Introduction
Estrogen has been shown to exert a variety of
pleiotropic effects in target tissues as diverse as bone, breast,
blood vessel, brain and the male and female gonads (1). Estrogen plays an important role in
the regulation of bone remodeling and maintenance of the skeleton
(2,3).
The ability of estrogens to stimulate the
transcriptional activity of the estrogen receptor may be inhibited
by a diverse range of estrogen antagonists (4). Fulvestrant, or ICI 182,780, is a
7α-alkylsulphinyl analog of estradiol that competes with endogenous
estrogen for binding to the estrogen receptor (5). Fulvestrant is a novel type of
endocrine treatment and is considered to be a potent inhibitor of
breast cancer cells (6). The
binding of fulvestrant to the estrogen receptor appears to prevent
receptor dimerization and impairs energy-dependent
nucleo-cytoplasmic shuttling, consequently blocking nuclear
localization of the receptor (4,7).
Additionally, the fulvestrant-estrogen receptor complex that enters
the nucleus is transcriptionally inactive since the two activation
domains AF-1 and -2 are disabled (8). Thus, fulvestrant works by
downregulating as well as degrading the estrogen receptor, leading
to an inhibition of estrogen signaling through the estrogen
receptor (5,9).
Previous studies investigating the effects of
fulvestrant on cell proliferation and differentiation have yielded
inconsistent results. Low doses of fulvestrant have been shown to
inhibit the proliferation of the MCF-7 breast cancer cell line
(10) and to inhibit insulin-like
growth factor-1 that stimulates cell proliferation (11). A concentration of 0.1 μM of
fulvestrant was reported to increase the proliferation of the human
fetal osteoblastic cell line, expressing high levels of estrogen
receptors (hFOB/ER9) (12).
However, no statistically significant difference occurred in the
number of cells in the treated (0.1 or 10 μM of fulvestrant) and
untreated groups using calvarial osteoblasts (9). The conflicting responses to
fulvestrant may be partly attributed to the species differences,
the system model, the stage of differentiation of the cells, the
culturing period and variable estrogen receptor content among
various cell lines and primary cultures (12,13).
Moreover, limited studies are available with regard to the effects
of fulvestrant on the differentiation and mineralization of
osteoblasts (9). The effects of
various doses of fulvestrant for bone cells have not yet been fully
investigated.
The present study aimed to examine the effects of
the fulvestrant at varying doses (0.01–10 μM) on cell
proliferation, differentiation and the mineralization of
preosteoblasts. Viability was evaluated using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
reagents, and the alkaline phosphatase activity (ALP) test and
Alizarin red S staining were used to assess the differentiation and
mineralization of treated cells, respectively. The expression of
proteins associated with bone formation and osteocalcin was
evaluated using a western blot analysis.
Materials and methods
Cell culture
MC3T3-E1 murine calvarial preosteoblasts were
maintained in α-minimum essential medium (αMEM; Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Invitrogen) and antibiotics (100 U/ml of penicillin and 100 μg/ml
of streptomycin; Invitrogen). Culture media were changed to an
osteogenic differentiation medium [αMEM supplemented with 50 μg/ml
ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA)] and 10 mM
β-glycerolphosphate (Sigma-Aldrich) to induce osteogenic
differentiation. Cells were maintained at 37°C in a humidified 5%
CO2 environment. The culture media were replenished with
fresh media every three to four days. Fulvestrant, or ICI 182,780,
was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and
filter-sterilized. An equal amount of DMSO was applied in the
control and test groups to minimize the effects of DMSO on cell
growth and for differentiation between the control and treated
cultures. The final concentration of DMSO in the culture never
exceeded 0.01%.
Cell proliferation
Cells were plated at a density of 1.0×104
cells/ml/well in 12-well plates and the cultures were stimulated
with fulvestrant at final concentrations ranging from 0.01 to 10
μM. The effects of fulvestrant on the proliferation of the
preosteoblasts were assessed at Day 4. Cells were incubated for 1 h
with the MTT reagent with a final concentration of 0.5 mg/ml
(13). Rinsing with
phosphate-buffered saline (PBS, pH 7.4) was followed by the
addition of DMSO. After complete dissolution with gentle agitation,
aliquots were transferred into 96-well plates and absorbance was
recorded at 560 and 670 nm using the microplate spectrophotometer
system (BioTek, Winooski, VT, USA).
Protein measurement
Cells were plated and cultured in αMEM in the
presence of ascorbic acid and β-glycerolphosphate for 14 days.
Protein content was determined using the Coomassie Plus Protein
Assay Reagent (Pierce Biotechnology, Rockford, IL, USA). Aliquots
of standard bovine serum albumin (BSA) or a sample were mixed with
the Coomassie reagent and the absorbance was measured at 595 nm
using the microplate spectrophotometer system. The results are
presented as a percentage of the control values.
ALP activity assays
The ALP assay for osteoblast differentiation was
performed at Day 14. Cells were lysed with a buffer containing 10
mM Tris-HCl pH 7.4 and 0.2% Triton X-100 and were then sonicated
for 20 sec at 4°C. Samples were incubated with 10 mM
p-nitrophenylphosphate as a substrate in 100 mM glycine buffer (pH
10.5) containing 1 mM MgCl2 at 37°C in a water bath
(14). Total protein content was
determined in comparison with a series of BSA as the internal
standards. The absorbance at 405 nm was measured using a microplate
reader, and ALP activities were normalized with respect to total
protein content (15).
Mineralization assay
A mineralized matrix was detected using Alizarin red
S staining for qualitative analysis (9). Cells were washed twice with PBS,
fixed with 70% ethanol in ice-cold PBS for 1 h and rinsed twice
with deionized water. The cultures were stained with 40 mM Alizarin
red S (Sigma-Aldrich) for 30 min under gentle agitation. For
quantification, cells stained with Alizarin red S were destained
with 10% cetylpyridinium chloride by agitation. Extracted stain
(200 μl) was transferred to a 96-well plate and the absorbance 562
nm was determined spectrophotometrically.
Western blot analysis
Cells were washed twice with ice-cold PBS and
solubilized with lysis buffer containing 10 mM Tris-HCl pH 7.4,
0.2% Triton X-100. The lysates were centrifuged at 14,000 rpm for
20 min at 4°C to remove the nuclear pellet. The supernatants were
boiled in a sodium dodecyl sulfate sample buffer containing
β-mercaptoethanol. Equal amounts of cell extracts were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred onto polyvinylidene fluoride microporous membrane
(Immobilon-P membranes; Millipore Corporation, Billerica, MA, USA).
Membranes were then blocked in 0.1% (v/v) PBS and Tween-20
containing 5% (w/v) powdered milk for at least 1 h. The membrane
was immunoblotted with the desired mouse antibodies against
osteocalcin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
diluted in the same buffer at the recommended concentrations. The
membrane was incubated with horseradish peroxidase-conjugated
secondary antibody and then the washed blot was developed using
enhanced chemiluminescence detection kits (16).
Statistical analysis
Results are presented as the mean ± standard
deviation of the experiments. One-way analysis of variance (ANOVA)
was performed to determine differences between groups using
commercially available software (SPSS 12 for Windows, SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell proliferation
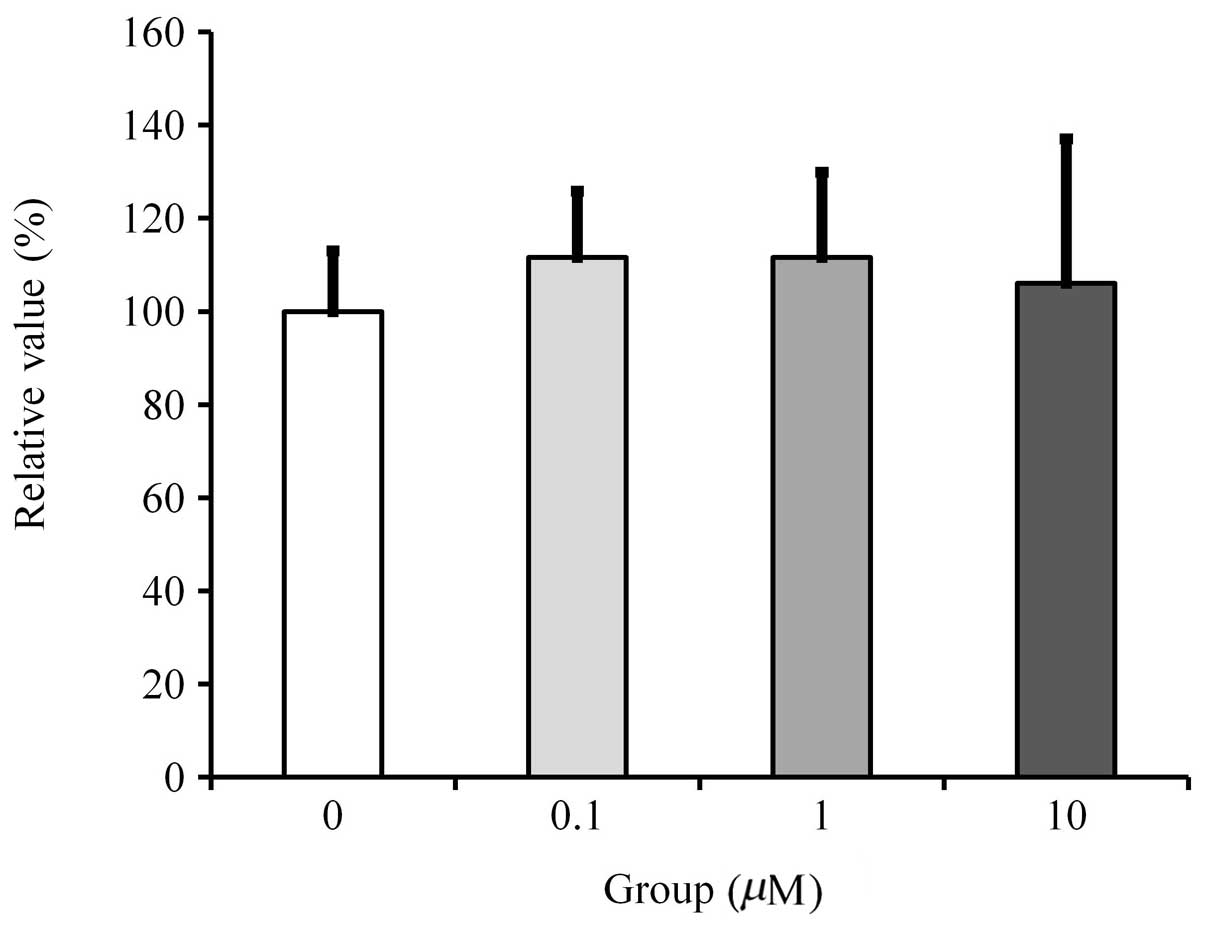
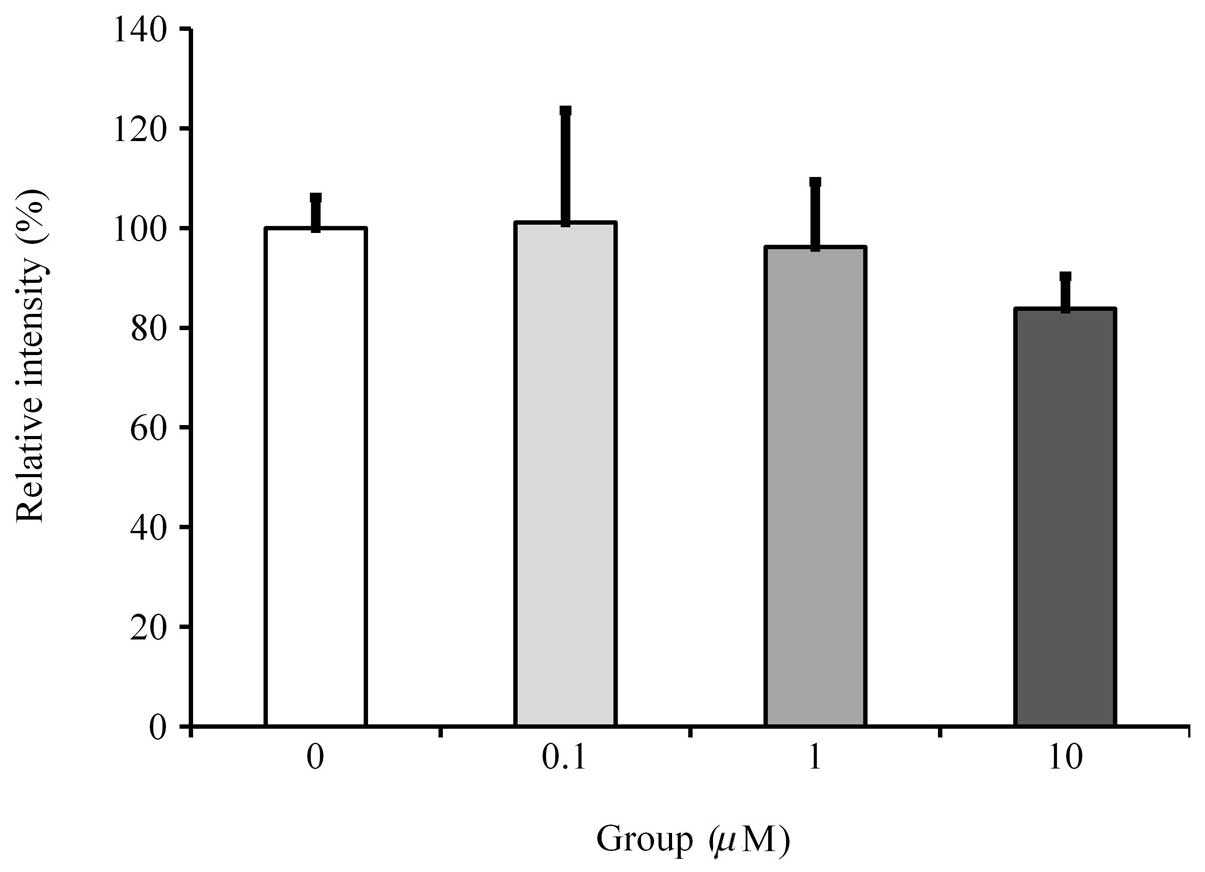
Cultures grown in the presence of fulvestrant at a
concentration of 0.1 to 10 μM did not show any significant change
in cell proliferation (Fig.
1).
Viability/proliferation of the cells with
protein measurement
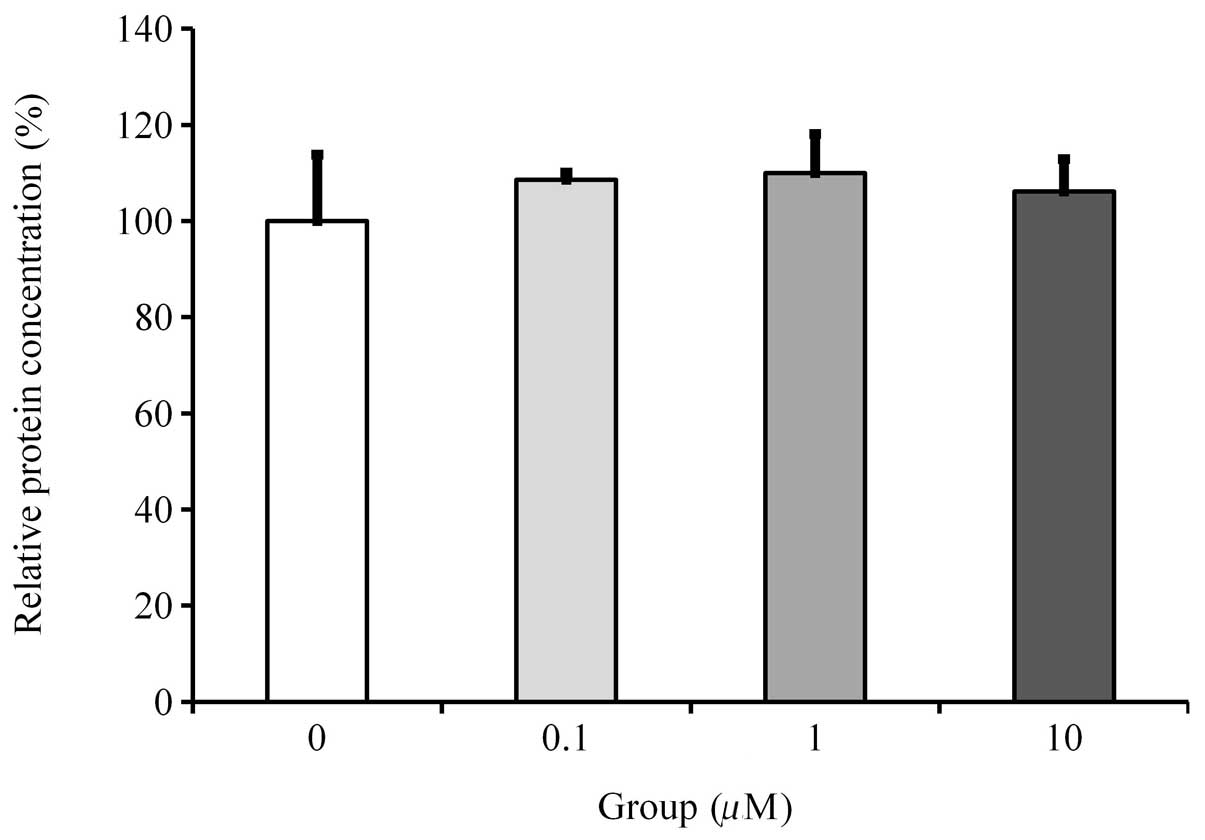
The protein concentration of the cultures grown in
osteogenic differentiation medium at Day 14 did not show any
significant differences between the observed groups (Fig. 2).
ALP activity assays
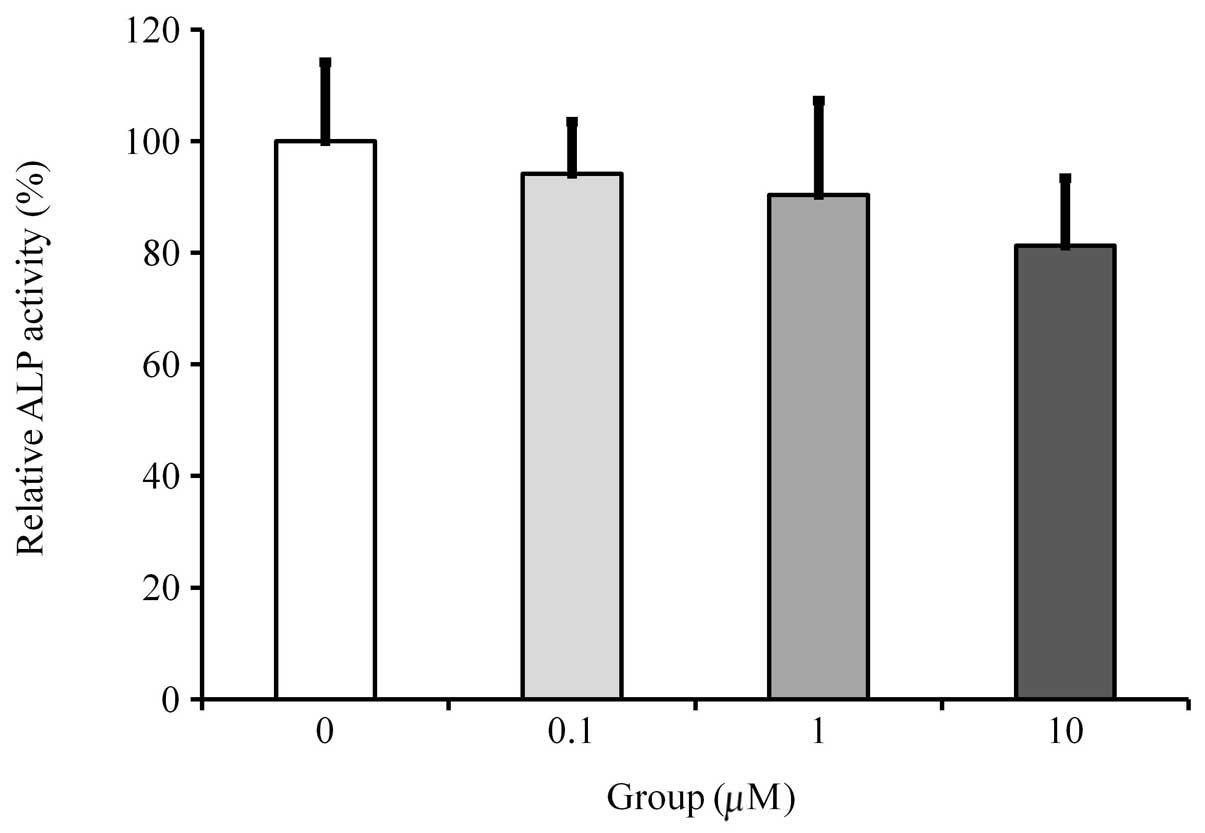
Cultures growing in the absence of fulvestrant
exhibited the highest value for the ALP activity. Cultures grown in
the presence of fulvestrant showed dose-dependent reductions in ALP
activity, however, statistically significant differences were not
achieved in the observed groups (Fig.
3).
Mineralization/calcium deposition
assay
Cultures without fulvestrant showed the highest rate
of mineralized nodule formation. Results showed that cultures grown
in the presence of fulvestrant presented with a dose-dependent
reduction in mineralization. However, statistically significant
differences were observed only in the 10 μM concentration group
(Fig. 4).
Western blot analysis
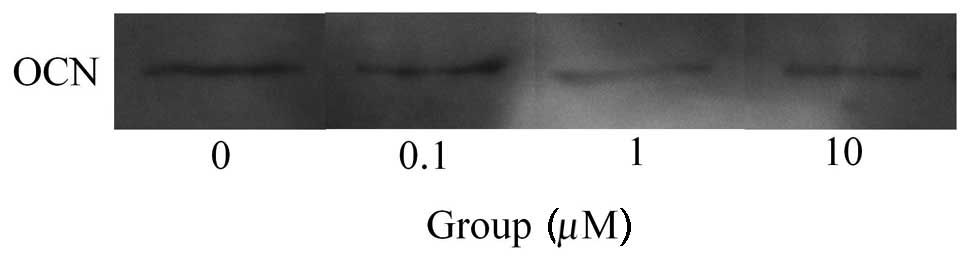
Western blot analysis was performed to detect
protein expression following treatment with fulvestrant (Fig. 5). The results showed that the
addition of fulvestrant decreased the expression of osteocalcin
(Fig. 6). Normalization of the
protein expression demonstrated that the group treated with 10 μM
fulvestrant yielded 83.8±6.5%, when cultures without fulvestrant
were considered to be 100%, however, this did not reach a
statistically significant level (P>0.05).
Discussion
The present study examined the effects of low doses
of fulvestrant on cell viability, and the differentiation and
mineralization of osteoblast progenitor cells under predetermined
concentrations (0.1–10 μM).
Viability and proliferation were evaluated using the
MTT assay and protein concentration. The MTT assay is an extremely
sensitive method for determining cell proliferation since this
assay allows mitochondrial dehydrogenases to oxidize MTT to an
insoluble blue formazan product (17,18).
The protein assay may be considered an indirect measurement of cell
viability as it measures the protein content of the viable cells
remaining after washing the cell plates (19). Equal amounts of DMSO were added to
each culture sample to offset the influence of the dissolving
vehicle (13). The results showed
no significant effect of fulvestrant on cell number. Thus, changes
in protein expression or mineral levels in the groups may be
attributed to changes in the ability of the osteoblasts to produce
minerals rather than to changes in the number of cells present
(9,20).
ALP activity, which is considered to be an early
marker of osteoblastic cell differentiation, was used to determine
osteoblast differentiation (14).
Cultures grown in the presence of fulvestrant showed a
dose-dependent reduction in ALP activity, yet statistically
significant differences were not achieved. Osteocalcin is an
osteoblast-specific gene expressed by fully differentiated
osteoblasts (21). Western blot
analysis showed that 10 μM fulvestrant yielded a decrease in
protein expression, however not at a statistically significant
level. In a previous study, osteocalcin expression was
significantly reduced in 0.1 μM fulvestrant at Day 28, which may be
the result of the longer period of culture (9).
Moreover, cultures grown in the presence of
fulvestrant presented with a dose-dependent reduction in
mineralization with a statistically significant difference at 10 μM
concentration. However, previous studies have showed that treatment
with fulvestrant alone at either 0.1 or 10 μM did not result in
significant changes in mineralization (9). The various responses to fulvestrant
may be attributed in part to the culturing period, the stage of
differentiation of the cells or the culturing condition (22).
The effect of fulvestrant on bone mineralization
remains controversial (5,9). Fulvestrant reduced bone volume at the
proximal tibial metaphysis by ~30%, associated with an increase in
osteoclast surface, while the authors suggested that this was
attributable to antagonization of the actions of estrogen (23). Likewise, the increase in calcium
content by 17β-estradiol was completely inhibited by the
fulvestrant (24), and
co-treatment of estrogen and fulvestrant resulted in a significant
reduction of mineralization (9).
However, bone mineral density in rats was not altered by
administration of fulvestrant at anti-uterotrophic doses, unlike
the significant reduction that occurred following an ovariectomy
(25), and mineralization by
osteoblastic cells was not altered in an estrogen-deficient
environment using calvarial osteoblasts (MC3T3 cells). Further
elucidation of the mechanisms by which fulvestrant affects bone may
have the potential to improve the clinical management of
fulvestrant usage.
These findings support the conclusion that the use
of fulvestrant may have negative effects on the mineralization of
osteoprecursor cells and that the long-term use of fulvestrant may
have detrimental effects on osteoblastic activity.
Acknowledgements
This study was supported by the Bioethics Expert
Development Fund of the Committee for Life in the Archdiocese of
Seoul.
References
|
1
|
Dechering K, Boersma C and Mosselman S:
Estrogen receptors alpha and beta: two receptors of a kind? Curr
Med Chem. 7:561–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song C, Wang J, Song Q, et al: Simvastatin
induces estrogen receptor-alpha (ER-alpha) in murine bone marrow
stromal cells. J Bone Miner Metab. 26:213–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JB: Effects of low dose of estrone on
the proliferation, differentiation and mineralization of
osteoprecursor cells. Exp Ther Med. 4:681–684. 2012.PubMed/NCBI
|
|
4
|
Dauvois S, Danielian PS, White R and
Parker MG: Antiestrogen ICI 164,384 reduces cellular estrogen
receptor content by increasing its turnover. Proc Natl Acad Sci
USA. 89:4037–4041. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curran M and Wiseman L: Fulvestrant.
Drugs. 61:807–814. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osborne CK, Wakeling A and Nicholson RI:
Fulvestrant: an oestrogen receptor antagonist with a novel
mechanism of action. Br J Cancer. 90(Suppl 1): S2–S6. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fawell SE, White R, Hoare S, Sydenham M,
Page M and Parker MG: Inhibition of estrogen receptor-DNA binding
by the ‘pure’ antiestrogen ICI 164,384 appears to be mediated by
impaired receptor dimerization. Proc Natl Acad Sci USA.
87:6883–6887. 1990.
|
|
8
|
Nicholson RI, Gee JM, Manning DL, Wakeling
AE, Montano MM and Katzenellenbogen BS: Responses to pure
antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and
-resistant experimental and clinical breast cancer. Ann NY Acad
Sci. 761:148–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brennan O, O’Brien FJ and McNamara LM:
Estrogen plus estrogen receptor antagonists alter mineral
production by osteoblasts in vitro. Horm Metab Res. 44:47–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lykkesfeldt AE, Larsen SS and Briand P:
Human breast cancer cell lines resistant to pure anti-estrogens are
sensitive to tamoxifen treatment. Int J Cancer. 61:529–534. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Cupis A, Noonan D, Pirani P, Ferrera A,
Clerico L and Favoni RE: Comparison between novel steroid-like and
conventional nonsteroidal antioestrogens in inhibiting oestradiol-
and IGF-I-induced proliferation of human breast cancer-derived
cells. Br J Pharmacol. 116:2391–2400. 1995.
|
|
12
|
Robinson JA, Harris SA, Riggs BL and
Spelsberg TC: Estrogen regulation of human osteoblastic cell
proliferation and differentiation. Endocrinology. 138:2919–2927.
1997.PubMed/NCBI
|
|
13
|
Park JB, Zhang H, Lin CY, et al:
Simvastatin maintains osteoblastic viability while promoting
differentiation by partially regulating the expressions of estrogen
receptors α. J Surg Res. 174:278–283. 2012.PubMed/NCBI
|
|
14
|
Park JB: The effects of dexamethasone,
ascorbic acid, and β-glycerophosphate on osteoblastic
differentiation by regulating estrogen receptor and osteopontin
expression. J Surg Res. 173:99–104. 2012.
|
|
15
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances differentiation of
osteoblasts by regulating the expression of phospho-Smad1/5/8. Exp
Ther Med. 4:303–306. 2012.PubMed/NCBI
|
|
16
|
Park JB: Effects of fibroblast growth
factor 2 on osteoblastic proliferation and differentiation by
regulating bone morphogenetic protein receptor expression. J
Craniofac Surg. 22:1880–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JB: Effects of doxycycline,
minocycline, and tetracycline on cell proliferation,
differentiation, and protein expression in osteoprecursor cells. J
Craniofac Surg. 22:1839–1842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almazin SM, Dziak R, Andreana S and
Ciancio SG: The effect of doxycycline hyclate, chlorhexidine
gluconate, and minocycline hydrochloride on osteoblastic
proliferation and differentiation in vitro. J Periodontol.
80:999–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fotakis G and Timbrell JA: In vitro
cytotoxicity assays: comparison of LDH, neutral red, MTT and
protein assay in hepatoma cell lines following exposure to cadmium
chloride. Toxicol Lett. 160:171–177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu Q, Perälä-Heape M, Kapanen A, et al:
Estrogen enhances differentiation of osteoblasts in mouse bone
marrow culture. Bone. 22:201–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi H, Aguiar DJ, Williams SM, La Pean A,
Pan W and Verfaillie CM: Identification of genes responsible for
osteoblast differentiation from human mesodermal progenitor cells.
Proc Natl Acad Sci USA. 100:3305–3310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: an in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallagher A, Chambers TJ and Tobias JH:
The estrogen antagonist ICI 182,780 reduces cancellous bone volume
in female rats. Endocrinology. 133:2787–2791. 1993.PubMed/NCBI
|
|
24
|
Sato K, Nohtomi K, Shizume K, et al: 17
beta-estradiol increases calcium content in fetal mouse parietal
bones cultured in serum-free medium only at physiological
concentrations. Bone. 19:213–221. 1996. View Article : Google Scholar
|
|
25
|
Wakeling AE: The future of new pure
antiestrogens in clinical breast cancer. Breast Cancer Res Treat.
25:1–9. 1993. View Article : Google Scholar : PubMed/NCBI
|