Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant
tumor in southern China and Southeast Asia (1,2).
Suramin is a naphthalene trisulfonic acid derivative originally
used as an agent for treating trypanosomiasis and onchocerciasis
(3). Previous studies suggested
that suramin inhibits the growth of various tumor models (4,5).
Suramin may interact with cell enzymes, such as DNA polymerase,
topoisomerase II and protein kinase C to inhibit tumor growth, and
has been tested in clinical trials (6). Osteopontin (OPN) is a secreted
arginine-glycine-asparic acid (RGD)-containing phosphoprotein which
contains a predicted thrombin cleavage site. By binding to several
integrins and CD44 variants, OPN plays a crucial role in
tumorigenesis, tumor invasion, tumor angiogenesis and metastasis in
several types of cancer (7–11).
Findings of previous studies have also demonstrated that OPN may
promote cell survival through the inhibition of apoptosis (12). A recent report has proven that OPN
has the potential to regulate the growth and migration of NPC cells
(13). Radiotherapy is the
principal treatment for patients with early-stage NPC. High
pre-treatment plasma osteopontin level in NPC patients has been
demonstrated to be a significant predictor of poor response to
radiotherapy (14,15). The effect of suramin on the OPN
expression in NPC cells, however, has yet to be reported.
Suramin has as yet not been studied in the framework
of NPC. Therefore, we used a poorly-differentiated NPC cell line
(CNE-2) to investigate the effects of suramin on proliferation,
apoptosis, cell cycle arrest and the changes of various related
proteins in NPC cells.
Materials and methods
Materials
Antibody against OPN was purchased from Sigma (St.
Louis, MO, USA), against β-actin from PTG (Chicago, IL, USA), while
other antibodies used in this investigation were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Suramin was
purchased from Alexis (Lausen, Switzerland).
Cell culture
The cell line CNE-2 (a gift from Dr Kewei Wu, State
Key Laboratory of Oncology in Southern China, SunYat-Sen
University, Guangzhou, China) was cultured in DMEM, supplemented
with heat-inactivated fetal calf serum supplemented with penicillin
(100 U/ml) and streptomycin (100 mg/ml) in 5% CO2 at
37°C.
MTT assay
The effects of a sustained application of suramin on
the viability of CNE-2 cells were determined by MTT assay. The
cells were seeded in 96-well plates at a concentration of 5,000
cells/well in a volume of 150 μl of cell culture medium per well.
After 24 h, suramin was added at different concentrations (50, 100
and 200 μM) to the wells in triplicate. The plates were incubated
at 37°C with 5% CO2 for 48 h, and cells were then
additionally treated with suramin (100 μM) and cultured for 24, 48
and 72 h. A 20 μl sample of MTT solution (5 g/l, dissolved in PBS)
was added to each well, and the plates were incubated at 37°C for
an additional 4 h. The supernatant was discarded, then l50 μl
dimethylsulfoxide was added to dissolve the insoluble MTT formazan.
The absorbance values at 570 nm (A570) were determined by a
multi-well plate reader (Tecan, Maennedorf, Switzerland).
Flow cytometric analysis
Cells were cultured with suramin (100 μM) for 24 or
48 h. Apoptosis and the cell cycle were determined by
cytofluorimetry. To detect apoptotic cells, labeling tests
involving both propidium iodide (PI) and annexin-V were conducted
using an Annexin-V staining kit (Invitrogen, Shanghai, China),
according to the manufacturer’s instructions. Briefly, at least
1×106 cells were harvested by trypsinization, incubated
with FITC-labeled annexin-V and PI stock solutions for 10 min at
room temperature and analyzed using a flow cytometer (Beckman
Counter, Miami, FL, USA). For analysis of the cell cycle, cells
treated in different ways were subjected to flow cytometric
analysis for chromosomal DNA. DNA labeling was performed using the
Cycle TEST™ PLUS DNA reagent kit (BD Biosciences Pharmingen, San
Diego, CA, USA). Briefly, the cells were washed 3 times with PBS,
then mixed with 250 μl of solution A, incubated for 10 min at 25°C,
and subsequently mixed with 200 μl of solution B for 10 min at
25°C. Solution C (200 μl) was added into each reaction, followed by
a 10 min incubation in the dark on ice. The samples were then
analyzed using a flow cytometer (Beckman Counter).
Western blot analysis
Cells were cultured with suramin (100 μM) for 24, 48
and 72 h, then harvested and lysed for total protein extraction.
Protein concentration was determined using a Bio-Rad protein assay
kit (Bio-Rad, China). Equal amounts of protein were separated by
12% SDS-PAGE and transferred onto PVDF membranes. The membranes
were rinsed with Tris-buffered saline and Tween (TBST, Probe Co.
Ltd., China) and incubated in blocking buffer (5% dried milk in
PBS) for 1 h at 37°C, followed by incubation with primary
antibodies at 4°C overnight. The polyclonal antibody against OPN
was used at a 1000-fold dilution. Monoclonal antibody against
β-actin was used at a 2000-fold dilution. After three washes with
TBST, the membranes were incubated with their corresponding
secondary antibodies for 1 h. The blots were visualized by an
enhanced chemiluminescence detection system (Invitrogen). The
expression of β-actin was used as an optimization regulator for
protein loading.
Statistical analysis
Statistical analysis was performed using the
unpaired Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
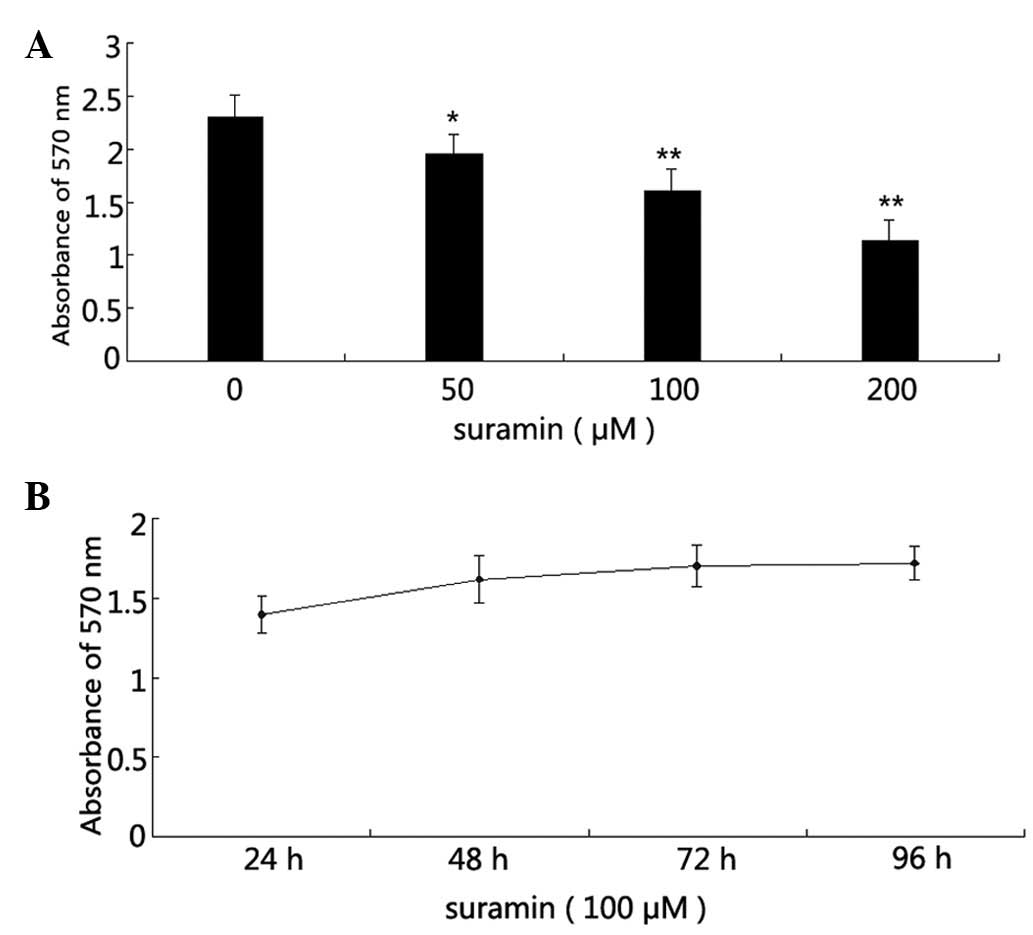
Effects of suramin on the proliferation
of NPC CNE-2 cells
Cells were cultured at a low cell density of ~5,000
cells/well. Cell viability was measured by MTT assay following
suramin treatment. The results showed that CNE-2 cell growth was
inhibited by suramin treatment in a dose- and time-dependent
manner, with a marked effect after 48 h (Fig. 1A and B).
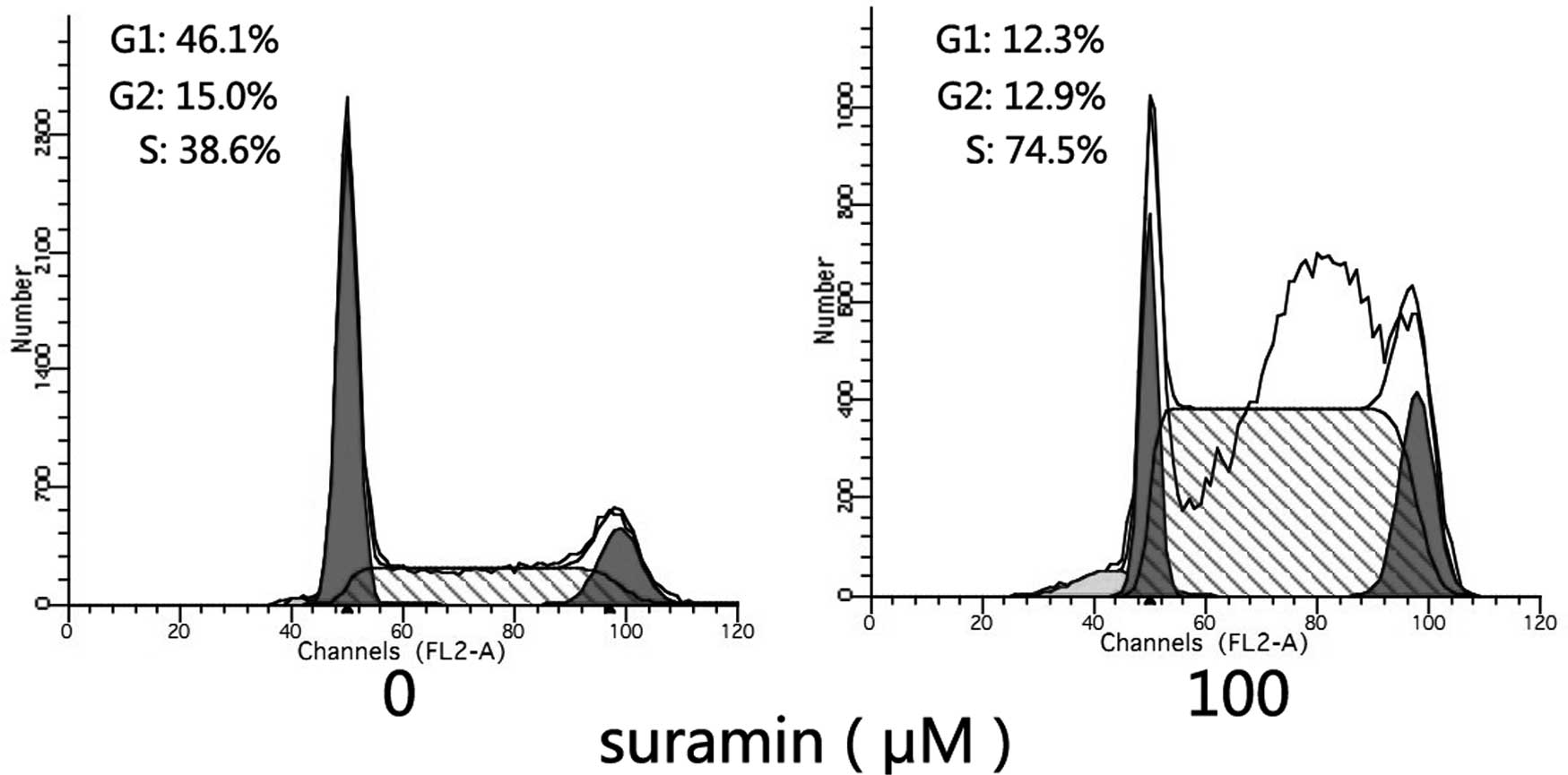
Effects of suramin on the cell cycle
Cell cycle analysis was carried out to investigate
whether the growth inhibitory effect of suramin on NPC cells was
induced by cell cycle arrest. Following sustained incubation of the
cells with suramin for 24 h, the proportion of CNE-2 cells in the S
phase of the cell cycle was found to have significantly increased
when exposed to suramin (Fig. 2).
This finding indicated that S-phase arrest was involved in the
growth inhibitory effect on CNE-2 cells. The concentration of
suramin used was 100 μmol/l.
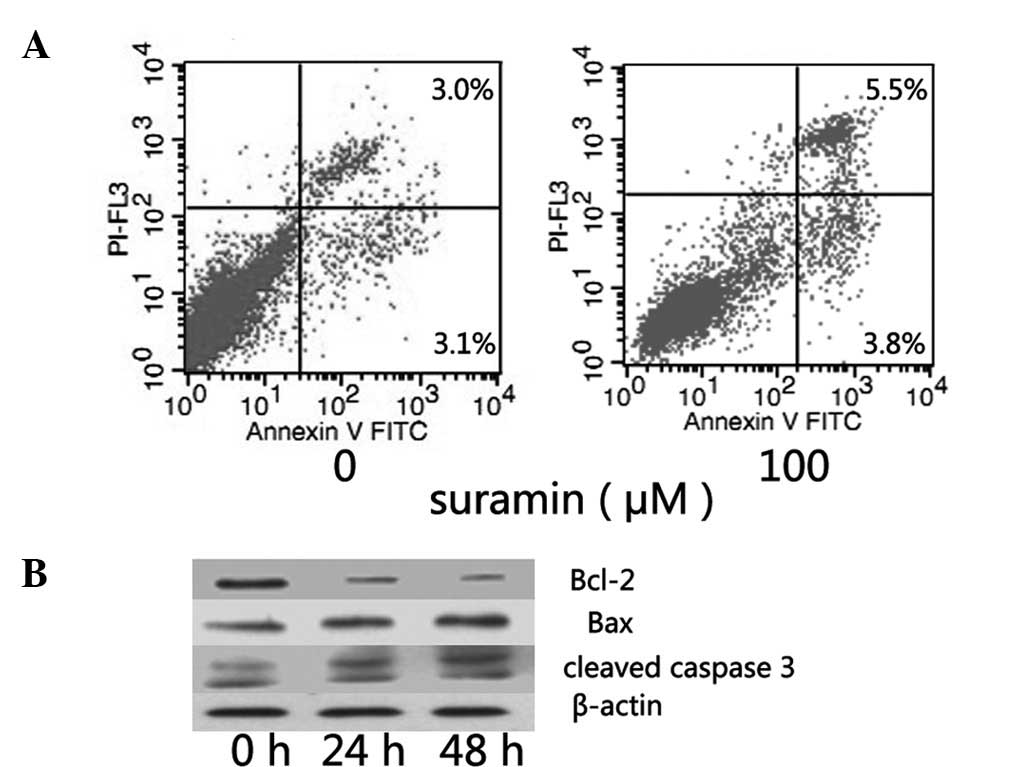
Determination of apoptosis by flow
cytometric analysis
Subsequent to 48-h incubation with suramin, flow
cytometric analysis with propidium iodide (PI) and annexin-V
staining was performed. A clear increase in the annexin V-positive
and PI-positive cell fractions was observed (Fig. 3A), indicating that suramin may
induce apoptosis in NPC cells. The level of some genes was also
found to be associated with CNE-2 cell apoptosis (bcl-2, bax and
caspase 3). Subsequent to suramin treatment, the level of bcl-2
decreased while that of bax increased, and caspase 3 also showed a
cleaved band (Fig. 3B). Thus, at a
concentration of 100 μmol/l suramin may induce apoptosis in NPC
cells.
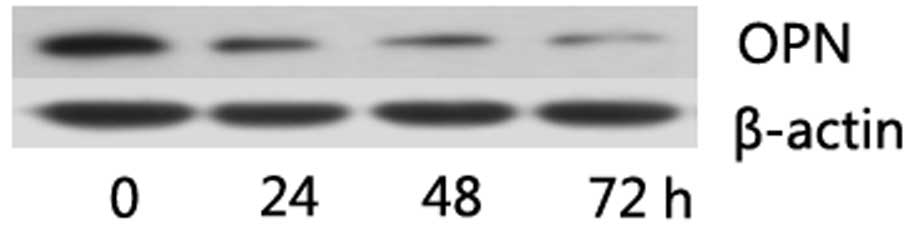
Suramin decreased OPN expression in NPC
cells
Downregulation of the OPN level in NPC cells may
increase the sensitivity of NPC to radiotherapy, thus the OPN level
of NPC cells when treated with suramin was measured. To determine
the change in OPN expression following culture with suramin, the
cells were incubated in medium with or without suramin content for
24, 48 and 72 h. Western blotting showed an obvious decrease of OPN
protein in CNE-2 cells, with the optimization of equal protein
loading regulated by β-actin (Fig.
4). Suramin was used at a concentration of 100 μM.
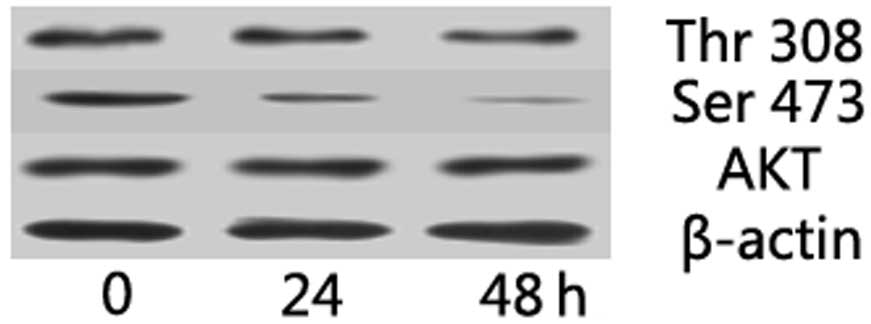
Akt pathway is involved in the growth
inhibitory effect of suramin on NPC cells
OPN expression is reportedly regulated by the Akt
pathway in several tumor models. In the present study, the effect
of suramin on Akt pathway in NPC cells was investigated by western
blotting. Akt phosphorylation at Thr 308 and Ser 473 was reduced in
suramin-treated NPC cells (Fig.
5). These results suggest that suramin may decrease the p-Akt
expression in NPC cells.
Discussion
Suramin is known to inhibit cancer cell growth in
several models through a number of mechanisms (4–6). The
present study aimed to investigate the effects of suramin on NPC
cell growth as well as on the potential mechanisms. Our results
showed that suramin reduced cell viability in a time- and
dose-dependent manner. Similar to other studies, we found that
suramin-induced cell cycle arrest in the S phase may be detected in
NPC cells, the reason of which has yet to be determined. This
finding may be associated with the inhibitory effects of various
cell cycle-related proteins (16).
Some chemotherapeutic drugs show more cytotoxicity to the cells
arrested in S phase (17,18), therefore suramin-induced
recruitment of cancer cells to the S phase may sensitise
poorly-differentiated NPC cells to those drugs. Clinically, the
poorly-differentiated models are more common than the
well-differentiated ones, thus, in this investigation the
poorly-differentiated CNE-2 cell line was used. Suramin-induced
apoptosis was also demonstrated by flow cytometry, whereas changes
in bcl-2, bax and caspase 3 were detected by western blotting.
A recent study also showed that OPN might regulate
NPC cell growth (13). The present
investigation demonstrates for the first time that suramin inhibits
NPC cell growth via the downregulation of OPN. Higher OPN levels
were detected in NPC patient serum, also proving that a higher OPN
level decreased the sensitivity of radiotherapy to NPC, provided
that radiotherapy is the principal therapy for NPC. Suramin-induced
downregulation of OPN in NPC cells may sensitise
poorly-differentiated NPC cells to radiotherapy. The role of AKT
pathway to cell proliferation was thoroughly studied in a number of
tumor models, and certain recent reports have demonstrated that OPN
levels may be regulated by the AKT pathway in several tumor cells
(19,20). We hypothesized that this might also
be the case for NPC cells, and investiged the effect of suramin on
AKT pathway in NPC cells. OPN is a secreted protein, often playing
an extracellular role by binding to certain membrane receptors,
given that suramin may interact with various growth and angiogenic
factors. To determine whether suramin is able to directly interact
with OPN, additional studies are required.
In conclusion, our data have shown that suramin has
a growth inhibitory effect and induces cell cycle arrest in NPC
cells. Given that additional related mechanisms are currently being
investigated, we believe that suramin is a promising alternative
for the adjunctive therapy of NPC.
Acknowledgements
The authors are grateful to Dr Kong at the
Department of Inspection of the First Affiliated Hospital at the
Sun Yat-sen University for his skillful assistance with our flow
cytometric analysis.
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guigay J: Advances in nasopharyngeal
carcinoma. Curr Opin Oncol. 20:264–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hawking F: Suramin: with special reference
to onchocerciasis. Adv Pharmacol Chemother. 15:289–322. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song S, Yu B, Wei Y, Wientjes MG and Au
JL: Low-dose suramin enhanced paclitaxel activity in
chemotherapy-naive and paclitaxel-pretreated human breast xenograft
tumors. Clin Cancer Res. 10:6058–6065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhargava S, Hotz B, Hines OJ, Reber HA,
Buhr HJ and Hotz HG: Suramin inhibits not only tumor growth and
metastasis but also angiogenesis in experimental pancreatic cancer.
J Gastrointest Surg. 11:171–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogelzang NJ, Karrison T, Stadler WM,
Garcia J, Cohn H, Kugler J, et al: A phase II trial of suramin
monthly × 3 for hormone-refractory prostate carcinoma. Cancer.
100:65–71. 2004.
|
|
7
|
Agrawal D, Chen T, Irby R, et al:
Osteopontin identified as lead marker of colon cancer progression
using pooled sample expression profiling. J Natl Cancer Inst.
94:513–521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rudland PS, Platt-Higgins A, El-Tanani M,
et al: Prognostic significance of the metastasis-associated protein
osteopontin in human breast cancer. Cancer Res. 62:3417–3427.
2002.PubMed/NCBI
|
|
9
|
Coppola D, Szabo M, Boulware D, Muraca P,
Alsarraj M, Chambers AF and Yeatman TJ: Correlation of osteopontin
protein expression and pathological stage across a wide variety of
tumor histologies. Clin Cancer Res. 10:184–190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang S, Plymoth A, Ge S, Feng Z, Rosen
HR, Sangrajrang S, Hainaut P, Marrero JA and Beretta L:
Identification of osteopontin as a novel marker for early
hepatocellular carcinoma. Hepatology. 55:483–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong M, Lu Z, Fang G, Bi J and Xue X: A
small interfering RNA targeting osteopontin as gastric cancer
therapeutics. Cancer Lett. 272:148–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geissinger E, Weisser C, Fischer P,
Schartl M and Wellbrock C: Autocrine stimulation by osteopontin
contributes to antiapoptotic signalling of melanocytes in dermal
collagen. Cancer Res. 62:4820–4828. 2002.PubMed/NCBI
|
|
13
|
Yang G, Zhang Y, Wu J, Xiong J, Deng H,
Wang J, Yang C and Zhu Z: Osteopontin regulates growth and
migration of human nasopharyngeal cancer cells. Mol Med Rep.
4:1169–1173. 2011.PubMed/NCBI
|
|
14
|
Wong TS, Kwong DL, Sham J, Wei WI, Kwong
YL and Yuen AP: Elevation of plasma osteopontin level in patients
with undifferentiated nasopharyngeal carcinoma. Eur J Surg Oncol.
31:555–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hui EP, Sung FL, Yu BK, et al: Plasma
osteopontin, hypoxia, and response to radiotherapy in
nasopharyngeal cancer. Clin Cancer Res. 14:7080–7087. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCain DF, Wu L, Nickel P, Kassack MU,
Kreimeyer A, Gagliardi A, Collins DC and Zhang ZY: Suramin
derivatives as Inhibitors and activators of protein-tyrosine
phosphatases. J Biol Chem. 279:14713–14725. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chevillard S, Pouillart P, Beldjord C,
Asselain B, Beuzeboc P, Magdelenat H and Vielh P: Sequential
assessment of multidrug resistance phenotype and measurement of
S-phase fraction as predictive markers of breast cancer response to
neoadjuvant chemotherapy. Cancer. 77:292–300. 1996. View Article : Google Scholar
|
|
18
|
Kolfschoten GM, Hulscher TM, Pinedo HM and
Boven E: Drug resistance features and S-phase fraction as possible
determinants for drug response in a panel of human ovarian cancer
xenografts. Br J Cancer. 83:921–927. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, He B and Weber GF: Growth factor
signaling induces metastasis genes in transformed cells: molecular
connection between Akt kinase and osteopontin in breast cancer. Mol
Cell Biol. 23:6507–6519. 2003. View Article : Google Scholar
|
|
20
|
Kim MS, Park MJ, Moon EJ, Kim SJ, Lee CH,
Yoo H, Shin SH, Song ES and Lee SH: Hyaluronic acid induces
osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to
enhance the motility of human glioma cells. Cancer Res. 65:686–691.
2005.PubMed/NCBI
|