Introduction
Parkinson’s disease (PD) is a degenerative disease
of the nervous system that occurs during middle age. Previous
studies showed that genetic factors are important in the etiology
of PD (1,2), with LRRK2 described as a significant
susceptibility gene. Approximately 20 mutations in the LRRK2 gene
have been confirmed to be associated with PD, with the mutations
having significant regional and ethnic variations. Few studies have
been performed on G2385R sites, which are considered to be specific
genetic risk factors for the East Asian population (3–6).
Xinjiang Uyghur individuals are known to have a
different genetic background compared with Xinjiang Han Chinese. A
study on the LRRK2 gene polymorphism in the Xinjiang region,
located in Central Asia, is the first to be performed concerning
the LRRK2 gene polymorphism of PD patients of various ethnicities
and regional backgrounds. Xinjian Uyghur and Han Chinese
individuals with PD and healthy controls were enrolled in the
current study. The correlation between the LRRK2 gene polymorphism
G2385R and PD in Uyghur and Han Chinese individuals was
examined.
Subjects and methods
Subjects
This study was conducted in accordance with the
declaration of Helsinki and with approval from the Ethics Committee
of the First Affiliated Hospital of Xinjiang Medical University.
Written informed consent was obtained from all participants.
PD patients in the patient group were confirmed
based on epidemiological survey (sporadic), whereas the healthy
individuals without PD in the control group were selected from the
survey population who were of identical age, gender, ethnicity and
background as PD patients, but not genetically related to the
patient group. The PD patients were screened using the diagnostic
criteria by BrainBank (United Kingdom) (7). The patients were examined by
specialists from the Neurological Department of the First
Affiliated Hospital of Xinjiang Medical University, China, in cases
of difficult diagnosis. When necessary, diagnosis was confirmed
using a head MRI or CT scan. Patients who were 50 years old were
divided into early- and late-onset PD groups. Secondary PD,
Parkinson’s syndrome, hyperthyroidism and other genetic or neural
diseases were excluded.
General data
There were 354 cases in the PD group, comprising 171
Uyghur and 183 Han individuals. For the Uyghur individuals, the
male:female ratio was 97:74 and their ages ranged from 31 to 95
(mean, 62.1±12.3) years. For the Han individuals, the male:female
ratio was 105:78 and their ages ranged from 25 to 85 (mean,
61.9±11.5) years. There were 340 cases in the control group,
comprising 160 Uyghur and 180 Han individuals. The Uyghur
male:female ratio was 90:70 and their ages ranged from 33 to 90
(mean, 61.1±11.4) years. The Han male:female ratio was 100:80 and
their ages ranged from 27 to 86 (mean, 60.9±11.4) years. There was
no significant difference in gender and age between the PD and
control groups (χ2 gender, 0.098; P>0.05; t-test for
age = 1.104; P>0.05).
DNA extraction
The patients and normal controls provided informed
consent prior to genomic DNA extraction from 2 ml of peripheral
venous blood using the conventional phenol/chloroform method. DNA
purity was 1.7–1.9 at ≥10 ng/μl and was stored at −20°C.
Primer design
According to the DNA sequence of exon 48 in the
G2385R of the LRRK2 gene from the National Center for Biotechnology
Information (NCBI) and ensemble, primers for G2385R were designed.
The upstream primer was 5′-TAGCCCTGTTGTGGAAGTG-3′ and the
downstream primer was 5′-TTCAGAGGCAGAAAGGAAG-3′. The length of the
amplified fragment was 170 bp. The primers were synthesized by the
Beijing Huata Company (Beijing, China).
Polymerase chain reaction (PCR)
amplification and identification
The total PCR volume was 25 μl, including 0.5 μl of
100 ng/μl upstream and downstream primers, 10 μl MIX, 3.0 μl of 50
ng/μl gDNA and 11 μl ddH2O. The PCR was performed on the
PE9600 thermal cycler (P.E. USA Inc., Cincinnati, OH, USA). The
reactions were incubated at 94°C for 2 min, followed by 35 cycles
of 94°C for 30 sec, annealing at 55.5°C for 30 sec and 72°C for 45
sec, then extended to 72°C for 5 min and preserved at 4°C.
Subsequently, 7 μl of the mixture was combined with loading buffer
and the sample was analyzed in 2% agarose gel. Subsequent to
staining, the gel was observed under a UV instrument and images
were captured. The amplified fragment length was 170 bp.
Restriction fragment length polymorphism
(RLFP)
The enzyme digestion reaction was performed using a
20-μl reaction system containing 10 μl PCR product, 2 μl 10×
buffer, 5 units AccI enzyme and 7.5 μl ddH2O. The
reaction system was incubated at 37°C and was digested overnight
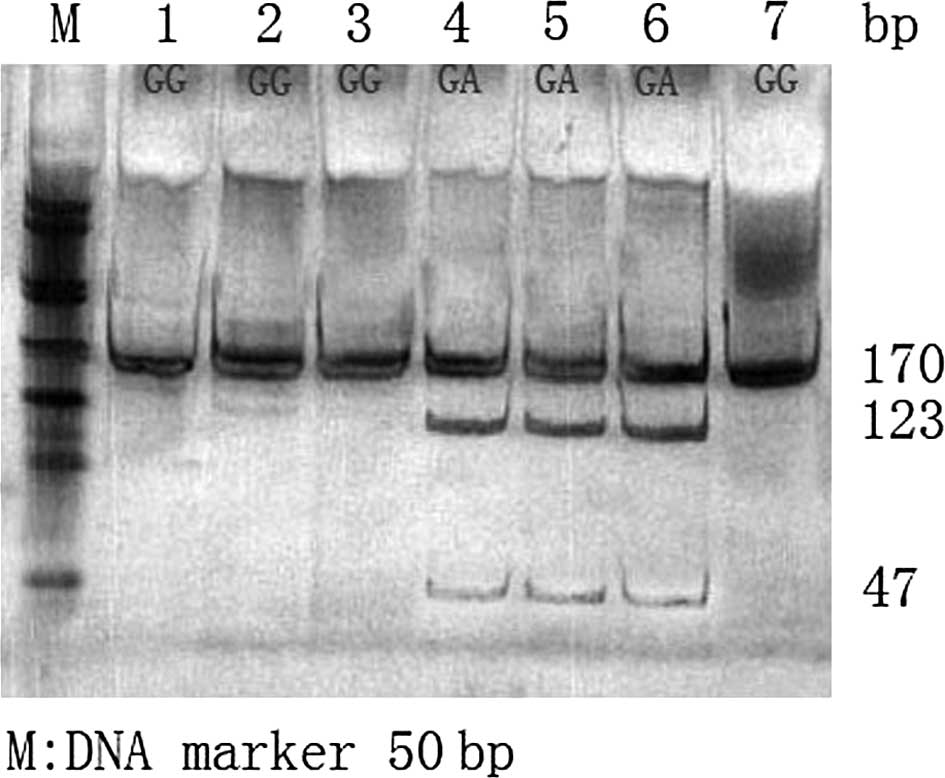
(16–24 h). The digestion products of the G2385R genotypes were
confirmed using 6% neutral polyacrylamide gel with D50 Marker as
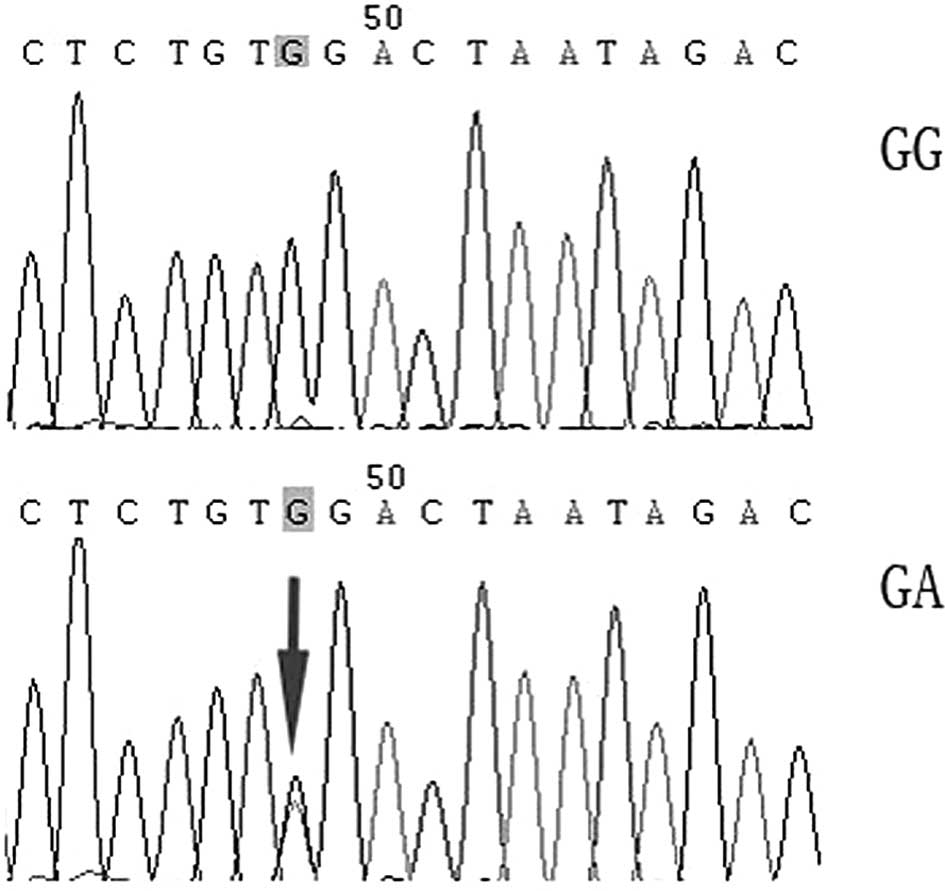
the standard, certain samples were confirmed through DNA
sequencing. Only 170 bp was identified in the GG homozygote
(wild-type, no restriction site), three fragments (170, 123 and 47
bp) were identified in the heterozygous GA-type and two fragments
(123 and 47 bp) were identified in the AA homozygotes.
Statistical analysis
SPSS 17.0 software was used for data analysis. The
frequencies of the genotypes and alleles were analyzed using the
gene counting method. Rates (%) were used to represent the counting
data. The allelic and genotypic frequencies of the two groups were
analyzed using a χ2 test. P<0.05 was regarded as
statistically significant.
Results
Goodness-of-fit test for Hardy-Weinberg
equilibrium
The distribution of the two genotypic polymorphisms
in the case and control groups were consistent with the
Hardy-Weinberg genetic equilibrium and results of the
goodness-of-fit test were regarded as excellent (PD group,
χ2=0.320, P>0.05; control group, χ2=0.036,
P>0.05).
Results of the LRRK2 gene G2385R polymorphism assay
showed the frequency of the GG genotype to be the highest, whereas
the frequency of the GA-type heterozygote was the lowest. No AA
genotype was identified (Figs. 1
and 2).
Comparison of the G2385R genotypic and
allelic frequency distribution
The comparison of the G2385R polymorphism genotypic
and allelic frequencies between the PD and control groups are shown
in Table I. The GA genotypic and A
allelic frequencies in the PD group were significantly higher than
those in the control group (χ2=6.720, P=0.01 and
χ2=6.582, P=0.01). The risk of occurrence of PD was
higher for individuals carrying the A allele than those without the
A allele (OR, 2.94; 95% CI, 1.29–6.69).
 | Table IComparison of the G2385R polymorphism
allele and genotype frequency between the PD and control
groups. |
Table I
Comparison of the G2385R polymorphism
allele and genotype frequency between the PD and control
groups.
| | Genotype frequency, n
(%) | Allele frequency, n
(%) |
|---|
| |
|
|
|---|
| Group | Cases (n) | GG | GA | AA | G | A |
|---|
| PD | 354 | 333 (94.1) | 21 (5.9) | 0 (0) | 687 (97) | 21 (3) |
| Control | 340 | 333 (97.9) | 7 (2.1) | 0 (0) | 673 (99) | 7 (1) |
The comparison of the G2385R genotypic and allelic
frequencies between the Uyghur and Han PD and control groups are
shown in Table II. The GA
genotypic and A allelic frequencies in the Han PD group were
significantly higher than those in the Uyghur PD group
(χ2=16.95, P=0.000 and χ2=16.432, P=0.000).
The risk of PD occurrence was higher among the Han Chinese
individuals carrying the A allele than for Uyghur individuals (OR,
19.71; 95% CI, 4.66–83.43). The GA genotypic and allelic
frequencies in the Han PD group were significantly higher than
those in the Han control group (χ2=7.873, P=0.005 and
χ2=7.581, P=0.006). The risk of PD occurrence among the
Han Chinese individuals carrying the A allele was significantly
higher than that in the Han Chinese without the A allele (OR, 3.41;
95% CI, 1.42–8.19). The difference in genotypic and allelic
frequencies was not statistically significant between the Uyghur PD
and control groups (χ2=0.002, P=0.962).
 | Table IIComparison of the G2385R polymorphism
allele and genotype frequency between the Uyghur and Han
ethnicities. |
Table II
Comparison of the G2385R polymorphism
allele and genotype frequency between the Uyghur and Han
ethnicities.
| | Genotype frequency, n
(%) | Allele frequency, n
(%) |
|---|
| |
|
|
|---|
| Groups | Cases (n) | GG | GA | AA | G | A |
|---|
| Uyghur PD | 171 | 170 (99.4) | 1 (0.6) | 0 (0) | 341 (99.7) | 1 (0.3) |
| Uyghur control | 160 | 159 (99.4) | 1 (0.6) | 0 (0) | 319 (99.7) | 1 (0.3) |
| Han PD | 183 | 163 (89.1) | 20 (10.9) | 0 (0) | 346 (94.5) | 20 (5.5) |
| Han control | 180 | 174 (96.7) | 6 (3.3) | 0 (0) | 354 (98.3) | 6 (1.7) |
A comparison was conducted of the G2385R genotypic
and allelic frequency distributions between the PD patients and the
controls with various (Table
III). The GA genotypic and A allelic frequencies were higher in
the late-onset PD group than in the control group (>50 years of
age) and the difference was statistically significant
(χ2=4.437, P=0.035 and χ2=4.436, P=0.037).
The risk of PD occurrence was significantly higher among
individuals carrying the A allele than in individuals without the A
allele (OR, 2.64; 95% CI, 1.07–6.50). No statistically significant
difference was detected in the genotypic and allelic frequencies
between the early-onset PD and the control group >50 years old
(χ2=2.456, P=0.117 and χ2=2.405,
P=0.121).
 | Table IIIComparison of G2385R polymorphism
allele and genotype frequency in the PD and control groups with
different age. |
Table III
Comparison of G2385R polymorphism
allele and genotype frequency in the PD and control groups with
different age.
| | Genotype frequency, n
(%) | Allele frequency, n
(%) |
|---|
| |
|
|
|---|
| Groups | Cases (n) | GG | GA | AA | G | A |
|---|
| Early-onset (≤50
years of age) |
| PD | 77 | 72 (93.5) | 5 (6.5) | 0 (0) | 149 (96.8) | 5 (3.2) |
| Control | 71 | 70 (98.6) | 1 (1.4) | 0 (0) | 141 (99.3) | 1 (0.7) |
| Late-onset (>50
years of age) |
| PD | 277 | 261 (94.2) | 16 (5.8) | 0 (0) | 538 (97.1) | 16 (2.9) |
| Control | 269 | 263 (97.8) | 6 (2.2) | 0 (0) | 532 (98.9) | 6 (1.1) |
A comparison was conducted of the G2385R genotypic
and allelic frequency distributions in the PD patient and control
groups with different gender (Table
IV). No statistically significant differences were found in the
genotypic and allelic frequencies between the male PD and the male
control groups (χ2=3.471, P=0.062 and
χ2=3.413, P=0.065) and the female PD and the female
control group (χ2=3.341, P=0.068 and
χ2=3.256, P=0.071).
 | Table IVComparison of G2385R polymorphism
allele and genotype frequency in PD group and control group with
different gender. |
Table IV
Comparison of G2385R polymorphism
allele and genotype frequency in PD group and control group with
different gender.
| | Genotype frequency, n
(%) | Allele frequency, n
(%) |
|---|
| |
|
|
|---|
| Groups | Cases (n) | GG | GA | AA | G | A |
|---|
| Male PD | 202 | 192 (95) | 10 (5) | 0 (0) | 394 (97.5) | 10 (2.5) |
| Male control | 190 | 187 (98.4) | 3 (1.6) | 0 (0) | 377 (99.2) | 3 (0.8) |
| Female PD | 152 | 141 (92.8) | 11 (7.2) | 0 (0) | 293 (96.4) | 11 (3.6) |
| Female control | 150 | 146 (97.3) | 4 (2.7) | 0 (0) | 296 (98.7) | 4 (1.3) |
Discussion
Among the neurodegenerative diseases, the incidence
of PD ranks second to Alzheimer’s disease. Genetic factors are
significant etiological factors. The LRRK2 gene, also known as
PARK8, causes disease and possesses the highest frequency of gene
mutation in autosomal dominant PD (8). It is also the disease-causing gene
most common in late-onset PD.
The LRRK2 gene mutation has significant geographic
and ethnic variations. G2019S and R1441C are common among Caucasian
populations in Europe and North Africa (9–13).
By contrast, this mutation is extremely rare in Asia, accounting
for only 0.1% of LRRK2 mutations. In previous studies, no G2019S,
R1441G or R1441C mutations were identified in a Chinese population
(14,15). Mata et al first reported
that G2385R is a Chinese-specific PD risk factor (16), making the study of this mutation
popular. The mutation frequency of G2385R in Malay PD patients was
2.0%, but the mutation was not correlated with the incidence of PD
(17). This type of polymorphism
is not detected in other ethnicities, including Southern Asian and
Caucasian populations (18–21).
This point mutation is common in the sporadic PD patients in East
Asian countries and regions, with a frequency of 10% in mainland
China, 7.27% in Singapore, 11.6% in Japan and 8% in Taiwan
(22). A study from mainland China
revealed that the mutation frequencies in the G2385R PD populations
from Shanghai and Sichuan were at 5.96 and 11.8%, respectively. The
mutation had a marked correlation with the incidence of PD
(4,22). A previous study performed by Ross
et al also confirmed that the mutation of G2385R increased
the PD risk of East Asian ethnicities (1.73, 1.20–2.49; P=0.0026)
(6).
The current study revealed that the mutation rate of
the G2385R polymorphism is extremely low among the Uyghur
population in the Xinjiang region. The mutation frequency of the GA
genotype was 0.58 (1/171) and 0.62% (1/160) in the Uyghur PD and
the Uyghur control groups, respectively. No statistically
significant differences were detected in the genotypic and allelic
frequencies between these two groups, suggesting that the G2385R
polymorphism does not correlation with PD in the Xinjiang Uyghur
population. Previous studies have confirmed that the G2385R
polymorphism is a common mutation among the Chinese PD population
and is, therefore, a specific genetic risk factor in East Asian
populations (17,23,24).
Uyghur and Han individuals in the Xinjiang region in Central Asia
have various genetic, geographic and ethinic backgrounds, leading
to varying results. The GA mutation frequencies were 10.9 (20/183)
and 3.3% (6/180) in the Han PD and Han control groups,
respectively. Statistically significant differences were found in
the genotypic and allelic frequencies between these two groups. The
risk of PD was higher among individuals carrying the A allele than
those without the A allele, suggesting that the G2385R polymorphism
is correlated with the occurrence of PD among the Han population in
the Xinjiang region. In the age subgrouping, statistically
significant differences were detected in the genotypic and allelic
frequencies between the late-onset PD group (>50 years old) and
the control group. The risk of PD was higher among individuals
carrying the A allele than those without the A allele, suggesting
that the G2385R polymorphism is correlated with late-onset PD. This
is consistent with reports that the LRRK2 gene is a common
disease-causing gene in late-onset PD. In the gender subgrouping,
there were no significant differences in the G2385R genotypic and
allelic frequencies between the male PD and control groups and the
female PD and control groups, respectively. The results contrast
with those reported by Li et al(22) where the frequency of the G2385R
mutation among female patients was significantly higher than that
among male patients. The varying results are caused by sampling
errors, inadequate sample size, regional differences and other
environmental and lifestyle differences.
In conclusion, the G2385R polymorphism is correlated
with PD among the Han population in Xinjiang, particularly among
those individuals >50 years old. However, the polymorphism is
not correlated with the incidence of PD among the Uyghur
population. The LRRK2 gene mutation has geographic and ethnic
variations. Expanding the sample size in other populations and
ethnic groups is necessary for further studies.
Acknowledgements
This study was supported by the Natural Science
Foundation of the Xinjiang Uyghur Autonomous Region (2009211A17)
and National Natural Science Foundation of China (81160143).
References
|
1
|
Gosal D, Ross OA and Toft M: Parkinson’s
disease: the genetics of a heterogeneous disorder. Eur J Neurol.
13:616–627. 2006.
|
|
2
|
Pankratz N and Foroud T: Genetics of
Parkinson disease. Genet Med. 9:801–811. 2007. View Article : Google Scholar
|
|
3
|
Di Fonzo A, Wu-Chou YH, Lu CS, et al: A
common missense variant in the LRRK2 gene, Gly2385Arg, associated
with Parkinson’s disease risk in Taiwan. Neurogenetics. 7:133–138.
2006.
|
|
4
|
An XK, Peng R, Li T, et al: LRRK2
Gly2385Arg variant is a risk factor of Parkinson’s disease among
Han Chinese from mainland China. Eur J Neurol. 15:301–305.
2008.
|
|
5
|
Tan EK, Zhao Y, Skipper L, et al: The
LRRK2 Gly2385Arg variant is associated with Parkinson’s disease:
genetic and functional evidence. Hum Genet. 120:857–863. 2007.
|
|
6
|
Ross OA, Soto-Ortolaza AI, Heckman MG, et
al: Genetic Epidemiology Of Parkinson’s Disease (GEO-PD)
Consortium: Association of LRRK2 exonic variants with
susceptibility to Parkinson’s disease: a case-control study. Lancet
Neurol. 10:898–908. 2011.
|
|
7
|
Hughes AJ, Daniel SE, Kilford L and Lees
AJ: Accuracy of clinical diagnosis of idiopathic Parkinson’s
disease: a clinico-pathological study of 100 cases. J Neurol
Neurosurg Psychiatry. 55:181–184. 1992.
|
|
8
|
Lesage S, Dürr A and Brice A: LRRK2: a
link between familial and sporadic Parkinson’s disease. Pathol Biol
(Paris). 55:107–110. 2007.
|
|
9
|
Deng H, Le WD, Guo Y, et al: Genetic and
clinical identification of Parkinson’s disease patients with LRRK2
G2019S mutation. Ann Neurol. 57:933–934. 2005.
|
|
10
|
Lesage S, Dürr A, Tazir M, et al; French
Parkinson’s Disease Genetics Study Group. LRRK2 G2019S as a cause
of Parkinson’s disease in North African Arabs. N Engl J Med.
354:422–423. 2006.
|
|
11
|
Ozelius LJ, Senthil G, Saunders-Pullman R,
et al: LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi
Jews. N Engl J Med. 354:424–425. 2006.
|
|
12
|
Paisán-Ruíz C, Jain S, Evans EW, et al:
Cloning of the gene containing mutations that cause PARK8-linked
Parkinson’s disease. Neuron. 44:595–600. 2004.PubMed/NCBI
|
|
13
|
Simón-Sánchez J, Martí-Massó JF,
Sánchez-Mut JV, et al: Parkinson’s disease due to the R1441G
mutation in Dardarin: a founder effect in the Basques. Mov Disord.
21:1954–1959. 2006.
|
|
14
|
Patra B, Parsian AJ, Racette BA, et al:
LRRK2 gene G2019S mutation and SNPs [haplotypes] in subtypes of
Parkinson’s disease. Parkinsonism Relat Disord. 15:175–180.
2009.
|
|
15
|
Cookson MR and Bandmann O: Parkinson’s
disease: insights from pathways. Hum Mol Genet. 19:R21–R27.
2010.
|
|
16
|
Mata IF, Kachergus JM, Taylor JP, et al:
Lrrk2 pathogenic substitutions in Parkinson’s disease.
Neurogenetics. 6:171–177. 2005.
|
|
17
|
Tan EK, Zhao Y, Tan L, et al: Analysis of
LRRK2 Gly2385Arg genetic variant in non-Chinese Asians. Mov Disord.
22:1816–1818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haubenberger D, Bonelli S, Hotzy C, et al:
A novel LRRK2 mutation in an Austrian cohort of patients with
Parkinson’s disease. Mov Disord. 22:1640–1643. 2007.PubMed/NCBI
|
|
19
|
Toft M, Haugarvoll K, Ross OA, et al:
LRRK2 and Parkinson’s disease in Norway. Acta Neurol Scand Suppl.
187:72–75. 2007.
|
|
20
|
Biskup S, Mueller JC, Sharma M, et al:
Common variants of LRRK2 are not associated with sporadic
Parkinson’s disease. Ann Neurol. 58:905–908. 2005.
|
|
21
|
Paisán-Ruíz C, Lang AE, Kawarai T, et al:
LRRK2 gene in Parkinson disease: mutation analysis and case control
association study. Neurology. 65:696–700. 2005.PubMed/NCBI
|
|
22
|
Li C, Ting Z, Qin X, et al: The prevalence
of LRRK2 Gly2385Arg variant in Chinese Han population with
Parkinson’s disease. Mov Disord. 22:2439–2443. 2007.
|
|
23
|
Farrer MJ, Stone JT, Lin CH, et al: Lrrk2
G2385R is an ancestral risk factor for Pakinson’s disease in Asia.
Parkinsonism Relat Disord. 13:89–92. 2007.PubMed/NCBI
|
|
24
|
Funayama M, Li Y, Tomiyama H, et al:
Leucine-rich repeat kinase 2 G2385R variant is a risk factor for
Parkinson disease in Asian population. Neuroreport. 18:273–275.
2007. View Article : Google Scholar : PubMed/NCBI
|