Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent and deadly human tumor types. The majority of HCC cases
occur in East and South-East Asia and in Middle and Western Africa,
but HCC incidence rates are increasing in many parts of the world,
including the United States and Central Europe in recent years
(1–3). Surgical resection is the most
effective treatment for HCC, but the tumor recurrence rate is
approximately 70% at 5 years after resection (4). The exact molecular mechanisms
responsible for HCC development have not yet been clarified.
Therefore, searching for HCC-associated molecules may enable the
identification of effective strategies for the chemoprevention and
treatment of HCC.
Nitric oxide (NO) is generated by the oxidation of
L-arginine under the catalytic activity of nitric oxide synthase
(NOS). NO is a highly reactive radical that exerts a wide range of
biological activities, including smooth muscle relaxation,
inhibition of platelet aggregation and neurotransmission. There are
three major isoforms of NOS: neuronal NOS (NOS-1), inducible NOS
(NOS-2) and endothelial NOS (NOS-3). NOS-1 and NOS-3 are
constitutively expressed at basal levels in various tissues,
whereas NOS-2 is transcriptionally regulated and may be induced by
various cytokines, such as tumor necrosis factor, interferon-γ and
interleukin-1 (5). NOS-2 is
expressed in various cell types and is capable of producing high
amounts of NO.
Excessive NO production by NOS-2 has been
demonstrated to be implicated in the pathogenesis of human
malignant tumors, such as breast cancer (6), colon cancer (7), melanoma(8) and lung cancer (9). A strong positive correlation between
tumor NOS-2 expression and higher tumor grade or poorer patient
survival has been reported in these studies. NO generated by NOS-2
may promote carcinogenesis through oxidative and nitrative DNA
lesions, inducing mutations in the p53 gene (10). Studies in mice confirmed that the
expression of mutant p53 protein may have a positive effect on cell
growth and drive the development of various types of
tumors(11). However, the
functions of NO or NOS-2 in cancer development and progression
remain controversial, with reports in the literature suggesting
they are both pro-neoplastic and anti-neoplastic effectors. Recent
evidence suggests that NO produced by NOS-2 can exert a negative
effect on the regulation of tumor cell behavior and an
antitumorigenic effect in vitro and in vivo (12–16).
To investigate the role of NO/NOS-2 in hepatocarcinogenesis, the
production of NO and the expression of NOS-2, mutant p53 protein
and proliferating cell nuclear antigen (PCNA) were examined in HCC
and non-tumor liver tissues.
Materials and methods
Tissue specimens
We obtained tumors and/or non-malignant liver
tissues from 30 HCC patients who underwent hepatectomies in Tongji
Hospital between 2010 and 2011. None of the patients had received
chemotherapy or radiotherapy prior to surgery. Informed consent was
obtained from all patients for subsequent use of their resected
liver tissues. Tissue samples were collected immediately following
liver resection. The non-malignant liver tissues were at least 2cm
in distance from the tumor margin. Half of the tissue was
immediately frozen in liquid nitrogen and stored at −80°C until
use. Thirty patients had paired tumors and non-malignant liver
tissues. The paired tumors and non-malignant specimens were not
always available for HCC, due to limited tissue material, physical
damage or necrosis in tissue. Part of the tissue (including
30pair-matched tumors and non-malignant liver tissues) was
immediately frozen in liquid nitrogen and stored at −80°C until use
for measurement of NO and NOS-2 mRNA. The other part of the tissue
(including 28 pair-matched tumors and non-malignant tissues, 1
patient only had HCC tissue, 1 patient only had non-malignant
tissue) was fixed in 4% paraformaldehyde and embedded in paraffin
for histopathological diagnosis and immunohistochemical staining.
The diagnoses were confirmed by histopathological study. Tumor
staging was determined by the 6th edition of the
Tumor-Node-Metastasis (TNM) Classification of the International
Union Against Cancer. Table I
shows the general clinicopathological features of the 30 patients
with HCC. The evidence of metastasis included vascular invasion,
particularly portal vein invasion and/or intrahepatic
dissemination. Twenty-eight HCC patients showed markers of
hepatitis B virus (HBV) infection, and 10 HCC patients had a
history of alcohol abuse (mean alcohol consumption of
215.0±168.4g/day; range 50–500g/day). In 2 HCC cases, the
underlying cause of liver disease remained unknown. No hepatitis C
virus (HCV)-related HCC was found in this study. The present study
was performed according to the guidelines of the ethics committee
of the Tongji Hospital and approved in accordance with the ethical
standards of the World Medical Association Declaration of
Helsinki.
 | Table IClinicopathological characteristics of
the 30 HCC patients. |
Table I
Clinicopathological characteristics of
the 30 HCC patients.
| Characteristics | Results |
|---|
| Gender |
| Male | 27 |
| Female | 3 |
| Age (years) |
| ≤45 | 16 |
| >45 | 14 |
| Etiology |
| HBV | 18 |
| HBV + alcohol | 10 |
| Unknown | 2 |
|
Alpha-fetoprotein |
| Normal | 3 |
| High | 27 |
| Tumor diameter
(cm) |
| ≤5 | 3 |
| >5 | 27 |
| No. of tumors |
| Single | 24 |
| Multiple | 6 |
| Pathological
grade |
|
Well-differentiated | 1 |
| Well- to moderately
differentiated | 3 |
| Moderately
differentiated | 15 |
| Moderately to poorly
differentiated | 4 |
| Poorly
differentiated | 7 |
| Metastasis |
| Yes | 8 |
| No | 22 |
| TNM |
| I | 17 |
| II + III | 13 |
Measurement of NO production
Frozen tissue samples (100mg) were mixed with 1.0ml
0.01M phosphate-buffered saline (PBS, pH7.4) and incubated on ice
and then homogenized. After centrifugation for 20min at 12,000rpm
at 4°C, the supernatants were transferred to fresh tubes. After the
protein concentrations were assayed, NO levels were estimated by
measuring the stable NO derivative, i.e. total nitrites, in tissue
supernatant with a commercially available kit according to the
manufacturer’s instructions (Beyotime Biotech Inc., Haimen,
Jiangsu, China). Briefly, 50μl of supernatant was mixed with 100μl
of Griess reagent in a 96-well plate. Optical density was
determined in a microplate spectrophotometer (Bio-Rad Laboratories,
Hercules, CA, USA) at 540nm to form a standard curve (0–100μM)
derived from NaNO2. Each experiment was performed in
triplicate.
Real-time polymerase chain reaction
(RT-PCR)
Total RNA was extracted from frozen tissue specimens
(50–100mg) using phenolchloroform and TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions,
and the isolated RNA was dissolved in 0.1% diethylpyrocarbonate
water for cDNA synthesis. RNA concentrations were examined by
NanoDrop 2000 spectrophotometry (Thermo Scientific, Waltham, MA,
USA). Reverse transcription was accomplished on 4μg of total RNA
using random primers Oligo(dT)18 (Fermentas China,
Shengzhen, China), dNTP Mix (Fermentas China) and M-MLV Reverse
Transcriptase (Promega, Madison, WI, USA). PCR reactions were
performed using Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan).
GAPDH was used as an internal control. The following gene-specific
primers were used: GAPDH sense 5′-TCATTGACCTCAACTA CATGGTTT-3′, and
antisense 5′-GAAGATGGTGATGG GATTTC-3′, yielding a 122-bp product;
NOS-2 sense 5′-ACAAGCCTACCCCTCCAGAT-3′, and antisense
5′-CCGGCCAGATGTTCCTCTA-3′, yielding a 104-bp product (17). PCR assays were performed in
triplicate on a ABI StepOne™ Real-time PCR System (Applied
Biosystems, Foster City, CA, USA) running the cycling conditions:
5min at 94°C, followed by 45 cycles of 10sec at 94°C and 40sec at
60°C. Reaction specificity was detected by melting curve analysis,
which was performed by heating the plate from 55 to 95°C and
measuring SYBR-Green I dissociation from the amplicons. The PCR
products were visualized on 2% agarose gels with GoldView staining
under UV transillumination. Relative mRNA levels of NOS-2 were
calculated and expressed as 2−ΔCt, according to the
formula ΔCt = Ct (NOS-2) − Ct (GAPDH) (18).
Immunohistochemical staining
Serial sections (5-μm thick) were prepared from
paraffin blocks. The sections were deparaffinized in xylene and
hydrated in graded alcohol, then incubated in 3%
H2O2 in absolute methanol for 10min to block
endogenous peroxidase. They were heated by microwaving in citrate
buffer (0.01M, pH6.0) for 3min at 800W, for 7min at 640W and for
3min at 480W. Slides were slowly cooled down to room temperature.
After washing with PBS (0.01M, pH7.4), non-specific binding sites
were blocked with 8% bovine serum albumin (BSA) and 2% horse serum
for 20min at room temperature. Slides were incubated with the
following primary antibodies at 4°C overnight: a rabbit anti-NOS-2
polyclonal antibody (Wuhan Boster Bio-engineering Co., Ltd., China)
at 1:100 dilution, a mouse anti-p53 monoclonal antibody (Wuhan
Boster Bio-engineering Co., Ltd.) at 1:100 dilution, and a mouse
anti-PCNA monoclonal antibody (Wuhan Boster Bio-engineering Co.,
Ltd.) at 1:400 dilution. After washing the slides with PBS for
10min, the antibody detection was performed using the Vectastain
Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according
to the manufacturer’s instructions. Immunoreactive cells were
visualized with diaminobenzidine (DAB; Dako, Glostrup, Denmark)
solution and then counterstained with hematoxylin. Finally, the
sections were coved with neutral balsam. All slides were subjected
to the same procedure under standardized conditions. Negative
controls were performed by replacing the primary antibody with
PBS.
The immunoreactive score of NOS-2 was assessed by
the percentage of positively stained cells (8): 0, no positive staining; 1, 1–25%
cells positive; 2, 26–50% cells positive; 3,51–75% cells positive;
4, 76–100% cells positive. Specimens with scores ≥2 were labeled as
‘positive’. The frequency of p53 and PCNA positively stained nuclei
was expressed as a percentage of stained cell nuclei over the total
number of cells counted, and 1,000 cells were observed in five or
more random fields at a magnification of ×200 to calculate the
percentage of positive cells. ‘p53 positive (+)’ was defined as
positive nuclear p53 staining ≥10%, whereas ‘p53 negative (−)’ was
for cases whose positive nuclear p53 staining was <10%. The
proliferation rate was shown as % PCNA-positive nuclei.
Statistical analysis
Quantitative data were expressed as the
means±standard deviation and qualitative data as the number of
cases. The quantitative data were calculated using the Wilcoxon
signed rank test for paired groups, unpaired groups were compared
by Mann-Whitney U test. The qualitative data were compared using
χ2 test and Fisher’s exact test. Correlation between
factors was evaluated using the Spearman’s rank correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS version 13.0 and GraphPad Prism version 5.0.
Results
Level of NO in HCC and pair-matched
non-tumor liver tissues
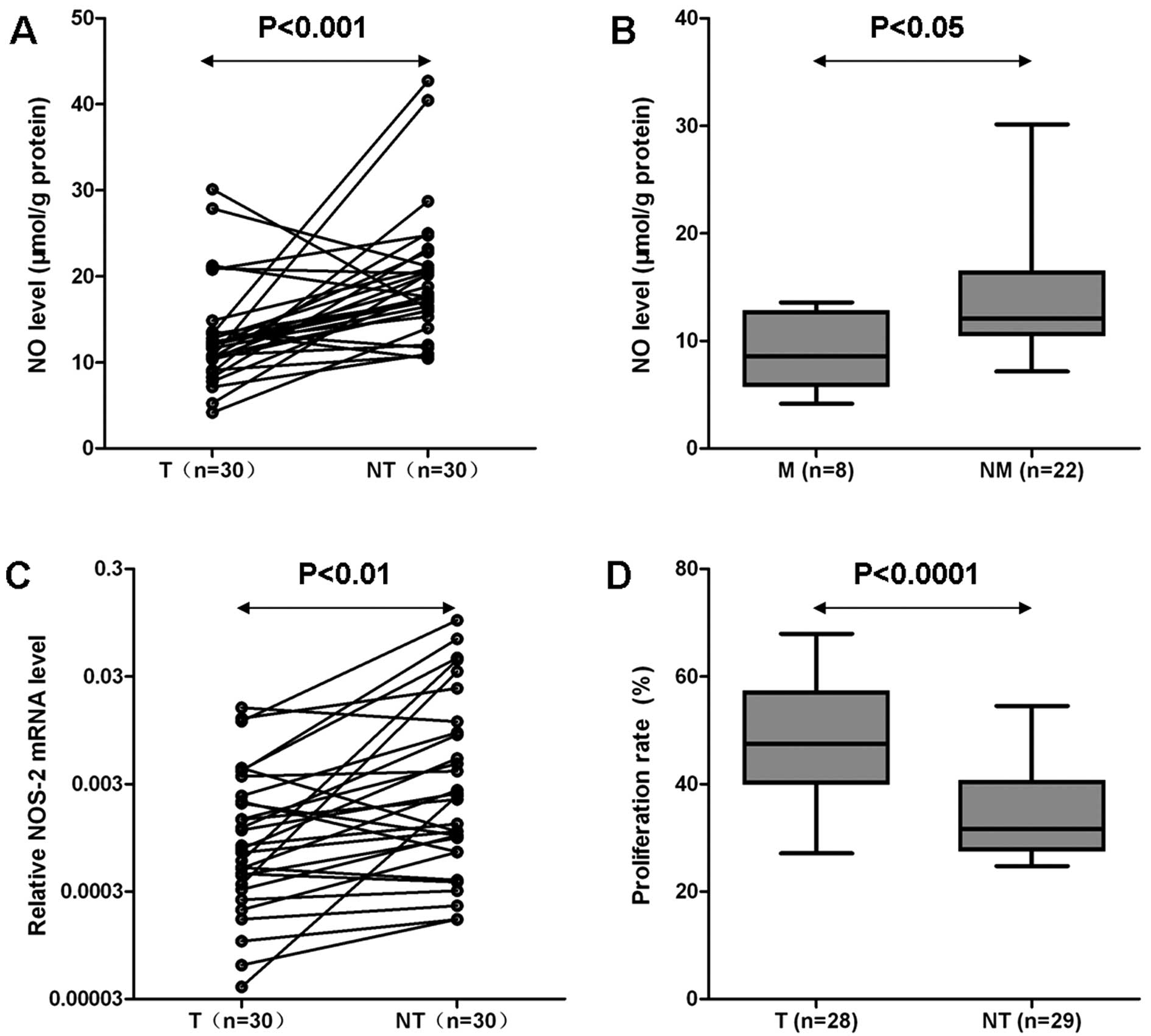
The content of NO was 12.96±5.89μmol/g protein in
HCC, while it was 19.58±7.46μmol/g protein in pair-matched
non-tumor liver tissues, and the difference was significant
(P<0.001; Fig. 1A). There was
no significant difference in NO level in association with age,
etiology, tumor number, differentiation and TNM stage (data not
shown), but the patients suffering from HCC metastasis had lower NO
levels than those without metastasis (9.11±3.48μmol/g protein vs.
14.36±6.01μmol/g protein; P<0.05; Fig. 1B).
NOS-2 mRNA expression in HCC and
pair-matched non-tumor liver tissues
Comparing the relative expression of NOS-2 mRNA in
HCC and pair-matched non-tumor liver tissues from 30 cases, HCC
tissues showed much lower expression than non-tumor liver tissues
(P<0.01; Fig. 1C).
NOS-2 protein expression in HCC and
non-tumor liver tissues
The expression of NOS-2 was observed mainly in the
hepatocytes or cancer cells, showing mainly cytoplasmic staining,
with the positive cells distributed in both HCC and non-tumor liver
tissues. The NOS-2 expression was not observed in inflammatory
cells, bile duct epithelium and vascular endothelium. NOS-2
immunoreactive score was significantly higher in non-tumor liver
than in HCC tissues (P<0.01; Table
II and Fig. 2B). In addition,
we found that NOS-2 expression was correlated with tumor diameter
(P<0.05; Table III) and
metastasis (P<0.05; Table III
and Fig. 2F–H). There were no
differences between the groups in terms of age, etiology, tumor
number, differentiation and TNM staging (Table III).
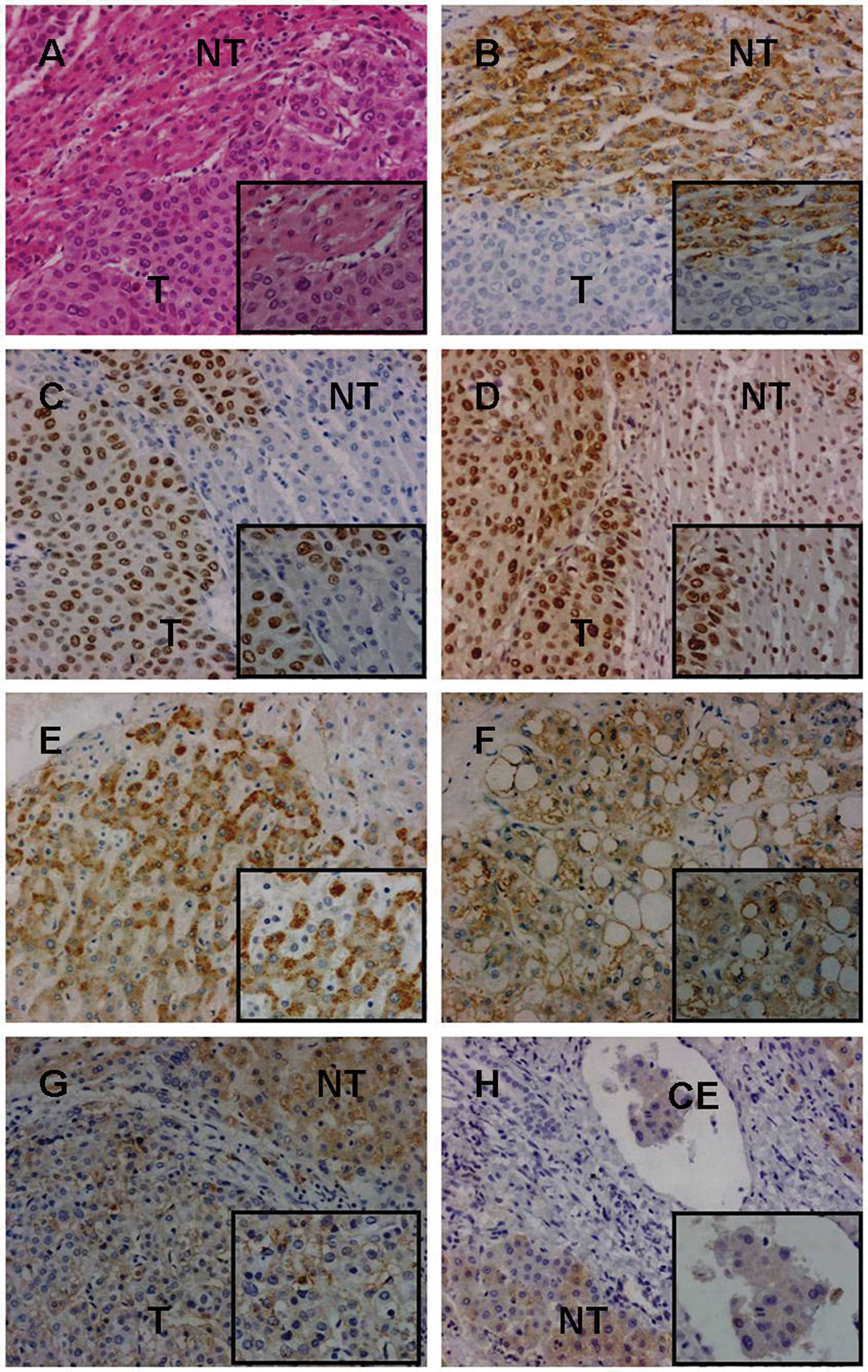 | Figure 2Immunohistochemical analysis of NOS-2,
mutant p53 protein and PCNA expression in liver tissues. (A-D) Case
no. 2100: (A) moderately differentiated HCC and adjacent non-tumor
liver tissue were examined by H&E staining. (B) The
immunoreactivity of NOS-2 in adjacent non-tumor liver tissue was
significantly stronger than in HCC. (C) Mutant p53 protein was
positively expressed in HCC, but was negative in adjacent non-tumor
liver tissue. (D) The positive rate of PCNA was significantly
greater in HCC than in adjacent non-tumor liver tissue. (E-F) Case
no. 9073: (F) moderately differentiated HCC without metastasis
(clear cell carcinoma subtype) and (E)non-tumor liver tissue (at
least 2cm in distance from the tumor margin); NOS-2 had
significantly positive expression in HCC and non-tumor liver
tissue. (G-H) Case no. 8894. (G)Moderately differentiated HCC (T,
primary tumor) and adjacent non-tumor liver tissue (NT).
(H)Pair-matched non- tumor liver tissue (NT, at least 2cm in
distance from the primary tumor margin) and metastatic cancer
embolus of portal vein (CE). The immunoreactivity of NOS-2 was
significantly stronger in HCC without metastasis than in that with
metastasis (F vs. G) and was also significantly stronger in primary
tumor than in metastatic cancer embolus (G vs. H). (Original
magnification, ×200 and ×400). T, HCC tissue; NT, non-tumor liver
tissue; CE, cancer embolus; NOS-2, inducible NOS; HCC,
hepatocellular carcinoma. |
 | Table IIExpression of NOS-2 in HCC and
adjacent non-tumor liver tissues. |
Table II
Expression of NOS-2 in HCC and
adjacent non-tumor liver tissues.
| NOS-2 expression | | |
|---|
|
| | |
|---|
| Tissue | 0 | 1 | 2 | 3 | 4 | Positive rate
(%) | P-value |
|---|
| HCC (n=29) | 11 | 4 | 9 | 5 | 0 | 48.3 | 0.0057a |
| Non-tumor (n=29) | 0 | 5 | 10 | 11 | 3 | 82.8 | |
 | Table IIIRelationship between NOS-2 expression
and clinicopathological features in HCC. |
Table III
Relationship between NOS-2 expression
and clinicopathological features in HCC.
| Variables | Positive (n=14) | Negative (n=15) | P-value |
|---|
| Age (years)a | 45.4±8.9 | 44.9±12.5 | 0.7931 |
| Etiology | | | 0.1201 |
| HBV | 6 | 11 | |
| HBV+ alcohol | 7 | 3 | |
| Unknown | 0 | 2 | |
| Tumor diameter
(cm)a | 6.5±3.0 | 9.4±3.8 | 0.0121 |
| Tumor no. | | | 0.6513 |
| Single | 12 | 11 | |
| Multiple | 2 | 4 | |
| Pathological
gradeb | | | 0.4497 |
| Group I | 10 | 8 | |
| Group II | 4 | 7 | |
| Metastasis | | | 0.0352 |
| Yes | 1 | 7 | |
| No | 13 | 8 | |
| TNM staging | | | 0.1394 |
| I | 10 | 6 | |
| II + III | 4 | 9 | |
Nuclear expression of mutant p53 protein
in liver tissues
The nuclear expression of mutant p53 protein was
detectable in 22 of 28 HCC tissues (78.6%, one case was not
available due to lack of material), whereas this was not found in
any non-tumor liver tissue (Fig.
2C). Only well-differentiated (n=1), well- to moderately
differentiated (n=1) and moderately differentiated (n=4) HCC showed
negative expression of the mutant p53 protein. No significant
differences were noted in mutant p53 expression in association with
any clinicopathological features in HCC (data not shown). The
positive expression of NOS-2 did not show a significant correlation
with the expression rate of mutant p53 protein in HCC tissues.
Positive expression of PCNA in liver
tissues
The proliferation rate in HCC (47.6±10.9%) was
significantly higher than that in non-tumor liver tissues
(34.1±8.2%, P<0.0001; Figs. 1D
and 2D). There was no significant
association between proliferation rate and any clinicopathological
features in HCC (data not shown).
Discussion
NO is a simple, inorganic, free radical gas that
exerts an important role in numerous physiological and
pathophysiological conditions. However, the functions of NO in the
development and progression of cancer remain controversial, with
reports in the literature of it acting as a pro-neoplastic or an
anti-neoplastic agent.
In the present study, we used in vivo
measurement of NO to determine the role it may play in the
development of HCC. We found the content of NO in HCC was
significantly lower than in non-tumor liver tissues. This suggested
that a systemic decrease in NOS expression may occur in HCC tissue.
On the contrary, a previous study reported that NO biosynthesis was
higher in tumor tissues obtained from primary human breast cancers
compared with benign lesions or normal breast tissue (6). Consistent with the biochemical
observation, we found that the mRNA and protein expression of NOS-2
was lower in HCC than in non-tumor liver tissues. Our result was in
agreement with that of another study, in which the authors also
observed that NOS-2 immunoreactivity was significantly lower in
tumor tissue than in the surrounding non-tumor liver tissues in
HCV-positive HCC cases (19).
These findings indicated that the decreased level of NO/NOS-2 may
be associated with hepatocarcinogenesis. However, this is in
contrast to the previous study, which demonstrated that the human
colon tumors contained higher levels of NOS-2 mRNA than the
surrounding normal tissues (7).
The variation in NO production and NOS-2 expression in different
types of tumor tissues suggested that NOS-2 may have a
tissue-specific expression pattern in human tumors.
In the present study, it was of note that HCC with
negative NOS-2 expression was more likely to have a large diameter
and a greater propensity for metastasis. These findings implied
that the deletion of NOS-2 could partially contribute to the growth
and metastasis of HCC. This observation was consistent with a
previous study in which NOS-2-null tumor cells that were injected
subcutaneously grew much faster, and when these cells were injected
intravenously (i.v.) there was more lung metastasis in
NOS-2−/− mice than in NOS-2 wild-type mice (20). In addition, recent reports found
that the high level of NO provided primarily by the NO donor could
inhibit the metastatic cascade, including epithelial to mesenchymal
transition (EMT), migration and invasion in multiple cancer cells.
These results suggested that NO/NOS-2 may be an important mediator
of an aggressive phenotype in HCC (13,14,21).
It was also of note that the immunoreactivity of
NOS-2 was significantly higher in non-tumor liver tissues than in
HCC tissues. The nuclear expression of mutant p53 protein was
detectable in 78.6% of HCC tissues, whereas this was not found in
any non-tumor liver tissues. This finding appears to be consistent
with the hypothesis that low concentrations of NO may induce p53
alteration or mutation, which cause tumor cell resistance; however,
at high concentrations, the DNA damage induced by NO increases
wild-type p53, leading to programmed cell death(10). As a transcription factor, wild-type
p53 protein has a crucial role in promoting apoptosis, senescence
or protective cell cycle arrest and suppressing tumorigenesis. The
p53 gene is one of the most frequently targeted for genetic
alterations in many cancers, and is found to be mutated and
accumulated in tumor tissues. Studies in vitro and in
vivo have confirmed that the expression of mutant p53 protein
can have a positive effect on cell growth and contribute to
carcinogenesis (11,22). In our study, we found the mutant
p53 protein was expressed in the majority of cases of HCC, and the
proliferation rate of HCC tissue was significantly higher than that
of adjacent non-tumor liver tissues. This implies that the
expression of mutant p53 protein had a positive effect on cell
proliferation and contributed to the development of HCC. Therefore,
the decreased expression of NOS-2 in liver tissues may stimulate
cell proliferation and promote hepatocarcinogenesis by inducing the
overexpression of mutant p53 protein.
In conclusion, we have demonstrated that the
production of NO and expression of NOS-2 in non-tumor liver tissues
were higher than in HCC tissues. The decreased level of NO/NOS-2 in
liver tissues may partly contribute to the mutant p53 protein
overexpression and excessive cell proliferation, eventually leading
to the development and progression of HCC.
Acknowledgements
The authors thank all the patients who participated
in this study. This study was funded by the National Natural
Science Foundation of China (grant no. 81071999).
Abbreviations:
|
NO
|
nitric oxide
|
|
NOS
|
nitric oxide synthase
|
|
HCC
|
hepatocellular carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitisC virus
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
PBS
|
phosphate-buffered saline
|
|
BSA
|
bovine serum albumin
|
|
DAB
|
diaminobenzidine
|
|
RT-PCR
|
real-time polymerase chain
reaction
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosetti C, Levi F, Boffetta P, Lucchini F,
Negri E and LaVecchia C: Trends in mortality from hepatocellular
carcinoma in Europe, 1980–2004. Hepatology. 48:137–145. 2008.
|
|
4
|
Adachi E, Maeda T, Matsumata T, et al:
Risk factors for intrahepatic recurrence in human small
hepatocellular carcinoma. Gastroenterology. 108:768–775. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kroncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase in human diseases. Clin Exp
Immunol. 113:147–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomsen LL, Miles DW, Happerfield L,
Bobrow LG, Knowles RG and Moncada S: Nitric oxide synthase activity
in human breast cancer. Br J Cancer. 72:41–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambs S, Merriam WG, Bennett WP, et al:
Frequent nitric oxide synthase-2 expression in human colon
adenomas: implication for tumor angiogenesis and colon cancer
progression. Cancer Res. 58:334–341. 1998.PubMed/NCBI
|
|
8
|
Ekmekcioglu S, Ellerhorst J, Smid CM, et
al: Inducible nitric oxide synthase and nitrotyrosine in human
metastatic melanoma tumors correlate with poor survival. Clin
Cancer Res. 6:4768–4775. 2000.PubMed/NCBI
|
|
9
|
Masri FA, Comhair SA, Koeck T, et al:
Abnormalities in nitric oxide and its derivatives in lung cancer.
Am J Respir Crit Care Med. 172:597–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huerta S, Chilka S and Bonavida B: Nitric
oxide donors: novel cancer therapeutics (Review). Int J Oncol.
33:909–927. 2008.PubMed/NCBI
|
|
11
|
Shaulsky G, Goldfinger N and Rotter V:
Alterations in tumor development in vivo mediated by expression of
wild type or mutant p53 proteins. Cancer Res. 51:5232–5237.
1991.PubMed/NCBI
|
|
12
|
Bonavida B and Baritaki S: Dual role of NO
donors in the reversal of tumor cell resistance and EMT:
downregulation of the NF-kB/Snail/YY1/RKIP circuitry. Nitric Oxide.
24:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hickok JR, Sahni S, Mikhed Y, Bonini MG
and Thomas DD: Nitric oxide suppresses tumor cell migration through
N-Myc downstream-regulated gene-1 (NDRG1) expression: role of
chelatable iron. J Biol Chem. 286:41413–41424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonavida B, Baritaki S, Huerta-Yepez S,
Vega MI, Chatterjee D and Yeung K: Novel therapeutic applications
of nitric oxide donors in cancer: roles in chemo- and
immunosensitization to apoptosis and inhibition of metastases.
Nitric Oxide. 19:152–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussain SP, Trivers GE, Hofseth LJ, et al:
Nitric oxide, a mediator of inflammation, suppresses tumorigenesis.
Cancer Res. 64:6849–6853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le X, Wei D, Huang S, Lancaster JR Jr and
Xie K: Nitric oxide synthase II suppresses the growth and
metastasis of human cancer regardless of its up-regulation of
protumor factors. Proc Natl Acad Sci USA. 102:8758–8763. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thilakawardhana S, Everett DM, Murdock PR,
Dingwall C and Owen JS: Quantification of apolipoprotein E
receptors in human brain-derived cell lines by real-time polymerase
chain reaction. Neurobiol Aging. 26:813–823. 2005. View Article : Google Scholar
|
|
18
|
Yang XR, Xu Y, Yu B, et al: CD24 is a
novel predictor for poor prognosis of hepatocellular carcinoma
after surgery. Clin Cancer Res. 15:5518–5527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahman MA, Dhar DK, Yamaguchi E, et al:
Coexpression of inducible nitric oxide synthase and COX-2 in
hepatocellular carcinoma and surrounding liver: possible
involvement of COX-2 in the angiogenesis of hepatitis C
virus-positive cases. Clin Cancer Res. 7:1325–1332. 2001.
|
|
20
|
Wei D, Richardson EL, Zhu K, et al: Direct
demonstration of negative regulation of tumor growth and metastasis
by host-inducible nitric oxide synthase. Cancer Res. 63:3855–3859.
2003.PubMed/NCBI
|
|
21
|
Sugita H, Kaneki M, Furuhashi S, Hirota M,
Takamori H and Baba H: Nitric oxide inhibits the proliferation and
invasion of pancreatic cancer cells through degradation of insulin
receptor substrate-1 protein. Mol Cancer Res. 8:1152–1163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bossi G, Lapi E, Strano S, Rinaldo C,
Blandino G and Sacchi A: Mutant p53 gain of function: reduction of
tumor malignancy of human cancer cell lines through abrogation of
mutant p53 expression. Oncogene. 25:304–309. 2006.PubMed/NCBI
|