Introduction
Ischemic cerebrovascular diseases are a common type
of aggressive disease with high rates of incidence, mortality and
morbidity. Therefore, studies on the pathophysiology of these
diseases and new treatment strategies are necessary, since there
are currently no effective clinical treatments. For example, the
factors that promote angiogenesis might be helpful for the recovery
of patients with cerebral ischemia.
VEGF plays a significant role in the increase of
vascular permeability (1,2). In the brain, VEGF is considered the
main medium of peritumoral vasogenic brain edema (3,4).
Animal and clinical experiments have shown that shortly after focal
cerebral ischemia, leakage in the vascular permeability barrier
occurs, leading to cerebral edema. VEGF expression peaks when
vasogenic brain edema following ischemia is most severe, so the
high VEGF expression may be associated with blood-brain barrier
leakage. It has already been suggested in previous studies that in
patients with cerebral ischemia, the microvascular density of the
ischemic hemisphere increased significantly when compared with
contralateral lesions, and the number of microvessels in the
penumbra area may be correlated with the survival time of the
stroke patients (5–7).
Results showed that following persistent cerebral
ischemia in rats, local injection of VEGF in the cerebral cortex
promoted angiogenesis 7 days following ischemia. The treatment
group of 30 μg/ml VEGF showed the most significant effect and the
group of 10 μg/ml showed the least significant effect, which
indicated that the effect of angiogenesis may be related to the
concentration of VEGF. Within a certain range, the higher the dose,
the greater the effect.
In this study, the effects of exogenous VEGF on the
infarct volume of cerebral ischemia, neurological deficits,
cerebral edema, microvascular density and apoptosis were
investigated. Electron microscopy of vascular endothelial cells and
apoptotic cell ultrastructure were performed to study whether
excessive proliferation causes formation of the vascular tumor-like
structures. The protective roles of VEGF suggest that exogenous
VEGF may be appropriate for clinical use.
Materials and methods
Animals
Forty healthy male Wistar rats 3–4 months old, each
weighing 250–300 g, were provided by the Experimental Animal Center
of Shandong Medical University (China). The rats were randomly
divided into 4 groups, administered PBS and 10, 20 or 30 μg/ml of
VEGF. The PBS group served as the control. The infarct volume ratio
and the neurological deficits were determined by MRI. Microvessel
density, apoptosis in brain slices and brain edema were determined.
The focal cerebral ischemia rat model was made using the middle
cerebral artery occlusion (MCAO) method described by Longa et
al(8). All animal experiments
were conducted according to the animal experimental guidelines of
Jining Medical College (Shandong, China). The study was approved by
the ethics committee of Jining Medical College.
Drug treatments
VEGF was diluted to concentrations of 10, 20 or 30
μg/ml using sterile 0.1 M PBS containing 0.1% fetal calf serum.
VEGF solutions were slowly injected into the left cerebral cortex
of rats. For the control group, sterile 0.1 M PBS containing 0.1%
fetal calf serum was injected into the left cerebral cortex of the
rats. Each rat was injected three times at 1, 24 and 48 h after
infarct. Observation was performed 7 days after infarction.
Neurological deficit score
The neurological deficit scores were calculated
according to the 0–5 grade scoring criteria described by Longa
et al(8), with the greater
number indicating more severe function deficits.
Infarct volume ratio
The 1.5T MRI machine was used to determine the
anatomy structure of the rat brains, with the following parameters:
3 inches of surface coils, a large matrix (256×256), small view
field (FOV, 8×4 cm), 3 mm of thickness and 0.3 mm of intervals.
According to the location and extent of the abnormal signal area on
each layer of the scanned image, the ratio of the infarct volume to
the contralateral cerebral hemisphere was calculated.
Microvessel density
The immunohistochemical SP method was performed to
detect microvessel density, using antibody against laminin
concentration (1:200; Santa Cruz Biotechnology, Inc., USA). Ten
high power microscopic fields (40×10) were randomly selected in the
infarct cortex and basal ganglia in each slice. The number of
positive vessels per field was calculated.
Apoptosis
The oligonucleotide terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling method (TUNEL) was used
to detect in situ apoptosis. At 40×10 high power, 10 fields
were randomly selected around the infarct cortex and basal ganglia
in each slice. The number of total cells and positive cells were
counted to calculate the positive cell rates.
Electron microscopy
Rat tissues were fixed in 4% glutaraldehyde and
examined by electron microscopy. The brain tissue was rinsed with
2% glutaraldehyde and then fixed with 1%
S4O4. Following acetone dehydration and the
EPON-821 embedment, 1-μm ultrathin sections were cut. With sodium
acetate and citrate double staining, the ultrastructure of
apoptotic cells, endothelial cell proliferation and deterioration
tendency were observed by transmission electron microscopy.
Statistical analysis
Data were analyzed by SPSS software and given as the
mean ± SD. Single-factor analysis of variance, q-test and t-test
were performed. P<0.05 was considered to indicate statistically
significant differences.
Results
VEGF reduces infarct volume and improves
neurological functions
To determine whether VEGF treatment affects focal
cerebral ischemia in rats, rats were administered PBS or VEGF at
concentrations of 10, 20 or 30 μg/ml. The effects of VEGF on rat
infarct volume and neurological deficits were investigated as
described in Materials and methods and the results are presented in
Table I.
 | Table ITreatment of rats with different
concentrations of VEGF. |
Table I
Treatment of rats with different
concentrations of VEGF.
| PBS | 10 μg/ml | 20 μg/ml | 30 μg/ml |
|---|
| Nos. of rats | 7 | 8 | 8 | 9 |
| Infarct volume
(%) | 43.21±7.12 | 37.26±5.81a | 33.62±8.24b | 27.64±6.77c |
| Neurological deficit
scores | 2.86±0.69 | 2.50±0.76a | 2.00±0.54b | 1.78±0.67c |
As shown in Table
I, there were no significant differences in infarct volume and
neurological deficit scores between the PBS and VEGF (10 μg/ml)
group (P>0.05). However, when rats were treated with 20 μg/ml
VEGF, infarct volume ratios and neurological deficit scores were
both decreased when compared with the PBS group. When rats were
treated with 30 μg/ml VEGF, the mean infarct volume ratio decreased
to 27.64±6.77%, while the mean infarct volume ratio of the PBS
group was 43.21±7.12%. The mean neurological deficit score of the
30 μg/ml VEGF group was 1.78±0.67, whereas the mean score of the
PBS group was 2.86±0.69. These results suggest that treatments with
VEGF reduce the infarct volume and improve neurological
functions.
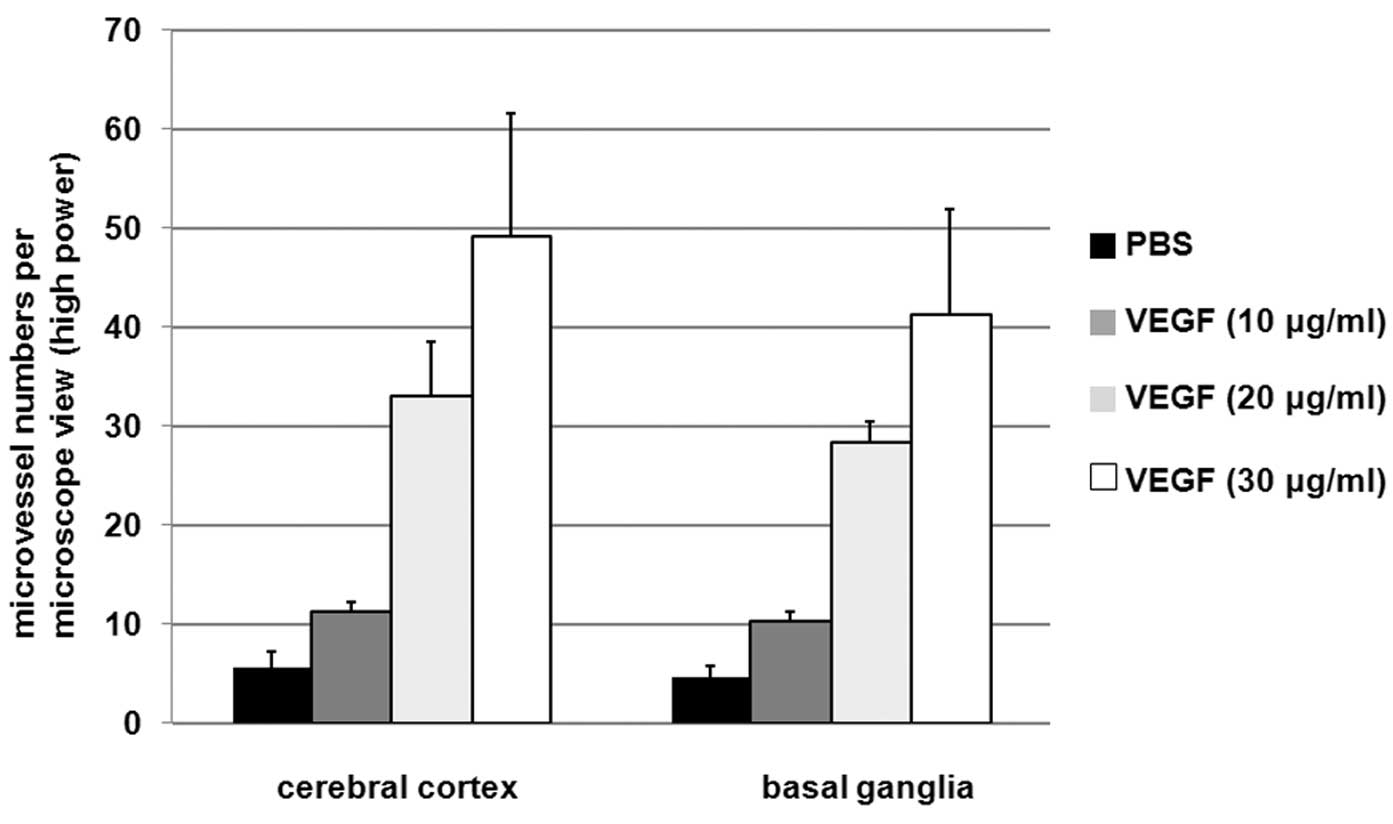
VEGF increases microvessel
generation
To determine the effects of VEGF treatment on
microvessel generation, the microvessel numbers in the cerebral
cortex and basal ganglia of rats administered PBS or VEGF were
detected. As shown in Fig. 1,
treatments with 20 and 30 μg/ml VEGF significantly improved
microvessel numbers compared with the PBS group. Treatment with 30
μg/ml VEGF increased microvessel numbers ~10-fold when compared
with the PBS group, suggesting that VEGF increases microvessel
generation.
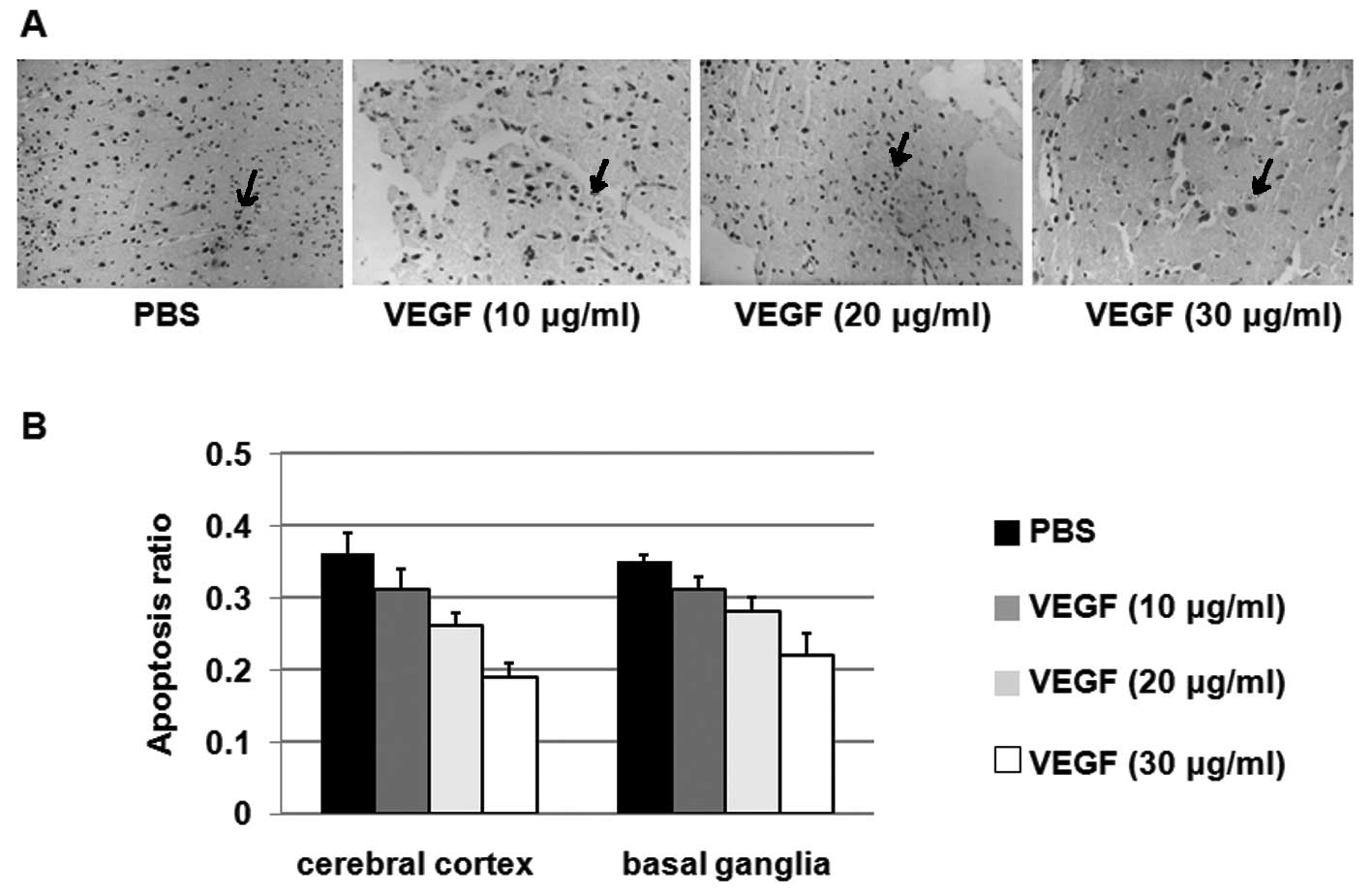
VEGF inhibits apoptosis in the cerebral
cortex and basal ganglia
To determine the effects of VEGF treatment on
apoptosis, the apoptotic cell numbers in the cerebral cortex and
basal ganglia from rats administered PBS or VEGF (10, 20 or 30
μg/ml) were determined using the TUNEL method. As shown in Fig. 2A, VEGF treatments with 20 and 30
μg/ml VEGF significantly decreased apoptotic cell numbers when
compared with the PBS group. The ratio of the apoptotic cell
numbers to the total cell numbers from rats in each group was
calculated and is shown in Fig.
2B. These results suggest that VEGF inhibits apoptosis in the
cerebral cortex and basal ganglia.
VEGF improves growth of vascular
endothelial cells
To determine the effects of VEGF on vascular
endothelial cells, electron microscopy was performed to detect
vascular endothelial cell changes in the ischemic peripheral brain
zone of rats administered PBS or VEGF (10, 20 or 30 μg/ml). As
shown in Fig. 3, for the rats in
the PBS group, vascular endothelium was thin with partial necrosis
and necrosis was observed in axons and dendrites. In rats
administered 10 μg/ml VEGF, similar results were observed when
compared with the PBS group. However, when the rats were
administered VEGF at a concentration of 20 μg/ml, more
proliferative endothelial cells were observed and the rats in the
30 μg/ml VEGF group presented the most proliferative endothelial
cells.
Discussion
In the present study we have shown that application
of VEGF reduced apoptosis 7 days after ischemia. Infarct volume was
reduced and neurological function was improved, which may be due to
the effects of VEGF. Thus, VEGF may play a protective role. This
effect is consistent with findings from previous studies. For
example, it has been reported that VEGF stimulates axon growth and
improves neuronal survival of mice on the neck and dorsal root
ganglion (9). Also, VEGF has been
found to promote the growth of cultured midbrain neurons (10,11).
Bilateral hippocampal ischemia test of rats indicated a direct
protective effect of VEGF on neurons (12). Jin et al(13) found the neurotrophic effect of VGEF
in studies of the mouse hippocampal HN33 cells (14).
The concentration and dosage of VEGF used in this
experiment were based on previous VEGF experiments (15,16).
For example, Hayashi et al(15) applied VEGF on the surface of the
cerebral cortex with similar concentrations. Zhang et
al(16) applied intravenous
infusion of 1 mg/kg VEGF165. In this study, slow and repeated
injections were used due to the short half-life of the VEGF
protein.
Previous studies focused on the effects of VEGF on
angiogenesis following cerebral ischemia (15,17),
and in ventricle and brain parenchyma (18,19).
It has been demonstrated that the application of VEGF in the
ventricle of non-ischemic adult rats would also promote
angiogenesis (20), but it is
generally accepted that VEGF would have a pro-angiogenic effect
only after it binds to its specific receptor on endothelial cells
(21,22). The effect of VEGF on brain
angiogenesis should be explored in future studies.
Cell death caused by ischemic brain damage may be
caused by the same pathway (23,24).
Neuronal death following ischemia presents with forms of necrosis
and apoptosis, related to active and passive cell death mechanisms,
respectively (25–27). Activation of the apoptotic response
may be an important step related to cell death, thus apoptosis may
affect the final infarct volume (28). The inhibition of apoptosis and the
reduction of ischemic penumbra would be effective in treating
ischemic vascular diseases.
References
|
1
|
Chen XL, Nam JO, Jean C, et al:
VEGF-induced vascular permeability is mediated by FAK. Dev Cell.
22:146–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weis SM: Evaluation of VEGF-induced
vascular permeability in mice. Methods Mol Biol. 763:403–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nassehi D, Dyrbye H, Andresen M, Thomsen
C, Juhler M, Laursen H and Broholm H: Vascular endothelial growth
factor A protein level and gene expression in intracranial
meningiomas with brain edema. APMIS. 119:831–843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmid S, Aboul-Enein F, Pfisterer W,
Birkner T, Stadek C and Knosp E: Vascular endothelial growth
factor: the major factor for tumor neovascularization and edema
formation in meningioma patients. Neurosurgery. 67:1703–1708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krupinski J, Kaluza J, Kumar P, Kumar S
and Wang JM: Role of angiogenesis in patients with cerebral
ischemic stroke. Stroke. 25:1794–1798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zan LK, Song YJ, Teng GX, et al:
Expression and function of vascular endothelial growth factor and
angiopoietins in rat brain after cerebral ischemia. Chin J Pathol.
40:834–839. 2011.(In Chinese).
|
|
7
|
Ma Y, Qu Y and Fei Z: Vascular endothelial
growth factor in cerebral ischemia. J Neurosci Res. 89:969–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sondell M, Lundborg G and Kanje M:
Vascular endothelial growth factor has neurotrophic activity and
stimulates axonal outgrowth, enhancing cell survival and Schwann
cell proliferation in the peripheral nervous systerm. J Neurosci.
19:5731–5740. 1999.
|
|
10
|
Silverman WF, Krum JM, Mani N and
Rosenstein JM: Vascular, glial and neuronal effects of vascular
endothelial growth factor in mesencephalic explant cultures.
Neuroscience. 90:1529–1541. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ku DD, Zaleski JK, Liu S and Brock TA:
VEGF induces EDRF-dependent relaxation in coronary arteries. Am J
Physiol. 265:H586–H592. 1993.PubMed/NCBI
|
|
12
|
Marti HJ, Bernaudin M, Bellail A, Schoch
H, Euler M, Petit E and Risau W: Hypoxia-induced vascular
endothelial growth factor expression precedes neovascularization
after cerebral ischemia. Am J Pathol. 156:965–976. 2000. View Article : Google Scholar
|
|
13
|
Jin KL, Mao XO and Greenberg DA: Vascular
endothelial growth factor: director neuroprotective effect in in
vitro ishemia. Proc Natl Acad Sci USA. 97:10242–10247. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin KL, Mao XO, Nagayama T, Goldsmith PC
and Greenberg DA: Induction of vascular endothelial growth factor
receptors and phosphatidylinositol 3′-kinase/Akt signaling by
global cerebral ischemia in the rat. Neuroscience. 100:713–717.
2000.
|
|
15
|
Hayashi T, Abe K and Itoyama Y: Reduction
of ischemic damage by application of vascular endothelial growth
factor in rat brain after transient ischemia. J Cereb Blood Flow
Metab. 18:887–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang ZG, Zhang L, Jiang Q, et al: VEGF
enhances angiogenesis and promotes blood-brain barrier leakage in
the ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayhan W: VEGF increases permeability of
the blood-brain barrier via a nitric oxide synthase/CGMP-dependent
pathway. Am J Physiol. 276:C1148–C1153. 1999.PubMed/NCBI
|
|
18
|
Rosenstein JM, Mani N, Silverman WF and
Krum JM: Patterns of brain angiogenesis after vascular endothelial
growth factor administration in vitro and in vivo. Proc Natl Acad
Sci USA. 95:7086–7091. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Proescholdt MA, Heiss JD, Walbridge S,
Mühlhauser J, Capogrossi MC, Oldfield EH and Merrill MJ: VEGF
modulates vascular permeability and inflammation in rat brain. J
Neuropathol Exp Neurol. 58:613–627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harrigan MR, Ennis SR, Masada T and Keep
RF: Intraventricular infusion of VEGF promotes cerebral
angiogenesis with minimal brain edema. Neurosurgery. 50:589–598.
2002.PubMed/NCBI
|
|
21
|
Plate KH, Breier G, Millauer B, Ullrich A
and Risau W: Up-regulation of vascular endothelial growth factor
and its cognate receptors in a rat glioma model of tumor
angiogenesis. Cancer Res. 53:5822–5827. 1993.PubMed/NCBI
|
|
22
|
Plate KH, Breier G, Weich HA, Mennel HD
and Risau W: VEGF and glioma angiogenesis: Coordinate induction of
VEGF receptors, distribution of VEGF protein and possible in vivo
regulatory mechanisms. Int J Cancer. 59:520–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furlan M, Marchal G, Viader F, Derlon JM
and Baron JC: Spontaneous neurological recovery after stroke and
the fate of the ischemic penumbra. Ann Neurol. 40:216–226. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosenblum WI: Histopathologic clues to the
pathways of neuronal death following ischemia/hypoxia. J
Neurotrauma. 14:313–326. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pulera MR, Adams LM, Liu H, et al:
Apoptosis in a neonatal rat model of cerebral hypoxia ischemia.
Stroke. 29:2622–2630. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beiharz EJ, Williams CE, Dragunow M, et
al: Mechanisms of delayed cell death following hypoxic-ischemic
injury in the immature rat: evidence for apoptosis during selective
neuronal loss. Brain Res Mol Brain Res. 29:1–14. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sei Y, Von Lubitz KJ, Basile AS, Borner
MM, Lin RC, Skolnick P and Fossom LH: Internucleosomal DNA
fragmentation in gerbil hippocampus following forebrain ischemia.
Neurosci Lett. 171:179–182. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linnik MD, Miller JA, Sprinkle-Cavallo J,
Mason PJ, Thompson FY, Montgomery LR and Schroeder KK: Apoptotic
DNA fragmentation in the rat cerebral cortex induced by permanent
middle cerebral artery occlusion. Brain Res Mol Brain Res.
32:116–124. 1995. View Article : Google Scholar : PubMed/NCBI
|