashmi SK, Kizilbash SH
and Barta SK: Central nervous system prophylaxis in diffuse large
B-cell lymphoma. Eur J Haematol. 97:108–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Haioun C, Besson C, Lepage E, Thieblemont
C, Simon D, Rose C, Tilly H, Sonet A, Lederlin P, Attal M, et al:
Incidence and risk factors of central nervous system relapse in
histologically aggressive non-Hodgkin's lymphoma uniformly treated
and receiving intrathecal central nervous system prophylaxis: A
GELA study on 974 patients. Groupe d'Etudes des Lymphomes de
l'Adulte. Ann Oncol. 11:685–690. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tilly H, Vitolo U, Walewski J, da Silva
MG, Shpilberg O, André M, Pfreundschuh M and Dreyling M: ESMO
Guidelines Working Group. Diffuse large B-cell lymphoma (DLBCL):
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23 (Suppl 7):vii78–82. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Feugier P, Virion JM, Tilly H, Haioun C,
Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, et
al: Incidence and risk factors for central nervous system
occurrence in elderly patients with diffuse large-B-cell lymphoma:
influence of rituximab. Ann Oncol. 15:129–133. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bernstein SH, Unger JM, Leblanc M,
Friedberg J, Miller TP and Fisher RI: Natural history of CNS
relapse in patients with aggressive non-Hodgkin's lymphoma: A
20-year follow-up analysis of SWOG 8516-the southwest oncology
group. J Clin Oncol. 27:114–119. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee KW, Yi J, Choi IS, Kim JH, Bang SM,
Kim DW, Im SA, Kim TY, Yoon SS, Lee JS, et al: Risk factors for
poor treatment outcome and central nervous system relapse in
diffuse large B-cell lymphoma with bone marrow involvement. Ann
Hematol. 88:829–838. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Villa D, Connors JM, Shenkier TN, Gascoyne
RD, Sehn LH and Savage KJ: Incidence and risk factors for central
nervous system relapse in patients with diffuse large B-cell
lymphoma: the impact of the addition of rituximab to CHOP
chemotherapy. Ann Oncol. 21:1046–1052. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chihara D, Oki Y, Matsuo K, Onoda H, Taji
H, Yamamoto K and Morishima Y: Incidence and risk factors for
central nervous system relapse in patients with diffuse large
B-cell lymphoma: Analyses with competing risk regression model.
Leuk Lymphoma. 52:2270–2275. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tai WM, Chung J, Tang PL, Koo YX, Hou X,
Tay KW, Quek R, Tao M and Lim ST: Central nervous system (CNS)
relapse in diffuse large B cell lymphoma (DLBCL): Pre- and
post-rituximab. Ann Hematol. 90:809–818. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
El-Galaly TC, Cheah CY, Bendtsen MD,
Nowakowski GS, Kansara R, Savage KJ, Connors JM, Sehn LH,
Goldschmidt N, Shaulov A, et al: Treatment strategies, outcomes and
prognostic factors in 291 patients with secondary CNS involvement
by diffuse large B-cell lymphoma. Eur J Cancer. 93:57–68.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Susanibar-Adaniya S and Barta SK: Update
on diffuse large B cell lymphoma: A review of current data and
potential applications on risk stratification and management. Am J
Hematol. 96:617–629. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cheah CY, Herbert KE, O'Rourke K, Kennedy
GA, George A, Fedele PL, Gilbertson M, Tan SY, Ritchie DS, Opat SS,
et al: A multicentre retrospective comparison of central nervous
system prophylaxis strategies among patients with high-risk diffuse
large B-cell lymphoma. Br J Cancer. 111:1072–1079. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Klanova M, Sehn LH, Bence-Bruckler I,
Cavallo F, Jin J, Martelli M, Stewart D, Vitolo U, Zaja F, Zhang Q,
et al: Integration of cell of origin into the clinical CNS
international prognostic index improves CNS relapse prediction in
DLBCL. Blood. 133:919–926. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kansara R: Central nervous system
prophylaxis strategies in diffuse large B cell lymphoma. Curr Treat
Options Oncol. 19(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gleeson M, Counsell N, Cunningham D,
Chadwick N, Lawrie A, Hawkes EA, McMillan A, Ardeshna KM, Jack A,
Smith P, et al: Central nervous system relapse of diffuse large
B-cell lymphoma in the rituximab era: Results of the UK NCRI
R-CHOP-14 vs. 21 trial. Ann Oncol. 28:2511–2516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abramson JS, Hellmann M, Barnes JA,
Hammerman P, Toomey C, Takvorian T, Muzikansky A and Hochberg EP:
Intravenous methotrexate as central nervous system (CNS)
prophylaxis is associated with a low risk of CNS recurrence in
high-risk patients with diffuse large B-cell lymphoma. Cancer.
116:4283–4290. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yhim HY, Yoon DH, Kim SJ, Yang DH, Eom HS,
Kim KH, Park Y, Kim JS, Kim HJ, Suh C, et al: First-line treatment
for primary breast diffuse large B-cell lymphoma using
immunochemotherapy and central nervous system prophylaxis: A
multicenter phase 2 trial. Cancers (Basel). 12(2192)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Garwood MJ, Hawkes EA, Churilov L and
Chong G: Patient selection and tolerability of high-dose
methotrexate as central nervous system prophylaxis in diffuse large
B-cell lymphoma. Cancer Chemother Pharmacol. 85:133–140.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC, Vandenbroucke JP and STROBE Initiative: The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. J Clin Epidemiol. 61:344–349.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee K, Yoon DH, Hong JY, Kim S, Lee K,
Kang EH, Huh J, Park CS, Lee SW and Suh C: Systemic HD-MTX for CNS
prophylaxis in high-risk DLBCL patients: A prospectively collected,
single-center cohort analysis. Int J Hematol. 110:86–94.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kuitunen H, Kaprio E, Karihtala P,
Makkonen V, Kauppila S, Haapasaari KM, Kuusisto M, Jantunen E,
Turpeenniemi-Hujanen T and Kuittinen O: Impact of central nervous
system (CNS) prophylaxis on the incidence of CNS relapse in
patients with high-risk diffuse large B cell/follicular grade 3B
lymphoma. Ann Hematol. 99:1823–1831. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wilson MR, Eyre TA, Martinez-Calle N,
Ahearne M, Parsons KE, Preston G, Khwaja J, Schofield J, Elliot J,
Mula Kh A, et al: Timing of high-dose methotrexate CNS prophylaxis
in DLBCL: An analysis of toxicity and impact on R-CHOP delivery.
Blood Adv. 4:3586–3593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eyre TA, Savage KJ, Cheah CY, El-Galaly
TC, Lewis KL, McKay P, Wilson MR, Evens AM, Bobillo S, Villa D, et
al: CNS prophylaxis for diffuse large B-cell lymphoma. Lancet
Oncol. 23:e416–e426. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bobillo S, Joffe E, Sermer D, Mondello P,
Ghione P, Caron PC, Hamilton A, Hamlin PA, Horwitz SM, Kumar A, et
al: Prophylaxis with intrathecal or high-dose methotrexate in
diffuse large B-cell lymphoma and high risk of CNS relapse. Blood
Cancer J. 11(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Glantz MJ, Cole BF, Recht L, Akerley W,
Mills P, Saris S, Hochberg F, Calabresi P and Egorin MJ: High-dose
intravenous methotrexate for patients with nonleukemic
leptomeningeal cancer: Is intrathecal chemotherapy necessary? J
Clin Oncol. 16:1561–1567. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vitolo U, Chiappella A, Ferreri AJ,
Martelli M, Baldi I, Balzarotti M, Bottelli C, Conconi A, Gomez H,
Lopez-Guillermo A, et al: First-line treatment for primary
testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS
prophylaxis, and contralateral testis irradiation: Final results of
an international phase II trial. J Clin Oncol. 29:2766–2772.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Eyre TA, Kirkwood AA, Wolf J, Hildyard C,
Mercer C, Plaschkes H, Griffith J, Fields P, Gunawan A, Oliver R,
et al: Stand-alone intrathecal central nervous system (CNS)
prophylaxis provide unclear benefit in reducing CNS relapse risk in
elderly DLBCL patients treated with R-CHOP and is associated
increased infection-related toxicity. Br J Haematol. 187:185–194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nazir A, Fawad Siddique N and Hameed A:
CNS relapse of diffuse large B cell lymphoma A single centre
experience. Pak J Med Sci. 33:1454–1458. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Holte H, Leppä S, Björkholm M, Fluge O,
Jyrkkiö S, Delabie J, Sundström C, Karjalainen-Lindsberg ML,
Erlanson M, Kolstad A, et al: Dose-densified chemoimmunotherapy
followed by systemic central nervous system prophylaxis for younger
high-risk diffuse large B-cell/follicular grade 3 lymphoma
patients: results of a phase II Nordic lymphoma group study. Ann
Oncol. 24:1385–1392. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Siegal T and Goldschmidt N: CNS
prophylaxis in diffuse large B-cell lymphoma: If, when, how and for
whom? Blood Rev. 26:97–106. 2012.PubMed/NCBI View Article : Google Scholar
|