Introduction
Studies on the molecular biological mechanisms of
bone healing have made several breakthroughs in recent years
(1,2), and it has been confirmed that the
bone morphogenetic proteins (BMPs) generated during the bone
healing process are capable of inducing bone regeneration and
promoting bone healing. At present, the BMP family has been found
to have over 20 members, and they can induce the proliferation and
differentiation of undifferentiated mesenchymal cells to form
cartilage and new bones; therefore, the research in this field has
great clinical application potential and broad prospects for
further study (1–4). BMPs are mostly homodimers in
vivo, but at the same time, small amounts of heterodimers have
also been identified. Several studies (5–8) have
shown that the BMP heterodimer has higher activity than the
homodimer, but the mechanism responsible for this requires further
study.
The cDNA for the mature peptides of BMP-4 and BMP-7
was cloned in tissues and cells, respectively, in this experiment,
and the encoding mature peptide genes of the two were connected to
produce the BMP-4/7 fusion gene. The AAV-BMP-4/7 recombinant
adeno-associated virus (AAV) was prepared and the expression of the
fusion gene was detected, as well as its effect on cell
differentiation and proliferation. In addition, the activity of
alkaline phosphatase (ALP) and osteocalcin (OC) was observed
following transfection and cultivation of the bone marrow stromal
cells (BMSCs).
Materials and methods
Rabbit bone marrow stromal cell (BMSC)
culture
Thirteen healthy male New Zealand rabbits were
provided by the Central Laboratory of the First Hospital of Harbin
Medical University. The rabbits weighed approximately 2.0 kg and
were 2.5 months old. Rabbit BMSCs were isolated and cultured using
the bone marrow method for primary culture. After the cells reached
confluence into a monolayer for digestion and passage, the third
generation cells that exhibited good growth were collected for use
in the experiment. This study was conducted with approval from the
Ethics Committee of the First Hospital of Harbin Medical
University, China.
Plasmid construction
The primers of the hBMP-7 and hBMP-7 genes were
designed according to the mature peptide sequences. The
EcoRI and BamHI sites were introduced upstream and
downstream of the hBMP4 gene. The BamHI and PstI
sites were inserted upstream and downstream of the hBMP-7 gene. The
cDNA sequences of BMP-4 and hBMP-7 were amplified through one-step
reverse transcriptase polymerase chain reaction (RT-PCR) from the
placental tissue, and were cloned into the pGEM-T vector to produce
the recombinant plasmids pGEM-BMP-4 and pGEM-BMP-7. The two
plasmids were digested with EcoRI/BamHI and
BamHI/PstI enzymes, and then ligated with T4 ligase
at 16°C overnight. This was followed by transformation into
Escherichia coli DH5α to screen the positive clones, which
were identified by EcoRI/BamHI and
BamHI/PstI double enzyme digestion, 1.5% agarose gel
electrophoresis and sequencing.
The BMP-4 and BMP-7 gene fragments were ligated to
produce the BMP-4/7 fusion gene, and were then cloned into the pGEM
plasmid identified by sequencing. The BMP-4/7 fusion gene was
cleaved from pGEM-BMP-4/7, the recombination was completed in E.
coli, and recombinant adeno-associated plasmids were produced
in 293 cells. To conduct EcoRI, BamHI and PstI
enzyme digestion identification and analysis, the AAV-BMP-4/7
plasmid was transformed into DH5α. A large number of AAV plasmids
were extracted, Lipofectamine 2000 was used to transfect the 293
cells according to the manufacturer’s instructions, and G418
selection and culture were conducted to obtain a drug-resistant
cloned cell line.
Expression of fusion gene and
purification
The AAV-BMP-4/7 plasmid was transformed into E.
coli DH5α cells and cultured at 37°C. When the OD reached 0.6,
IPTG was added until the ultimate volume concentration became 0.4
mmol/l, and the temperature was increased to 42°C to induce the
expression of recombinant protein for 4–6 h. Protein purification
was performed using a conventional method. SDS-PAGE electrophoresis
was carried out for the protein in the supernatant and the
inclusion body to detect the protein expression.
ELISA
A large number of AAV plasmids were extracted, and
liposome Lipofectamine 2000 was used to transfect 293 cells
according to the manufacturer’s instructions. The cells were then
screened under G418 selection and cultured until the drug-resistant
cell colonies appeared. Rabbit BMSCs were seeded in 24-well plates
at 1×105/well, with each group containing 6 duplicate
holes, and virus solution was added with different doses of 10, 50,
100 and 200 μl, respectively, and the supernatant was removed after
24 h for detection with the human BMP ELISA kit.
Cell morphology
After digestion of 105 cells in a 24-well
plate with 0.25% trypsinase, third generation cells in a good state
were selected and inoculated. The cells were then cultured until
70% confluence and inoculated with the virus. Four groups with
different multiplicity of infection (MOI) values of AAV
transfection, namely 5×104, 1×105,
5×105 and 1×106 vg/cell, respectively, were
selected for the experiment. Each group contained six wells. Based
on their MOI values, the required amount of virus and serum
Dulbecco’s modified Eagle’s medium (DMEM) were added into each
well. The virus solution was aspirated 2 h after transfection and
added to ordinary DMEM for conventional culture. The cell changes
were observed every 24 h following transfection.
Osteogenic activity
The cells were cultured for 7 and 14 days after
transfection with AAV-BMP-4/7 for 2 h (experimental group).
Morphological cellular changes and osteogenic differentiation were
observed with an inverted phase contrast microscope. AAV-EGFP was
taken as the control group (six samples each). Approximately 7 and
14 days after transfection, the supernatant of the two groups of
cells were collected. According to the instructions of the alkaline
phosphatase (ALP) assay kit, colorimetric determination (520 mm, 1
cm light path colorimetry) was conducted, and the ALP content was
measured in the supernatants. The supernatants of the two groups of
cells were collected at 7 and 14 days after transfection. The
osteocalcin (OC) values were tested according to the kit
instructions. Approximately 100 μl of the cell culture medium was
collected, then mixed with 100 μl 125I-labeled OC antibodies, and
stored at 4°C for 24 h. Separated agent from the kit was then added
and mixed, which was placed at room temperature and centrifuged at
4°C. The radiation dose of the detected precipitation in the
supernatants was discarded, and then the average OC content of each
group was calculated (repeated six times).
Statistical analysis
Data were analyzed using the statistical package
SPSS 11.5, and the data were presented as the means ± SD, comparing
the concentration of protein expression in different doses of virus
solution using analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Plasmid construction
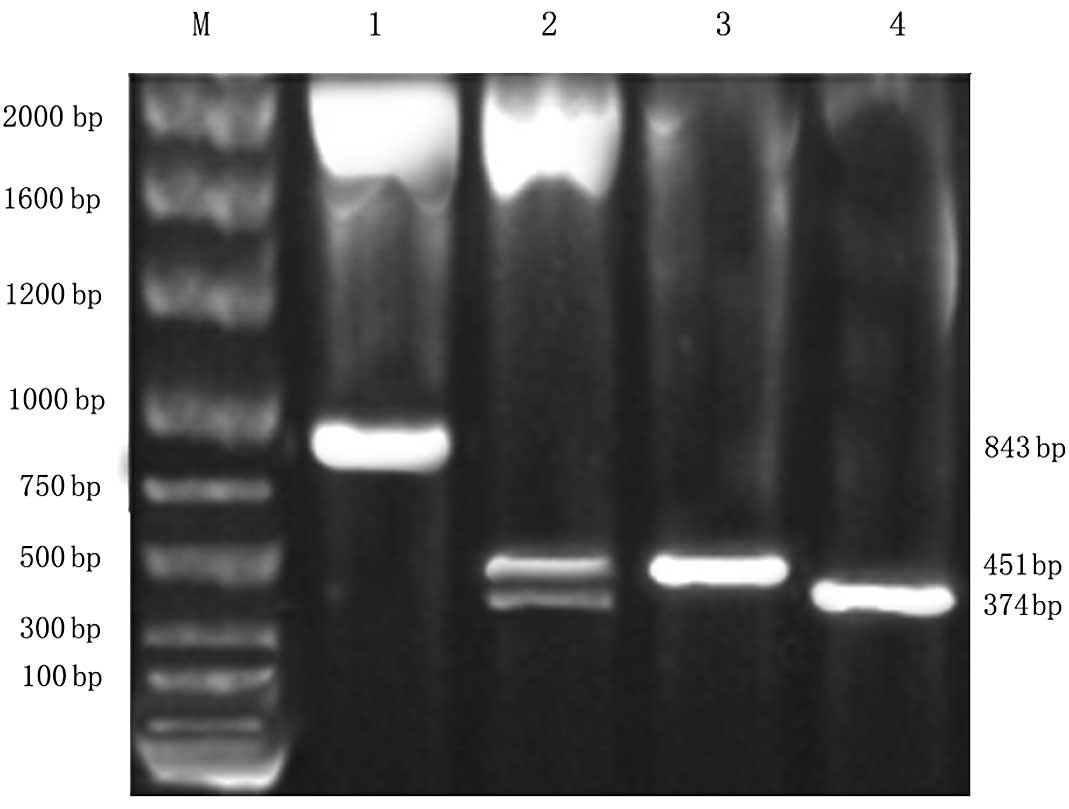
The pGEM-BMP-4 and pGEM-BMP-7 plasmids were
extracted and identified through agarose gel electrophoresis and
PCR primer expansion identification. Two bright bands of 374 and
451 bp were observed. The sequencing result was consistent with the
report in GenBank and the sizes of the mature peptide gene
sequences of BMP-4 and BMP-7.
AAV-BMP-4/7 was successfully obtained. A band of 826
bp was observed through double enzyme digestion. Two bright bands
of approximately 374 and 451 bp on the agarose gel electrophoresis
appeared following EcoRI, BamHI and PstI
identification and PCR primer expansion (Fig. 1), which indicated that the fusion
gene of BMP-4 and BMP-7 was successfully obtained. The AAV plasmid
carrying the BMP-4/7 fusion gene was generated following
recombination.
Expression of fusion gene and
purification
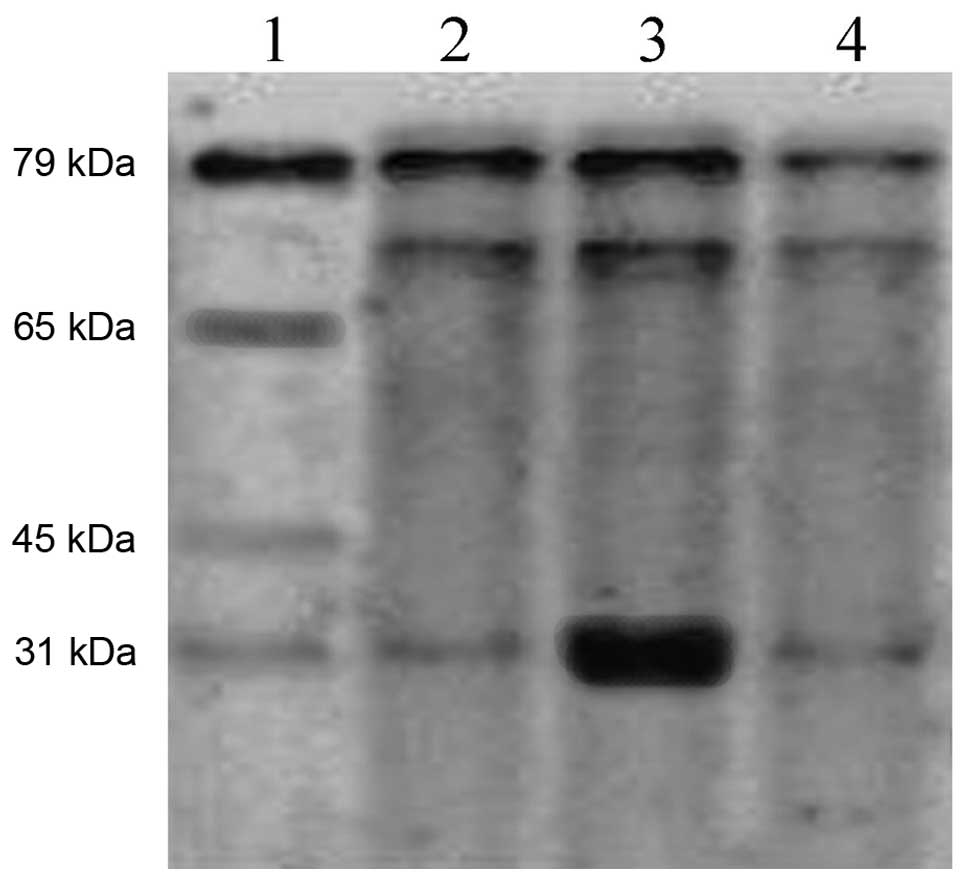
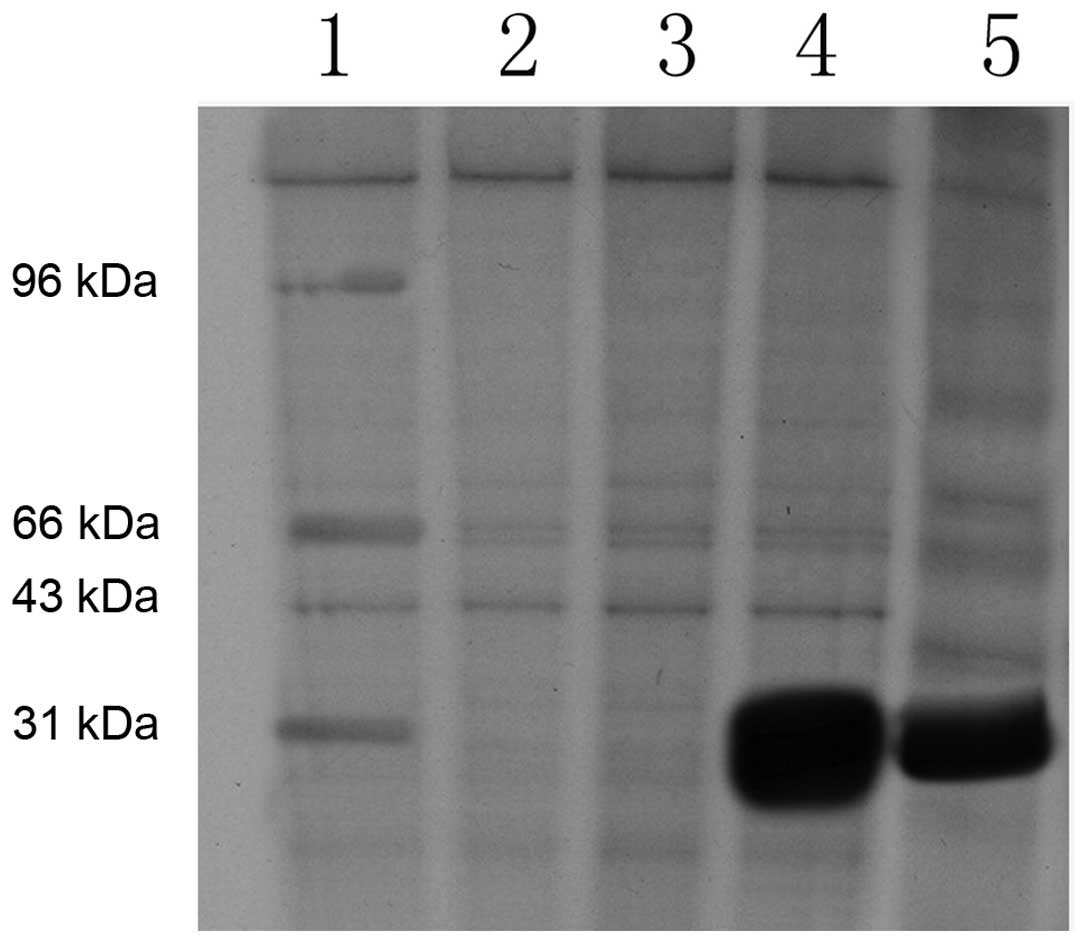
AAV-BMP-4/7 recombinant plasmid was transformed into
E. coli for induced expression, and after conducting
SDS-PAGE electrophoresis, the protein band could be observed at
29–30 kDa (Fig. 2). The bacteria,
supernatants and precipitates were dissolved and purified, and the
majority of proteins were determined to precipitate in the
inclusion body by SDS-PAGE electrophoresis (Fig. 3).
The expression of BMP-4/7 increased significantly
after cells were transfected with 10, 50, 100 and 200 μl of virus
solution, with the expression concentration being 5.614±0.167 ml,
13.698±0.523, 17.214±0.312 and 19.003±0.276 μg/ml respectively, and
the difference had statistical significance (F=1937.276,
P<0.001).
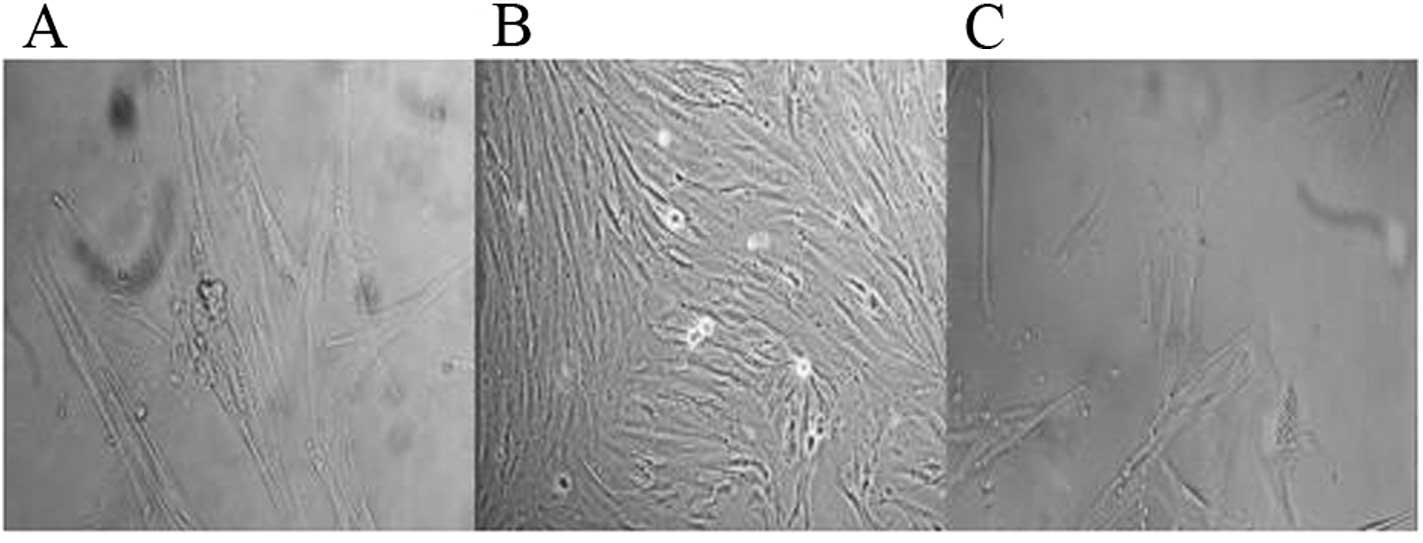
Cell morphology
We removed the cells from the
AAV-BMP-4/7-transfected group and the AAV-EGFP-transfected group,
and after transfection for 7 and 14 days, observed them using an
inverted phase contrast microscope. It was observed that after the
AAV-BMP-4/7 group was transfected for 7 days, the cell morphology
changed significantly, and notably, the distribution of the cells
was uneven, showing partial intensive and local porosity, with the
cells being polygonal, while it was observed under a high-power
field that there were brown particles in the cytoplasm, showing a
significant osteogenic change. It was observed after 14 days that
the cells showed multiple-layer growth, and the brown particles in
the cytoplasm were more evident, which indicates that the cell
calcium nodule was formed. Van Gieson (VG) staining dyed the
nodules black, and there may be bone cells attached around the
nodules (Figs. 4 and 5). The untransfected group only showed
cell contraction and irregular margins, but no specific changes in
osteogenic cell differentiation (Fig.
6).
ALP content determination
Two groups of cells were selected for transfection.
The cell supernatants were removed after 7 and 14 h to detect the
ALP concentration. The content of the AAV-BMP-4/7 group was
67.2±8.4 and 106.5±12.1 Kim units, whereas the contents of the
AAV-EGFP group were 10.1±2.7 and 23.6±4.8 Kim units. The difference
between the two groups was statistically significant (t=896.88,
P<0.001).
OC content determination
Two groups of cells were selected for transfection.
We removed the cell supernatants after 7 and 14 h to calculate the
OC contents. The concentrations of the AAV-BMP-4/7 group were
0.289±0.014 and 0.363±0.076 ng/ml, whereas those of the
untransfected group were 0.011±0.007 and 0.017±0.010 ng/ml. The
difference between the two groups was statistically significant
(t=543.24, P<0.01).
Discussion
At present, bone tissue regeneration is the first of
a variety of studies of tissue regeneration, and its mechanism is
more clear (9–12). The basic concepts of bone
regeneration, such as bone induction, bone conduction, bone
generation and bone reconstruction, have been widely accepted by
many researchers who have reached a consensus. The aim of
constructing tissue engineered bones in vivo is to enable
bone regeneration at bone defect sites, and for the tissue
engineered bones to gradually develop similar structures to
surrounding bones under stress stimulation and to integrate with
them, so as to ultimately restore the normal functions of bone
defect sites.
BMSCs belong to the osteogenic stem cells and have
the potential to transform into various cell lines. They can
achieve cross-system differentiation under certain external
environment conditions and stimulating factors, and as they have
the characteristics of being convenient to obtain and easy to
culture, they are a good source of seed cells for tissue
engineering (5,13,14).
BMSCs consist of two types of osteogenic precursor cells: i)
Oriented osteogenic precursor cells (OOPCs) that can perform
oriented differentiation into osteogenic cells without induction;
ii) induced osteogenic precursor cells (IOPCs) that can only
perform oriented differentiation into osteogenic cells with
induction by specific factors. When cultured in osteogenic culture
solution, BMSCs transform into osteogenic cells and have active
osteogenic ability.
The bone induction abilities of BMP-2, BMP-4 and
BMP-7 are the strongest of more than 20 types of BMPs that have
been found, particularly BMP-4. Recent studies confirm that the
role of recombinant human BMP-4 in promoting spinal fusion is
stronger than recombinant human BMP-2, and its required dose is
only one tenth of the recombinant BMP-2, with the osteogenic volume
being positively correlated with the dose (15,16).
As BMP heterodimers have higher activity than homodimers in
vivo, the mechanism of action remains unclear. In order to
investigate the relationship between the structure and function of
BMPs, this experiment selected BMP-4 and BMP-7 as target genes, and
used DNA recombinant technology to connect BMP-4 and BMP-7, so as
to successfully clone the fusion gene into the shutter vector and
then obtain the recombinant AAV-BMP-4/7 plasmid, which successfully
and stably expressed BMP-4/7 in 293 cells. It was detected that the
recombinant gene is expressed in the cytoplasm of E. coli
and the inclusion body, and after division and purification
identification, high levels of target proteins were detected in the
inclusion body. According to these results, the fusion gene of the
mature peptide BMP-4/7 is expressed in E. coli through the
AAV vector, and some of the expression products exist in the
supernatant, while most exist in the form of an inclusion body.
This lays the experimental foundation for further study on whether
the BMP-4/7 heterodimer is capable of promoting the osteogenic
effect of BMSCs.
Adeno-associated virus (AAV) (17–20)
belongs to the parvoviridae, and the following features draw
attention in gene therapy research: i) It has a high population
infection rate (80–90%), but has no pathogenicity, with minor
immune response; ii) it can perform site-specific integration, and
exist in a relatively stable form; iii) it has a wide range of
hosts, including a variety of cells in division and non-division
phases; iv) the exogenous genes it carries can be regulated due to
long-term sustained and stable expression; v) it has relatively
good thermal stability, anti-acid alkali performance (pH 3.0–9.0)
and resistance to organic solvents, therefore it is easy to store.
However, the conventional procedure of constructing recombinant AAV
is to use the transfected human embryonic kidney 293 cells with
recombinant plasmid and helper plasmid, and this method requires
cumbersome procedures, is expensive, produces low amounts and is
easily contaminated by wild-type adenovirus (21–23).
Various studies expect to achieve a higher rate of virus production
through modifying the gene structure of the AAV vector, packaging
cells and improving the purification method of virus particles.
This experiment successfully constructed the AAV-BMP-4/7 vector
using the AAV virus vector system, making the screening of
recombinant vectors simpler, and the recombinant virus can be
generated in two steps. This overcomes the shortcomings of past
traditional methods, such as low homologous recombination
efficiency in mammalian cells and the lengthy cycle of virus
production caused by multiple plaque purification steps. This
experiment adopted the homologous recombination system, and it
successfully prepared the recombinant AAV-BMP-4/7 adeno-associated
virus carrying the BMP-4/7 fusion gene in a relatively short time.
It confirmed using the ELISA method that transfected BMSCs express
exogenous BMP-4 and BMP-7 recombinant protein after using
AAV-BMP-4/7 to transfect BMSCs, and the expression of BMP-4/7
improves following the increase in virus content at a certain
range, thus confirming the efficiency and simplicity of using the
AAV system to prepare BMP-4/7 AAV and the feasibility of
transfecting BMSCs.
After the recombinant AAV-BMP-4/7 transfected the
BMSCs, the cell morphology transformed from spindle form to
polygonal and cubic forms relative to the untransfected group, and
the cell calcium nodule was formed. Van Gieson’s staining dyed the
nodules black, and there may be bone cells attached around the
nodules, showing marked osteogenic changes. With the increased
transfection time, the above change is more significant, and this
indicates that the BMP-4/7 fusion gene has osteogenic activity and
is positively correlated with the transfection time.
According to previous studies (24–28),
BMPs mainly play three roles in the osteogenic process, and these
roles are to promote the cell chemotaxis, mitosis and
differentiation. The complicated process of BMSCs differentiating
into mature osteogenic cells is completed by the interactions of
many system factors, paracrine factors and autocrine factors. BMPs
are added to the cultured BMSCs for induction of osteogenic cells,
and when differentiation into osteogenic cells begins, cell
synthesis and secretion of osteogenic proteins and extracellular
matrix components occurs. ALP and OC are commonly used test
indicators for this process. Following transfection, AAV-BMP-4/7
was induced in culture for 7 and 14 days, and the ALP and OC
contents in the supernatants both increased significantly and
increased further with longer culture time. However, the ALP and OC
contents in the AAV-EGFP control group did not show a significant
increase, so the results confirm that the BMP-4/7 fusion gene has
induced osteogenic activity after being transfected via AAV.
This experiment confirms that the BMP-4/7 fusion
gene is capable of being efficiently expressed in target cells via
AAV, and can accelerate the transformation of BMSCs to osteogenic
cells in vitro to promote bone formation. The experiment
further confirms that the BMP-4/7 fusion gene has the biological
capability of inducing BMSCs to form bones, and this provides a new
way of thinking for the gene therapy of bone tissue
engineering.
References
|
1
|
Gautschi OP, Frey SP and Zellweger R: Bone
morphogenetic proteins in clinical applications. ANZ J Surg.
77:626–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaibhav B, Nilesh P, Vikram S and Anshul
C: Bone morphogenic protein and its application in trauma cases: a
current concept update. Injury. 38:1227–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo X, Lee KM, Law LP, Chow HK, Rosier R
and Cheng CY: Recombinant human bone morphogenetic protein-4
(rhBMP-4) enhanced posterior spinal fusion without decortication. J
Orthop Res. 20:740–746. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hidaka C, Goodrich LR, Chen CT, Warren RF,
Crystal RG and Nixon AJ: Acceleration of cartilage repair by
genetically modified chondrocytes over expressing bone
morphogenetic protein-7. J Orthop Res. 21:573–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L, Liu W, Cui L and Cao Y:
Tissue-engineered bone repair of goat-femur defects with
osteogenically induced bone marrow stromal cells. Tissue Eng.
12:423–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carpenter RS, Goodrich LR, Frisbie DD, et
al: Osteoblastic differentiation of human and equine adult bone
marrow-derived mesenchymal stem cells when BMP-2 or BMP-7 homodimer
genetic modification is compared to BMP-2/7 heterodimer genetic
modification in the presence and absence of dexamethasone. J Orthop
Res. 28:1330–1337. 2010. View Article : Google Scholar
|
|
7
|
Valera E, Isaacs MJ, Kawakami Y, Izpisúa
Belmonte JC and Choe S: BMP-2/6 heterodimer is more effective than
BMP-2 or BMP-6 homodimers as inductor of differentiation of human
embryonic stem cells. PLoS One. 5:e111672010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Y, Wu G, Zhao J, Wang L, Sun P and
Gu Z: rhBMP2/7 heterodimer: an osteoblastogenesis inducer of not
higher potency but lower effective concentration compared with
rhBMP2 and rhBMP7 homodimers. Tissue Eng Part A. 16:879–887. 2010.
View Article : Google Scholar
|
|
9
|
Shiau AL, Liu PS and Wu CL: Novel strategy
for generation and titration of recombinant adeno-associated virus
vectors. J Virol. 79:193–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang SC, Chuang H, Chen YR, et al:
Cranial repair using BMP-2 gene engineered bone marrow stromal
cells. J Surg Res. 119:85–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang SC, Chung HY, Tai CL, Chen PK, Lin
TM and Jeng LB: Repair of large cranial defects by hBMP-2
expressing bone marrow stromal cells: comparison between alginate
and collagen type I systems. J Biomed Mater Res A. 94:433–441.
2010.PubMed/NCBI
|
|
12
|
Oreffo RO and Triffitt JT: Future
potentials for using osteogenic stem cells and biomaterials in
orthopedics. Bone. 25:S5–S9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar S, Mahendra G, Nagy TR and
Ponnazhagan S: Osteogenic differentiation of recombinant
adeno-associated virus 2-transduced murine mesenchymal stem cells
and development of an immunocompetent mouse model for ex vivo
osteoporosis gene therapy. Hum Gene Ther. 15:1197–1206. 2004.
View Article : Google Scholar
|
|
14
|
Shang Q, Wang Z, Liu W, Shi Y, Cui L and
Cao Y: Tissue-engineered bone repair of sheep cranial defects with
autologous bone marrow stromal cells. J Craniofac Surg. 12:586–593.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gregory CA, Prockop DJ and Spees JL:
Non-hematopoietic bone marrow stem cells: molecular control of
expansion and differentiation. Exp Cell Res. 306:330–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li GH, Hou XK and Wu XF: Experimental
research on spine fusion induced by tissue engineered bone.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 25:39–42. 2003.(In
Chinese).
|
|
17
|
Chen Y, Luk KD, Cheung KM, et al: Gene
therapy for new bone formation using adeno-associated viral bone
morphogenetic protein-2 vectors. Gene Ther. 10:1345–1353. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao H and Walsh CE: AAV vectors for
hemophilia B gene therapy. Mt Sinai J Med. 71:305–313.
2004.PubMed/NCBI
|
|
19
|
McCarty DM, Young SM Jr and Samulski RJ:
Integration of adeno-associated virus (AAV) and recombinant AAV
vectors. Annu Rev Genet. 38:819–845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang M, Ma QJ, Dang GT, Ma KT, Chen P and
Zhou CY: Adeno-associated virus-mediated bone morphogenetic
protein-7 gene transfer induces C2C12 cell differentiation into
osteoblast lineage cells. Acta Pharmacol Sin. 26:963–968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu BY and Zhao DW: Treatment for
osteonecrosis of femoral head by hVEGF-165 gene modified marrow
stromal stem cells under arthroscope. Zhonghua Yi Xue Za Zhi.
89:2629–2633. 2009.(In Chinese).
|
|
22
|
Jiang X, Yang CY, Mao M, Liu QQ and Wang
L: In vitro study on two kind of recombinant adeno-associated
virus-mediated transfection efficiency with enhanced green
fluorescent protein into rats osteoblasts. Sichuan Da Xue Xue Bao
Yi Xue Ban. 41:203–207. 2010.(In Chinese).
|
|
23
|
Li LH, Weng XS, Qiu GX, et al: In vitro
gene transfection into rabbit articular chondrocytes mediated by
recombinant adeno-associated virus vector. Zhonghua Yi Xue Za Zhi.
86:1489–1492. 2006.(In Chinese).
|
|
24
|
Stiehler M, Duch M, Mygind T, et al:
Optimizing viral and non-viral gene transfer methods for genetic
modification of porcine mesenchymal stem cells. Adv Exp Med Biol.
585:31–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levy O, Ruvinov E, Reem T, Granot Y and
Cohen S: Highly efficient osteogenic differentiation of human
mesenchymal stem cells by eradication of STAT3 signaling. Int J
Biochem Cell Biol. 42:1823–1830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montezano AC, Zimmerman D, Yusuf H, et al:
Vascular smooth muscle cell differentiation to an osteogenic
phenotype involves TRPM7 modulation by magnesium. Hypertension.
56:453–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou T, Luo F, Liu J, Bian B and Xu J:
Experimental comparative study on osteogenic activity between
freeze-dried tissue engineered bone and tissue engineered bone.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 24:779–784. 2010.(In
Chinese).
|
|
28
|
Trubiani O, Fulle S, Traini T, et al:
Functional assay, expression of growth factors and proteins
modulating bone-arrangement in human osteoblasts seeded on an
anorganic bovine bone biomaterial. Eur Cell Mater. 20:72–83.
2010.PubMed/NCBI
|