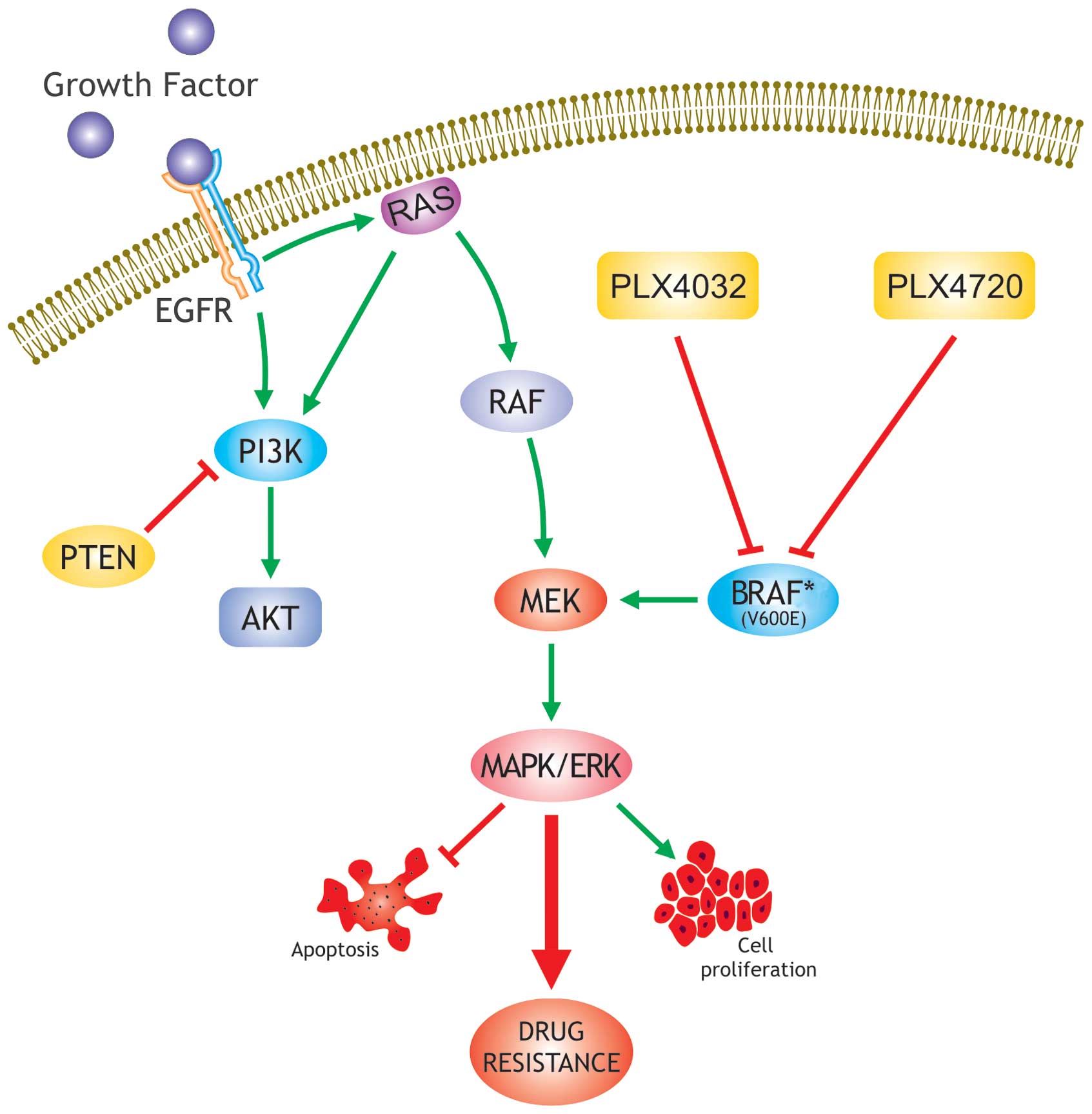
Papillary thyroid carcinoma (PTC) is the most common
histotype among thyroid tissue malignancies, accounting for
approximately 80% of all thyroid cancers (1). The growing incidence of thyroid
cancer is almost entirely ascribed to PTC (2,3). The
epidemiology of PTC has evoked much interest. Dietary iodine
deficiency appears to influence the incidence of the disease, and
in some cases, the morphology of the papillary carcinomas (4). However, as regards the etiology of
PTC, a number of studies have revealed an association between its
incidence and development with radiation exposure (5–7).
Differentiated thyroid cancer, including PTC, tends
to be biologically indolent, highly curable and has an excellent
prognosis. A number of clinicopathological features are considered
as high-risk factors in PTC (8,9).
These include: old patient age at the time of diagnosis, male
gender, large tumor size, extrathyroidal invasion, lymph node
metastasis, distant metastasis, and advanced disease stages
(8,10–12).
All of these clinical characteristics are associated with poor
prognosis as they reflect an aggressive tumor behavior with an
increased rate of progression and recurrence. Finally, the
histological criteria are important to stratify the risk of disease
aggressiveness for the patients (13–16).
All the cited elements are useful for a more appropriate
therapeutic approach of PTC and subsequent follow-up. It has been
shown that the accuracy of this clinicopathological criteria-based
method can be unreliable for patients with conventionally low
clinicopathological stages (11,17).
Although PTC is highly curable with standard surgical treatment and
radioiodine ablation therapy, a significant recurrence rate
(approximately 20% at 10 years and 30% at 30 years of follow-up) is
observed after treatment and many patients still succumb to the
disease. Additionally, there are no therapeutic options for those
patients who develop radioiodine resistance and are not eligible
for surgery. Risk stratification is the chief consideration in
determining the appropriate management of PTC-affected patients.
Therefore, it is important to improve the reliability of this
system in order to reduce the recurrence rate as well as the
mortality of PTC.
Thyroid cancer carries several highly prevalent
genetic alterations, some of which are observed only in this type
of cancer. These oncogenic alterations include: Ras mutations
(19,20), RET/PTC rearrangements (21,22)
and PAX8-peroxisome proliferator-activated receptor γ (PPARγ)
fusion oncogene (23,24). In PTC tumors, RET/PTC rearrangement
and point mutations of the BRAF and RAS genes are found in over 70%
of papillary carcinomas and they rarely overlap in the same tumor
(25).
The BRAF gene is highly mutated in tumor cells and
over 40 different mutations have been identified. The BRAF V600E
mutation is the most common and accounts for more than 90% of all
the mutations found in the BRAF gene (26). Moreover, this mutation has been
found to occur frequently in thyroid cancer (27–32).
Intriguingly, the BRAF mutation in thyroid cancer occurs
exclusively in PTC and PTC-derived anaplastic thyroid carcinoma
(ATC) and it does not occur in follicular thyroid carcinoma (FTC)
or other types of thyroid tumors (33). As highlighted in the study by Xing,
the prevalence and specificity of the BRAF mutation in PTC cells
may be associated with a pathogenic role of this mutation in this
tumor. The fine-needle aspiration is a pre-operative approach
useful for diagnosis that should be completed by screening for the
BRAF V600E mutation. Targeted therapy may prove to be advantageous
for PTC patients harboring this molecular aberration (33).
In the present review, the role of BRAF mutations in
the development and progression of thyroid cancer and their
implications for novel therapeutic strategies are summarized.
The ‘MAPK pathway’ [the RET/PTC → Ras → Raf →
mitogen extracellular kinase (MEK) → MAPK/ERK pathway] is a
fundamental intracellular signaling pathway that plays a central
role in cellular functions, such as proliferation, differentiation,
apoptosis and survival (34–38).
The aberrant activation of the MAPK pathway, through the activation
of genetic alterations of its elements, has been observed in many
human cancers (39–43) (Fig.
1). The B-type Raf kinase (BRAF) mutation was discovered to be
a leading cause of aberrant activation of the MAPK pathway in human
cancers (44). There are three Raf
kinases, A-Raf, BRAF (BRAF) and C-Raf. Among the three, BRAF is the
most potent activator of the MAPK pathway in many cells (37).
The wild-type BRAF gene is localized on chromosome 7
and is composed of 119 base pairs (bp). Most of BRAF mutations are
clustered within exons 11 and 15 (44). The T1799A mutation results in a
V600E amino acid substitution in the protein product and subsequent
constitutive activation of the BRAF kinase and ERK phosphorylation
(44–46). Accordingly, dysregulated signaling
through the activation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR
pathways is often the result of genetic alterations in critical
components in these pathways, as well as mutations in upstream
growth factor receptors (47).
Another BRAF mutation detected in thyroid tumors is the K601E
mutation which has been observed in two benign thyroid adenomas
(31,48) and three follicular-variant PTCs
(49). The mutations in exon 11 of
the BRAF gene found in other human cancers have not been found in
thyroid cancer (27–30,50–52).
The in vivo fusion of the BRAF gene with the AKAP9 gene
through a paracentric inversion of the long arm of chromosome 7 may
cause BRAF activation. This aberration has been observed in PTC
patients with a history of radiation exposure (53,54).
Strong molecular bases have now been revealed for
BRAF mutation-promoted invasiveness and the progression of PTC. It
has been shown that transgenic mice develop PTC after injection of
the BRAF V600E protein in the thyroid gland (55). Previous studies have shown that
BRAF V600E promotes the invasion of thyroid cells in a rat cell
line model (56). Ouyang et
al demonstrated that BRAF inhibitors can inhibit the growth and
proliferation of cells harboring the BRAF V600E mutation (57). As previously shown, siRNA
transfection of PTC cells harboring the BRAF mutation resulted in
the persistent suppression of BRAF, sustained the inhibition of
cell proliferation, prevented transformation even after long-term
culture and inhibited xenograft tumor growth in nude mice (58,59).
As already mentioned above, the activating genetic
alterations in the MAPK pathway, including RET/PTC rearrangement,
Ras mutation and BRAF mutation, are mutually exclusive in PTC,
suggesting that each of these genetic alterations in PTC may be
sufficient on their own to drive PTC tumorigenesis (25). Conversely, as shown in a previous
study, the expression of RET/PTC did not cause genetic instability
that was observed in rat thyroid cells after induction of BRAF
V600E (60). Mesa et al
showed that in rat thyroid cell lines, an increased Matrigel cell
invasion was observed with the induced expression of BRAF V600E,
but not with RET/PTC rearrangement (56).
Microarray gene expression analyses have displayed
different gene expression patterns in cells harboring the BRAF
V600E mutation or RET/PTC rearrangement, suggesting a difference in
the genes affected by the two genetic alterations and indicating
that the BRAF mutant is possibly a stronger activator of the MAPK
pathway than RET/PTC (56,61,62).
It has been also demonstrated in PTC that the BRAF
mutation is linked with the aberrant methylation of different tumor
suppressor genes, including the genes, tissue inhibitor of matrix
metalloproteinase-3 (TIMP3), death-associated protein kinase
(DAPK), SLC5A8 and retinoic acid receptor β2 (RARβ2) (63–66).
It is known that TIMP3 suppresses tumor growth, angiogenesis,
invasion and metastasis by preventing the interstitial matrix
destruction promoted by matrix metalloproteinase (MMP)-3 (67) and by blocking the binding of
vascular endothelial growth factor (VEGF) to the VEGF receptor
(68). The BRAF mutation has been
reported to be associated with alterations in the expression of
various micro-RNAs that appear to have tumor-suppressor potential
in PTC (69–71). The BRAF V600E mutation in thyroid
cell lines is also involved in the upregulation of tumor-promoting
molecules, such as MMPs (56,61,72).
In the study by Palona et al, the authors also showed that
BRAF V600E promoted the activation of the nuclear transcription
factor κB (NF-κB)-coupled signaling, which in turn promoted the
Matrigel invasion of thyroid cancer cells (72). Intriguingly, BRAF V600E promoted
the activation of NF-κB through signaling directly from BRAF,
independently of the downstream MEK/MAPK/ERK signaling pathway
(72–74). Moreover, it has been demonstrated
that the overexpression of VEGF is associated with BRAF mutation in
PTC (75). Recently, Grabellus
et al showed that glucose transporter 1 (GLUT1) is a target
of the constitutive activation of the RAF/MEK/ERK pathway,
hypothesizing that the BRAF mutation in PTC may contribute to the
initiation of the glycolytic phenotype and may confer growth
advantages in cancer cells (76).
Taken together, all these elements suggest the
critical role played by the BRAF mutation in promoting
extrathyroidal invasion and metastasis (which involves vigorous
angiogenesis and tissue invasion) and the mutation-mediated
progression and aggressiveness of PTC.
The efficacy of radioiodine ablation therapy for the
treatment of thyroid cancer depends on the ability of the cancer
cells to absorb and accumulate radioiodine, which in turn relies on
the integrity of the iodide-metabolizing system of the thyroid cell
(77). The expression of the
thyroid iodide-metabolizing genes is often impaired or lost in
thyroid cancer (78,79). Intriguingly, the BRAF mutation has
ben found to be associated with the decreased expression of
thyroperoxidase (62,80–82),
sodium/iodide symporter (80,83),
thyroglobulin (80) and pendrin
(81) in primary or recurrent PTC
tumors. The conditional expression of BRAF V600E in rat thyroid
cell lines has led to the silencing of all these thyroid-specific
iodide-metabolizing genes (60,83,84).
The MAPK inhibitors or BRAF siRNA could re-establish the expression
of these genes (83,84). Several studies have demonstrated
that the thyroid-stimulating hormone (TSH) receptor gene is
silenced in a promoter methylation-dependent manner in rat thyroid
cell lines (85,86), as well as human thyroid cancer
cells (87,88). Xing suggested that the silencing of
thyroid-specific genes associated with the BRAF mutation supports
the notion that the BRAF mutation promotes the progression and
aggressiveness of PTC (89).
A positive correlation between the BRAF V600E
mutation with the clinicopathological characteristics of PTC,
including extrathyroidal invasion, lymph node metastasis and
advanced stages has been identified in a number of studies
(83,90,93–97,99,100,102,103,105,107,109–111,113,115,117). Accordingly, the metanalysis
performed by Xing in 2007 confirmed such an association, displaying
overall odds ratios of 2.50 [95% confidence interval (CI)
2.11–2.97], 1.83 (95% CI 1.58–2.13) and 2.14 (95% CI 1.79–2.56)
(89). BRAF mutations have also
been observed in 77, 60 and 12% of tall cell PTCs, conventional
PTCs and follicular variant PTCs, respectively. These data support
the notion that the V600E mutation detected in BRAF is associated
with the aggressive variants of PTC (33,110). Furthermore, it has previously
been suggested that the BRAF mutation renders PTC prone to progress
into the more aggressive ATC (105,118–120).
As considered above, the association between BRAF
mutation and the conventional high-risk clinicopathological factors
in PTCs is supported by several studies; however, other studies
have failed to reveal such an association (54,75,80,90,
92,98,101,104,106, 108,112,114,115,121).
Conflicting data have also been generated regarding
the correlation between BRAF mutations, old age, male gender,
thyroid cancer progression and aggressiveness (32,91,92,95,96,
100,105,107,109,111,114,115,117,122). This controversy may be supported
by different hypotheses that have been properly considered by Xing
in 2007, such as different diagnostic criteria and the small number
of cases analyzed in some published studies (89).
The association between BRAF mutation and PTC
recurrence has been explored in several studies. Kim et al
demonstrated a close association between the BRAF mutation and
tumor recurrence in 203 patients with conventional PTC (99). A strong association between the
BRAF mutation and the recurrence of PTC has also been confirmed by
Riesco-Eizaguirre et al (83). In a large study by Xing et
al, an odds ratio of 4.0 (95% CI 1.1–14.1; P=0.03) for cancer
recurrence with the BRAF mutation was obtained by multivariate
analysis with adjustment for all the confounding
clinicopathological factors, including tumor subtypes and a history
of radioiodine treatment. Interestingly, such an association was
even established in a subgroup of patients with low-grade initial
clinicopathological stages I and II, usually linked with a low risk
of recurrence (110). Despite
this evidence however, in 2011, Cañadas Garre et al found no
association between BRAF mutation and the recurrence of PTC
(114).
The core of the current medical treatment of PTC
following thyroidectomy is radioiodine ablation therapy. The
medical treatment of recurrent PTC is also largely confined to
radioiodine therapy (8,9,123).
Although various clinicopathological factors are known to predict
an increased risk of recurrence of thyroid cancer, no factors have
been associated with the loss of radioiodine avidity, mainly
responsible for treatment failure. On this regard, Xing et
al showed that patients harboring BRAF mutations in the primary
PTC tumor developed a recurrence with an aggressive course of
disease, to be treated with surgery and external radiation therapy.
However, PTC patients with recurrent tumors that did not display
any BRAF mutation in the primary tumor may be treated with
radioiodine therapy, suggesting a better clinical behavior
(110). The association between
BRAF mutation in primary or recurrent PTC and the loss of
radioiodine avidity in the recurrent tumor, was observed in two
studies; however, such an association did not reach statistical
significance (81,83).
From both the clinicopathological and molecular
biological evaluations, it is evident that BRAF mutations are
fundamentally associated with the increased progression and
aggressiveness of PTC. Several studies have shown that BRAF
mutations may be considered as powerful markers for PTC recurrence
risk (83,95,99,110). BRAF mutations can be easily
detected in tumor DNA from thyroid fine-needle aspiration biopsy
(FNAB). Surgical and medical treatments can be better applied if
the BRAF mutation status is determined from thyroid FNAB (33,94,124–128).
It is evident that the MAPK pathway plays a major
role in tumorigenesis and the progression of PTC and may be
consequently considered as a potentially effective therapeutic
target for PTC.
In recent years, the inhibition of MAPK pathway has
been investigated in human cancers, generating interesting results
(reviewed in refs. 34–38,42).
The silencing of the MAPK pathway by Raf kinase inhibitors, such as
AAL-881 and LBT-613, has been shown to result in the inhibition of
BRAF-mutated thyroid cancer cell proliferation (57–59).
Moreover, Liu et al showed that through a stable siRNA
knockdown of BRAF, thyroid cells were able to differentiate as
reflected by the restoration of the expression of some
thyroid-specific genes (84).
The results from an advanced human thyroid cancer
preclinical study suggest that PLX4720, an orally available BRAF
V600E inhibitor, may be used in clinical trials for the treatment
of patients with BRAF V600E-positive thyroid cancers, refractory to
conventional therapy (129–131). MEK inhibitors are another class
of highly promising therapeutic agents for thyroid cancer. Liu
et al showed that CI-1040, a potent small molecule
MEK-selective inhibitor, which inhibits both MEK-1 and MEK-2,
inhibited the growth and proliferation of thyroid cancer cells
harboring mutant BRAF but not wild-type BRAF. The study also
demonstrated that the CI-1040 compound preferentially induced the
differentiation of some thyroid cancer cells that harbored the BRAF
mutation (132). Namba et
al showed that another MEK-inhibitor, U0126, inhibited cell
proliferation in thyroid cancer cells harboring the BRAF mutation
(30) and these findings were
confirmed in the study by Henderson et al (133). This chemically synthesized
compound, however, cannot be developed clinically due to its
limited solubility and bioavailability (134). More potent and pharmaceutically
superior second-generation MEK inhibitors, such as the PD-0325901
compound and the ARRY-142886 (AZD6244) compound (134,135), are currently under development
and are being used in clinical trials. Several clinical trials with
BRAF inhibitors and MEK inhibitors are currently under evaluation,
presenting a new perspective in PTC treatment. Therefore, it is
evident that the detection of the BRAF V600E mutation is crucial in
order to identify novel avenues for thyroid cancer treatment.
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006.
|
|
3
|
Leenhardt L, Grosclaude P and
Cherie-Challine L: Increased incidence of thyroid carcinoma in
France: a true epidemic or thyroid nodule management effects?
Report from the French Thyroid Cancer Committee. Thyroid.
14:1056–1060. 2004. View Article : Google Scholar
|
|
4
|
Williams ED, Abrosimov A, Bogdanova T,
Demidchik EP, Ito M, LiVolsi V, Lushnikov E, Rosai J, Tronko MD,
Tsyb AF, Vowler SL and Thomas GA: Morphologic characteristics of
Chernobyl-related childhood papillary thyroid carcinomas are
independent of radiation exposure but vary with iodine intake.
Thyroid. 18:847–852. 2008. View Article : Google Scholar
|
|
5
|
Hunt JL: Radiation induced thyroid
diseases. Pathol Case Rev. 14:224–230. 2009. View Article : Google Scholar
|
|
6
|
Nakachi K, Hayashi T, Hamatani K, Eguchi H
and Kusunoki Y: Sixty years of follow-up of Hiroshima and Nagasaki
survivors: cancer progress in molecular epidemiology studies. Mutat
Res. 659:109–117. 2008.PubMed/NCBI
|
|
7
|
Williams D: Radiation carcinogenesis:
lessons from Chernobyl. Oncogene. 27:9–18. 2009. View Article : Google Scholar
|
|
8
|
Sherman SI, Angelos P, Ball DW, Beenken
SW, Byrd D, Clark OH, Daniels GH, Dilawari RA, Ehya H, Farrar WB,
et al: Thyroid carcinoma. J Natl Compr Canc Netw. 3:404–457.
2005.PubMed/NCBI
|
|
9
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI and
Tuttle RM: Management guidelines for patients with thyroid nodules
and differentiated thyroid cancer. Thyroid. 16:109–142. 2006.
View Article : Google Scholar
|
|
10
|
Mazzaferri EL: Long-term outcome of
patients with differentiated thyroid carcinoma: effect of therapy.
Endocr Pract. 6:469–476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazzaferri EL and Kloos RT: Clinical
review 128: current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka K, Sonoo H, Hirono M, Ohkubo S,
Nomura T, Ikeda M, Nakajima K and Kurebayashi J: Retrospective
analysis of predictive factors for recurrence after curatively
resected papillary thyroid carcinoma. Surg Today. 35:714–719. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JK: Papillary carcinoma of thyroid:
classical and variants. Histol Histopathol. 5:241–257.
1990.PubMed/NCBI
|
|
14
|
Lang BH, Lo CY, Chan WF, Lam AK and Wan
KY: Classical and follicular variant of papillary thyroid
carcinoma: a comparative study on clinicopathologic features and
long-term outcome. World J Surg. 30:752–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keelawat S and Poumsuk U: Association
between different variants of papillary thyroid carcinoma and
risk-group according to AMES (age, metastasis, extent and size)
classification system. J Med Assoc Thai. 89:484–489. 2006.
|
|
16
|
Michels JJ, Jacques M, Henry-Amar M and
Bardet S: Prevalence and prognostic significance of tall cell
variant of papillary thyroid carcinoma. Hum Pathol. 38:212–219.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazzaferri EL: Managing small thyroid
cancers. JAMA. 295:2179–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fagin JA: Minireview: branded from the
start-distinct oncogenic initiating events may determine tumor fate
in the thyroid. Mol Endocrinol. 16:903–911. 2002.PubMed/NCBI
|
|
20
|
Bongarzone I and Pierotti MA: The
molecular basis of thyroid epithelial tumorigenesis. Tumori.
89:514–516. 2003.PubMed/NCBI
|
|
21
|
Nikiforov YE: RET/PTC rearrangement in
thyroid tumors. Endocrine Pathology. 13:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tallini G: Molecular pathobiology of
thyroid neoplasms. Endocr Pathol. 13:271–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kroll TG, Sarraf P, Pecciarini L, Chen CJ,
Mueller E, Spiegelman BM and Fletcher JA: PAX8- PPARgamma1 fusion
oncogene in human thyroid carcinoma. Science. 289:1357–1360. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McIver B, Grebe SK and Eberhardt NL: The
PAX8/PPARgamma fusion oncogene as a potential therapeutic target in
follicular thyroid carcinoma. Curr Drug Targets Immune Endocr
Metabol Disord. 4:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikiforova MN and Nikiforov YE: Molecular
genetics of thyroid cancer: implications for diagnosis, treatment
and prognosis. Expert Rev Mol Diagn. 8:83–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garnett MJ and Marais R: Guilty as
charged: B-RAF is a human oncogene. Cancer Cell. 6:313–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen Y, Xing M, Mambo E, Guo Z, Wu G,
Trink B, Beller U, Westra WH, Ladenson PW and Sidransky D: BRAF
mutation in papillary thyroid carcinoma. J Natl Cancer Inst.
95:625–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukushima T, Suzuki S, Mashiko M, Ohtake
T, Endo Y, Takebayashi Y, Sekikawa K, Hagiwara K and Takenoshita S:
BRAF mutations in papillary carcinomas of the thyroid. Oncogene.
22:6455–6457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA,
Nikiforov YE and Fagin JA: High prevalence of BRAF mutations in
thyroid cancer: genetic evidence for constitutive activation of the
RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma.
Cancer Res. 63:1454–1457. 2003.
|
|
30
|
Namba H, Nakashima M, Hayashi T, Hayashida
N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T and
Yamashita S: Clinical implication of hot spot BRAF mutation, V599E,
in papillary thyroid cancers. J Clin Endocrinol Metab.
88:4393–4397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soares P, Trovisco V, Rocha AS, Lima J,
Castro P, Preto A, Maximo V, Botelho T, Seruca R and
Sobrinho-Simões M: BRAF mutations and RET/PTC rearrangements are
alternative events in the etiopathogenesis of PTC. Oncogene.
22:4578–4580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu X, Quiros RM, Gattuso P, Ain KB and
Prinz RA: High prevalence of BRAF gene mutation in papillary
thyroid carcinomas and thyroid tumor cell lines. Cancer Res.
63:4561–4567. 2003.PubMed/NCBI
|
|
33
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pritchard C and McMahon M: Raf revealed in
life-or-death decisions. Nat Genet. 16:214–215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steelman LS, Abrams SL, Shelton JG,
Chappell WH, Bäsecke J, Stivala F, Donia M, Nicoletti F, Libra M,
Martelli AM and McCubrey JA: Dominant roles of the Raf/MEK/ERK
pathway in cell cycle progression, prevention of apoptosis and
sensitivity to chemotherapeutic drugs. Cell Cycle. 9:1629–1638.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011.PubMed/NCBI
|
|
38
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011.PubMed/NCBI
|
|
39
|
Hoshino R, Chatani Y, Yamori T, Tsuruo T,
Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J and Kohno
M: Constitutive activation of the 41-/43-kDa mitogen-activated
protein kinase signaling pathway in human tumors. Oncogene.
18:813–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciampi R and Nikiforov YE: RET/PTC
rearrangements and BRAF mutations in thyroid tumorigenesis.
Endocrinology. 148:936–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santoro M, Melillo RM and Fusco A: RET/PTC
activation in papillary thyroid carcinoma: European Journal of
Endocrinology Prize Lecture. Eur J Endocrinol. 155:645–653. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Russo AE, Torrisi E, Bevelacqua Y,
Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F and
Malaponte G: Melanoma: molecular pathogenesis and emerging target
therapies (Review). Int J Oncol. 34:1481–1489. 2009.PubMed/NCBI
|
|
43
|
Steelman LS, Navolanic PM, Franklin RA,
Bonati A, Libra M, Stivala F, Martelli AM and McCubrey JA:
Combining chemo-, hormonal and targeted therapies to treat breast
cancer (Review). Mol Med Report. 1:139–160. 2008.PubMed/NCBI
|
|
44
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dhillon AS and Kolch W: Oncogenic B-Raf
mutations: crystal clear at last. Cancer Cell. 5:303–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hubbard SR: Oncogenic mutations in B-Raf:
some losses yield gains. Cell. 116:764–766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McCubrey JA, Steelman LS, Kempf CR,
Chappell WH, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra
M, Bäsecke J, et al: Therapeutic resistance resulting from
mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways.
J Cell Physiol. 226:2762–2781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lima J, Trovisco V, Soares P, Máximo V,
Magalhães J, Salvatore G, Santoro M, Bogdanova T, Tronko M,
Abrosimov A, et al: BRAF mutations are not a major event in
post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol
Metab. 89:4267–4271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trovisco V, Vieira de Castro I, Soares P,
Maximo V, Silva P, Magalhaes J, Abrosimov A, Guiu XM and
Sobrinho-Simões M: BRAF mutations are associated with some
histological types of papillary thyroid carcinoma. J Pathol.
202:247–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Frattini M, Ferrario C, Bressan P,
Balestra D, De Cecco L, Mondellini P, Bongarzone I, Collini P,
Gariboldi M, Pilotti S, Pierotti MA and Greco A: Alternative
mutations of BRAF, RET and NTRK1 are associated with similar but
distinct gene expression patterns in papillary thyroid cancer.
Oncogene. 23:7436–7740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Perren A, Schmid S, Locher T, Saremaslani
P, Bonvin C, Heitz PU and Komminoth P: BRAF and endocrine tumors:
mutations are frequent in papillary thyroid carcinomas, rare in
endocrine tumors of the gastrointestinal tract and not detected in
other endocrine tumors. Endocr Relat Cancer. 11:855–860. 2004.
View Article : Google Scholar
|
|
52
|
Puxeddu E, Moretti S, Elisei R, Romei C,
Pascucci R, Martinelli M, Marino C, Avenia N, Rossi ED, Fadda G, et
al: BRAF(V599E) mutation is the leading genetic event in adult
sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab.
89:2414–2420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ciampi R, Knauf JA, Kerler R, Gandhi M,
Zhu Z, Nikiforova MN, Rabes HM, Fagin JA and Nikiforov YE:
Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway
activation in thyroid cancer. J Clin Invest. 115:94–101. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fusco A, Viglietto G and Santoro M: A new
mechanism of BRAF activation in human thyroid papillary carcinomas.
J Clin Invest. 115:20–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Knauf JA, Ma X, Smith EP, Zhang L,
Mitsutake N, Liao XH, Refetoff S, Nikiforov YE and Fagin JA:
Targeted expression of BRAFV600E in thyroid cells of transgenic
mice results in papillary thyroid cancers that undergo
dedifferentiation. Cancer Res. 65:4238–4245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mesa C Jr, Mirza M, Mitsutake N, Sartor M,
Medvedovic M, Tomlinson C, Knauf JA, Weber GF and Fagin JA:
Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells
is associated with gene expression profiles that predict a
preferential role of BRAF in extracellular matrix remodeling.
Cancer Res. 66:6521–6529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ouyang B, Knauf JA, Smith EP, Zhang L,
Ramsey T, Yusuff N, Batt D and Fagin JA: Inhibitors of Raf kinase
activity block growth of thyroid cancer cells with RET/PTC or BRAF
mutations in vitro and in vivo. Clin Cancer Res. 12:1785–1793.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu D, Liu Z, Condouris S and Xing M: BRAF
V600E maintains proliferation, transformation, and tumorigenicity
of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol
Metab. 92:2264–2271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Salvatore G, Falco V, Salerno P, Nappi T,
Pepe S, Troncone G, Carlomagno F, Melillo R, Wilhelm SM and Santoro
M: BRAF is a therapeutic target in aggressive thyroid carcinoma.
Clin Cancer Res. 12:1623–1629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mitsutake N, Knauf JA, Mitsutake S, Mesa C
Jr, Zhang L and Fagin JA: Conditional BRAFV600E expression induces
DNA synthesis, apoptosis, dedifferentiation, and chromosomal
instability in thyroid PCCL3 cells. Cancer Res. 65:2465–2473. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Melillo RM, Castellone MD, Guarino V, De
Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R,
Kruhoffer M, et al: The RET/PTC-RAS-BRAF linear signaling cascade
mediates the motile and mitogenic phenotype of thyroid cancer
cells. J Clin Invest. 115:1068–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Giordano TJ, Kuick R, Thomas DG, Misek DE,
Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al:
Molecular classification of papillary thyroid carcinoma: distinct
BRAF, RAS, and RET/PTC mutation-specific gene expression profiles
discovered by DNA microarray analysis. Oncogene. 24:6646–6656.
2005. View Article : Google Scholar
|
|
63
|
Hoque MO, Rosenbaum E, Westra WH, Xing M,
Ladenson P, Zeiger MA, Sidransky D and Umbricht CB: Quantitative
assessment of promoter methylation profiles in thyroid neoplasms. J
Clin Endocrinol Metab. 90:4011–4018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu S, Liu DX, Tufano RP, Carson KA,
Rosenbaum E, Cohen Y, Holt EH, Kiseljak-Vassiliades K, Rhoden KJ,
Tolaney S, et al: Association of aberrant methylation of tumor
suppressor genes with tumor aggressiveness and BRAF mutation in
papillary thyroid cancer. Int J Cancer. 119:2322–2329. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hou P, Liu D and Xing M: Genome-wide
alterations in gene methylation by the BRAF V600E mutation in
papillary thyroid cancer cells. Endocr Relat Cancer. 18:687–697.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Porra V, Ferraro-Peyret C, Durand C,
Selmi-Ruby S, Giroud H, Berger-Dutrieux N, Decaussin M, Peix JL,
Bournaud C, Orgiazzi J, et al: Silencing of the tumor suppressor
gene SLC5A8 is associated with BRAF mutations in classical
papillary thyroid carcinomas. J Clin Endocrinol Metab.
90:3028–3035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Anand-Apte B, Bao L, Smith R, Iwata K,
Olsen BR, Zetter B and Apte SS: A review of tissue inhibitor of
metalloproteinases-3 (TIMP-3) and experimental analysis of its
effect on primary tumor growth. Biochem Cell Biol. 74:853–862.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qi JH, Ebrahem Q, Moore N, Murphy G,
Claesson-Welsh L, Bond M, Baker A and Anand-Apte B: A novel
function for tissue inhibitor of metalloproteinases-3 (TIMP3):
inhibition of angiogenesis by blockage of VEGF binding to VEGF
receptor-2. Nat Med. 9:407–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chou CK, Chen RF, Chou FF, Chang HW, Chen
YJ, Lee YF, Yang KD, Cheng JT, Huang CC and Liu RT: miR-146b is
highly expressed in adult papillary thyroid carcinomas with high
risk features including extrathyroidal invasion and the BRAF(V600E)
mutation. Thyroid. 20:489–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cahill S, Smyth P, Denning K, Flavin R, Li
J, Potratz A, Guenther SM, Henfrey R, O’Leary JJ and Sheils O:
Effect of BRAFV600E mutation on transcription and
post-transcriptional regulation in a papillary thyroid carcinoma
model. Mol Cancer. 6:212007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yoon H, He H, Nagy R, Davuluri R, Suster
S, Schoenberg D, Pellegata N and Chapelle AL: Identification of a
novel noncoding RNA gene, NAMA, that is downregulated in papillary
thyroid carcinoma with BRAF mutation and associated with growth
arrest. Int J Cancer. 121:767–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Palona I, Namba H, Mitsutake N, Starenki
D, Podtcheko A, Sedliarou I, Ohtsuru A, Saenko V, Nagayama Y,
Umezawa K and Yamashita S: BRAFV600E promotes invasiveness of
thyroid cancer cells through nuclear factor kB activation.
Endocrinology. 147:5699–5707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Karin M: Nuclear factor-kB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
75
|
Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha
SY, Lee HJ, Sul JY, Kweon GR, Ro HK, Kim JM and Shong M: Influence
of the BRAF V600E mutation on expression of vascular endothelial
growth factor in papillary thyroid cancer. J Clin Endocrinol Metab.
91:3667–3670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Grabellus F, Worm K, Schmid KW and
Sheu-Grabellus SY: The BRAF V600E mutation in papillary thyroid
carcinomas is associated with glucose transporter 1 (GLUT1)
overexpression. Thyroid. 22:377–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nilsson M: Iodide handling by the thyroid
epithelial cell. Exp Clin Endocrinol Diabetes. 109:13–17. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Arturi F, Russo D, Bidart JM, Scarpelli D,
Schlumberger M and Filetti S: Expression pattern of the pendrin and
sodium/iodide symporter genes in human thyroid carcinoma cell lines
and human thyroid tumors. Eur J Endocrinol. 145:129–135. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ringel MD, Anderson J, Souza SL, Burch HB,
Tambascia M, Shriver CD and Tuttle RM: Expression of the sodium
iodide symporter and thyroglobulin genes are reduced in papillary
thyroid cancer. Mod Pathol. 14:289–296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Durante C, Puxeddu E, Ferretti E, Morisi
R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A,
et al: BRAF mutations in papillary thyroid carcinomas inhibit genes
involved in iodine metabolism. J Clin Endocrinol Metab.
92:2840–2843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mian C, Barollo S, Pennelli G, Pavan N,
Rugge M, Pelizzo MR, Mazzarotto R, Casara D, Nacamulli D, Mantero
F, et al: Molecular characteristics in papillary thyroid cancers
(PTCs) with no (131)I uptake. Clin Endocrinol. 68:108–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Di Cristofaro J, Silvy M, Lanteaume A,
Marcy M, Carayon P and De Micco C: Expression of tpo mRNA in
thyroid tumors: quantitative PCR analysis and correlation with
alterations of ret, Braf, ras and pax8 genes. Endocr Relat Cancer.
13:485–495. 2006.PubMed/NCBI
|
|
83
|
Riesco-Eizaguirre G, Gutierrez-Martinez P,
Garcia-Cabezas MA, Nistal M and Santisteban P: The oncogene BRAF
V600E is associated with a high risk of recurrence and less
differentiated papillary thyroid carcinoma due to the impairment of
Na+/I−targeting to the membrane. Endocr Relat
Cancer. 13:257–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu D, Hu S, Hou P, Jiang D, Condouris S
and Xing M: Suppression of BRAF/MEK/MAP kinase pathway restores
expression of iodide-metabolizing genes in thyroid cells expressing
the V600E BRAF mutant. Clin Cancer Res. 13:1341–1349. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ikuyama S, Niller HH, Shimura H, Akamizu T
and Kohn LD: Characterization of the 5-flanking region of the rat
thyrotropin receptor gene. Mol Endocrinol. 6:793–804.
1992.PubMed/NCBI
|
|
86
|
Yokomori N, Tawata M, Saito T, Shimura H
and Onaya T: Regulation of the rat thyrotropin receptor gene by the
methylationsensitive transcription factor GA-binding protein. Mol
Endocrinol. 12:1241–1249. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo
Z, Westra WB, Tong BC, Tallini G, Udelsman R, Califano JA, Ladenson
PW and Sidransky D: Methylation of the thyroid-stimulating hormone
receptor gene in epithelial thyroid tumors: a marker of malignancy
and a cause of gene silencing. Cancer Res. 63:2316–2321.
2003.PubMed/NCBI
|
|
88
|
Schagdarsurengin U, Gimm O, Dralle H,
Hoang-Vu C and Dammann R: CpG island methylation of tumor-related
promoters occurs preferentially in undifferentiated carcinoma.
Thyroid. 16:633–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xing M: BRAF mutation in papillary thyroid
cancer: pathogenic role, molecular bases, and clinical
implications. Endocr Rev. 28:742–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Abrosimov A, Saenko V, Rogounovitch T,
Namba H, Lushnikov E, Mitsutake N and Yamashita S: Different
structural components of conventional papillary thyroid carcinoma
display mostly identical BRAF status. Int J Cancer. 120:196–200.
2007. View Article : Google Scholar
|
|
91
|
Adeniran AJ, Zhu Z, Gandhi M, Steward DL,
Fidler JP, Giordano TJ, Biddinger PW and Nikiforov YE: Correlation
between genetic alterations and microscopic features, clinical
manifestations, and prognostic characteristics of thyroid papillary
carcinomas. Am J Surg Pathol. 30:216–222. 2006. View Article : Google Scholar
|
|
92
|
Fugazzola L, Mannavola D, Cirello V,
Vannucchi G, Muzza M, Vicentini L and Beck-Peccoz P: BRAF mutations
in an Italian cohort of thyroid cancers. Clin Endocrinol.
61:239–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fugazzola L, Puxeddu E, Avenia N, Romei C,
Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S,
et al: Correlation between B-RAFV600E mutation and
clinico-pathologic parameters in papillary thyroid carcinoma: data
from a multicentric Italian study and review of the literature.
Endocr Relat Cancer. 13:455–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jin L, Sebo TJ, Nakamura N, Qian X,
Oliveira A, Majerus JA, Johnson MR and Lloyd RV: BRAF mutation
analysis in fine needle aspiration (FNA) cytology of the thyroid.
Diagn Mol Pathol. 15:136–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kebebew E, Weng J, Bauer J, Ranvier G,
Clark OH, Duh QY, Shibru D, Bastian B and Griffin A: The prevalence
and prognostic value of BRAF mutation in thyroid cancer. Ann Surg.
246:466–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kim KH, Kang DW, Kim SH, Seong IO and Kang
DY: Mutations of the BRAF gene in papillary thyroid carcinoma in a
Korean population. Yonsei Med J. 45:818–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kim KH, Suh KS, Kang DW and Kang DY:
Mutations of the BRAF gene in papillary thyroid carcinoma and in
Hashimoto’s thyroiditis. Pathol Int. 55:540–545. 2005.
|
|
98
|
Kim TY, Kim WB, Song JY, Rhee YS, Gong G,
Cho YM, Kim SY, Kim SC, Hong SJ and Shong YK: The BRAF mutation is
not associated with poor prognostic factors in Korean patients with
conventional papillary thyroid microcarcinoma. Clin Endocrinol
(Oxf). 63:588–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim TY, Kim WB, Rhee YS, Song JY, Kim JM,
Gong G, Lee S, Kim SY, Kim SC, Hong SJ and Shong YK: The BRAF
mutation is useful for prediction of clinical recurrence in
low-risk patients with conventional papillary thyroid carcinoma.
Clin Endocrinol (Oxf). 65:364–368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kim J, Giuliano AE, Turner RR, Gaffney RE,
Umetani N, Kitago M, Elashoff D and Hoon DS: Lymphatic mapping
establishes the role of BRAF gene mutation in papillary thyroid
carcinoma. Ann Surg. 244:799–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu RT, Chen YJ, Chou FF, Li CL, Wu WL,
Tsai PC, Huang CC and Cheng JT: No correlation between BRAFV600E
mutation and clinicopathological features of papillary thyroid
carcinomas in Taiwan. Clin Endocrinol (Oxf). 63:461–466. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lupi C, Giannini R, Ugolini C, Proietti A,
Berti P, Minuto M, Materazzi G, Elisei R, Santoro M, Miccoli P and
Basolo F: Association of BRAF V600E mutation with poor
clinicopathologic outcomes in 500 consecutive cases of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 92:4085–4090. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee JH, Lee ES, Kim YS, Won NH and Chae
YS: BRAF mutation and AKAP9 expression in sporadic papillary
thyroid carcinomas. Pathology. 38:201–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mitsiades CS, Negri J, McMullan C,
McMillin DW, Sozopoulos E, Fanourakis G, Voutsinas G,
Tseleni-Balafouta S, Poulaki V, Batt D and Mitsiades N: Targeting
BRAFV600E in thyroid carcinoma: therapeutic implications. Mol
Cancer Ther. 6:1070–1078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Nikiforova MN, Kimura ET, Gandhi M,
Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G,
Fusco A, et al: BRAF mutations in thyroid tumors are restricted to
papillary carcinomas and anaplastic or poorly differentiated
carcinomas arising from papillary carcinomas. J Clin Endocrinol
Metab. 88:5399–5404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Park SY, Park YJ, Lee YJ, Lee HS, Choi SH,
Choe G, Jang HC, Park SH, Park do J and Cho BY: Analysis of
differential BRAF (V600E) mutational status in multifocal papillary
thyroid carcinoma: evidence of independent clonal origin in
distinct tumor foci. Cancer. 107:1831–1838. 2006. View Article : Google Scholar
|
|
107
|
Rodolico V, Cabibi D, Pizzolanti G,
Richiusa P, Gebbia N, Martorana A, Russo A, Amato MC, Galluzzo A
and Giordano C: BRAF(V600E) mutation and p27(kip1) expression in
papillary carcinomas of the thyroid ≤1 cm and their paired lymph
node metastases. Cancer. 110:1218–1226. 2007.PubMed/NCBI
|
|
108
|
Sedliarou I, Saenko V, Lantsov D,
Rogounovitch T, Namba H, Abrosimov A, Lushnikov E, Kumagai A,
Nakashima M, Meirmanov S, et al: The BRAFT1796A transversion is a
prevalent mutational event in human thyroid microcarcinoma. Int J
Oncol. 25:1729–1735. 2004.PubMed/NCBI
|
|
109
|
Trovisco V, Soares P, Preto A, de Castro
IV, Lima J, Castro P, Maximo V, Botelho T, Moreira S, Meireles AM,
et al: Type and prevalence of BRAF mutations are closely associated
with papillary thyroid carcinoma histotype and patients’ age but
not with tumour aggressiveness. Virchows Arch. 446:589–595.
2005.PubMed/NCBI
|
|
110
|
Xing M, Westra WH, Tufano RP, et al: BRAF
mutation predicts a poorer clinical prognosis for papillary thyroid
cancer. J Clin Endocrinol Metab. 90:6373–6379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Powell N, Jeremiah S, Morishita M, Dudley
E, Bethel J, Bogdanova T, Tronko M and Thomas G: Frequency of BRAF
T1796A mutation in papillary thyroid carcinoma relates to age of
patient at diagnosis and not to radiation exposure. J Pathol.
205:558–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sapio MR, Posca D, Troncone G, Pettinato
G, Palombini L, Rossi G, Fenzi G and Vitale M: Detection of BRAF
mutation in thyroid papillary carcinomas by mutant allele-specific
PCR amplification (MASA). Eur J Endocrinol. 154:341–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Frasca F, Nucera C, Pellegriti G, Gangemi
P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, et
al: BRAF(V600E) mutation and the biology of papillary thyroid
cancer. Endocr Relat Cancer. 15:191–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cañadas Garre M, López de la Torre Casares
M, Becerra Massare P, López Nevot MÁ, Villar Del Moral J, Muñoz
Pérez N, Vílchez Joya R, Montes Ramírez R and Llamas Elvira JM:
BRAF(T1799A) mutation in the primary tumor as a marker of risk,
recurrence, or persistence of papillary thyroid carcinoma.
Endocrinol Nutr. 58:175–184. 2011.PubMed/NCBI
|
|
115
|
Chakraborty A, Narkar A, Mukhopadhyaya R,
Kane S, D’Cruz A and Rajan MG: BRAF (V600E) Mutation in papillary
phyroid carcinoma: significant association with node metastases and
extra thyroidal invasion. Endocr Pathol. 23:83–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nam JK, Jung CK, Song BJ, Lim DJ, Chae BJ,
Lee NS, Park WC, Kim JS, Jung SS and Bae JS: Is the BRAF(V600E)
mutation useful as a predictor of preoperative risk in papillary
thyroid cancer? Am J Surg. 203:436–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, Park SY, Jung KC, Koo do H and Youn YK: BRAF(V600E)
mutation is associated with tumor aggressiveness in papillary
thyroid cancer. Word J Surg. 36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Begum S, Rosenbaum E, Henrique R, Cohen Y,
Sidransky D and Westra WH: BRAF mutations in anaplastic thyroid
carcinoma: implications for tumor origin, diagnosis and treatment.
Mod Pathol. 17:1359–1363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Quiros RM, Ding HG, Gattuso P, Prinz RA
and Xu X: Evidence that one subset of anaplastic thyroid carcinomas
are derived from papillary carcinomas due to BRAF and p53
mutations. Cancer. 103:2261–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Takano T, Ito Y, Hirokawa M, Yoshida H and
Miyauchi A: BRAF V600E mutation in anaplastic thyroid carcinomas
and their accompanying differentiated carcinomas. Br J Cancer.
96:1549–1553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ito Y, Yoshida H, Maruo R, et al: BRAF
mutation in papillary thyroid carcinoma in a Japanese population:
its lack of correlation with high-risk clinicopathologic features
and disease-free survival of patients. Endocrine J. 56:89–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Howell GM, Carty SE, Armstrong MJ, Lebeau
SO, Hodak SP, Coyne C, Stang MT, McCoy KL, Nikiforova MN, Nikiforov
YE and Yip L: Both BRAF V600E mutation and older age (≥65 years)
are associated with recurrent papillary thyroid cancer. Ann Surg
Oncol. 18:3566–3571. 2011.
|
|
123
|
Ain KB: Management of undifferentiated
thyroid cancer. Baillieres Best Pract Res Clin Endocrinol Metab.
14:615–629. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chung KW, Yang SK, Lee GK, Kim EY, Kwon S,
Lee SH, Park do J, Lee HS, Cho BY, Lee ES and Kim SW: Detection of
BRAFV600E mutation on fine needle aspiration specimens of thyroid
nodule refines cyto-pathology diagnosis, especially in BRAF600E
mutation-prevalent area. Clin Endocrinol (Oxf). 65:660–666. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Patel A, Klubo-Gwiezdzinska J, Hoperia V,
Larin A, Jensen K, Bauer A and Vasko V: BRAF(V600E) mutation
analysis from May-Grünwald Giemsa-stained cytological samples as an
adjunct in identification of high-risk papillary thyroid carcinoma.
Endocr Pathol. 22:195–199. 2011.
|
|
126
|
Rowe LR, Bentz BG and Bentz JS: Utility of
BRAF V600E mutation detection in cytologically indeterminate
thyroid nodules. Cytojournal. 3:102006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Marchetti I, Lessi F, Mazzanti CM,
Bertacca G, Elisei R, Coscio GD, Pinchera A and Bevilacqua G: A
morpho-molecular diagnosis of papillary thyroid carcinoma: BRAF
V600E detection as an important tool in preoperative evaluation of
fine-needle aspirates. Thyroid. 19:837–842. 2009. View Article : Google Scholar
|
|
128
|
Colanta A, Lin O, Tafe L, Ghossein R, Nafa
K, Mitchell T, Ladanyi M and Arcila M: BRAF mutation analysis of
fine-needle aspiration biopsies of papillary thyroid carcinoma:
Impact on diagnosis and prognosis. Acta Cytol. 55:563–569. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bollag G, Hirth P, Tsai J, et al: Clinical
efficacy of a RAF inhibitor needs broad target blockade in
BRAF-mutant melanoma. Nature. 467:596–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Nucera C, Porrello A, Antonello ZA, Mekel
M, Nehs MA, Giordano TJ, Gerald D, Benjamin LE, Priolo C, Puxeddu
E, et al: B-Raf(V600E) and thrombospondin-1 promote thyroid cancer
progression. Proc Natl Acad Sci USA. 107:10649–10654. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nucera C, Nehs MA, Mekel M, Zhang X, Hodin
R, Lawler J, Nose V and Parangi S: A novel orthotopic mouse model
of human anaplastic thyroid carcinoma. Thyroid. 19:1077–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liu D, Liu Z, Jiang D, Dackiw AP and Xing
M: Inhibitory effects of the mitogen-activated protein kinase
kinase inhibitor CI-1040 on the proliferation and tumor growth of
thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol
Metab. 92:4686–4695. 2007. View Article : Google Scholar
|
|
133
|
Henderson YC, Fredrick MJ and Clayman GL:
Differential responses of human papillary thyroid cancer cell lines
carrying the RET/PTC1 rearrangement or a BRAF mutation to MEK1/2
inhibitors. Arch Otolaryngol Head Neck Surg. 133:810–815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wallace EM, Lyssikatos JP, Yeh T, Winkler
JD and Koch K: Progress towards therapeutic small molecule MEK
inhibitors for use in cancer therapy. Curr Top Med Chem. 5:215–229.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang D, Boerner SA, Winkler JD and LoRusso
PM: Clinical experience of MEK inhibitors in cancer therapy.
Biochim Biophys Acta. 1773:1248–1255. 2007. View Article : Google Scholar : PubMed/NCBI
|