Introduction
Anterior cruciate ligament reconstruction has been
performed on autologous tendons for a long time, however, complete
healing of the graft is difficult. Regardless of whether the graft
is autologous or allogeneic, the healing process following
reconstruction includes graft necrosis, cellular migration,
revascularization and remodeling (1). Compared with the contralateral normal
tendon, regenerated tendons contain less glycosaminoglycan and
collagen. Regenerated tendons transmit the external force by the
myotendinous junction, however, conduction velocities are slower
than that of contralateral normal tendons and their maximum
anti-drag force is 32% of that of contralateral normal tendons
(2). Therefore, a new method of
tendon reconstruction must be designed to ensure that regenerated
tendons resemble the tissue structures and biomechanics of normal
tendons more closely.
Materials and methods
Ethics
All study methods were approved by the ethics
committee of the First People’s Hospital of Guangzhou (Guangzhou,
China).
Animals and equipment
Forty-eight New Zealand adult white rabbits of
either gender (age, 12 or 16 months old; weight, 2.0–3.0 kg) were
provided by the Experimental Animal Center of Guangzhou Chinese
Medical University (Guangzhou, China). The Olympus electron
microscope was purchased from Shinjuku Monolith (Tokyo, Japan), the
incubator was from Tai Site Test Equipment Co., Ltd. (Nanjing,
China), the histotome was purchased from Cixi Yide Electronic
Instrument Co. (Shenyang, China), the electric oven was provided by
Xinghuo Electric Factory (Shanghai, China) and the water-heated
incubator (220 V, 200–600°C) was obtained from Guoguang Medical
Instrument Factory (Shanghai, China).
Grouping
The 48 New Zealand white rabbits were randomly
divided into groups A and B (each group with 24 rabbits) and used
as models of anterior cruciate ligament reconstruction. In group A,
transforming growth factor (TGF)-β1 was locally injected into the
bone tunnel, while in group B, empty vector was injected. Tendons
were removed from 8 rabbits to observe the histology and
ultrastructure and to evaluate biomechanics at postoperative months
1, 3 and 6 in the 2 groups.
Gene-activated matrix (GAM)
preparation
GAM was prepared by mixing 6 μl liposomes
(Invitrogen Life Technologies, Carlsbad, CA, USA) and 6 μg
EGFP-N1-hTGF-β1 plasmids (Gene Institutes, Guangzhou, China) at
room temperature and incubating for 15–30 min. Following this, the
mixture was administered to group A. Liposomes (6 μl) and 6 μg
EGFP-N1-hTGF (empty vector) was used in group B.
Surgical procedures
Rabbits were anesthetized by ear vein injection of
3% pentobarbital sodium (3 mg/kg) and placed in the supine
position. With the patella as the centre, a 6-cm incision was made
to expose the joint capsule. The joint capsule was opened and,
following removal of the infrapatellar fat pad, the patella was
raised to completely excise the anterior cruciate ligament. The
semitendinosus insertion was exposed and retained. The proximal
semitendinosus was separated and the myotendinous junction was cut.
Interlacing suture was performed with silk suture in the free end
of the semitendinosus to be used for tendon graft. The tibial
tunnel was constructed with a 5-mm biopsy needle entering 5 mm
above the semitendinosus insertion inside the tibial tubercle and
exiting from the tibial plateau insertion of the anterior cruciate
ligament. Similarly, the femoral tunnel was constructed entering
from the fossa intercondylaris femoris of anterior cruciate
ligament and exiting from the lateral condyle of the femur. The
semitendinosus graft was passed through the tibial and femoral
tunnels and fixed by suture with the knee lateral collateral
ligament and surrounding soft tissue, performing anterior cruciate
ligament reconstruction.
In group A, 2 mm3 GAM was locally
injected into the bone tunnel with a 1-mm diameter needle, while in
group B, 2 mm3 empty vector was injected.
The incision was closed and then the surgical limb
was fixed in an extended position with a plaster cast. The plaster
external fixation was removed 2 weeks later and the local
conditions and limb activity were observed.
Histological observation of tendons
HE-stained tissue sections were generated to observe
collagen fiber morphology, tendon cells, matrix content, the number
of abnormal cells and fibrous bands.
The tissue samples were prepared for transmission
electron microscopy by cutting the tendons into 5×4-mm tissue
samples, fixing with 3% glutaral (0.2 M/l) for 2 h, washing with
0.2 M/l PBS 3 times every 15 min and fixing again with 1% osmic
acid (0.2 M/l) for 2 h. The tissue underwent gradient dehydration
with 30, 50, 70 and 90% alcohol, a mixture of 90% alcohol and 90%
acetone and 90% acetone and 100% acetone, each time for 10–15 min.
Dehydration was performed 3 times. Finally, the tissue was soaked
in 100% acetone for 2 h, followed by slicing. Sections were stained
with uranyl acetate for 10–15 min, washed with water 1–2 times,
stained with lead nitrate for 10–15 min and washed with water.
Cellular and nuclear ultrastructures were observed.
Biomechanical analysis of tendons
In the surgical limb, complete thigh and tarsal
amputation were performed to retain the femur and tibia. With the
exception of the anterior cruciate ligament, all other ligaments,
skin, muscle and joint capsule of the knee joint were removed. The
ends of the bone were fixed with 3-cm Kirschner wires and embedded
in denture base resin for biomechanical analysis.
For biomechanical analysis, specimens were loaded
into a MTS-858 biological material testing machine. There was a
computer in front of the testing machine and data were produced as
a direct readout by the computer. The decay rate was set at 1, the
measuring range of sensor at 25 N and the loading speed at 10
mm/min. The testing machine was operated until tendons were broken
and the maximum force, displacement of maximum load, the stress
force, strain and elastic modulus were recorded.
Statistical analysis
Statistical analysis was performed using SPSS 12.0
software. Numerical data are presented as the mean ± SD.
Homogeneity test for variance was used and significant values were
analyzed further by t-tests. Measurement data are presented as
constituent ratios (n%). A Chi-square test of 4-fold table was used
in the comparison of the groups. When the theoretical frequency was
<5, the Chi-square test correction formula was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
General conditions of transplanted
tendons
Graft rupture occurred in one rabbit in group A at
postoperative month 1. In group B, no graft rupture occurred. The
overall success rate of grafts was 98.6%.
Gross observation on successfully
transplanted tendons
Gross morphology was similar between the 2
groups.
Histological observation on transplanted
tendons
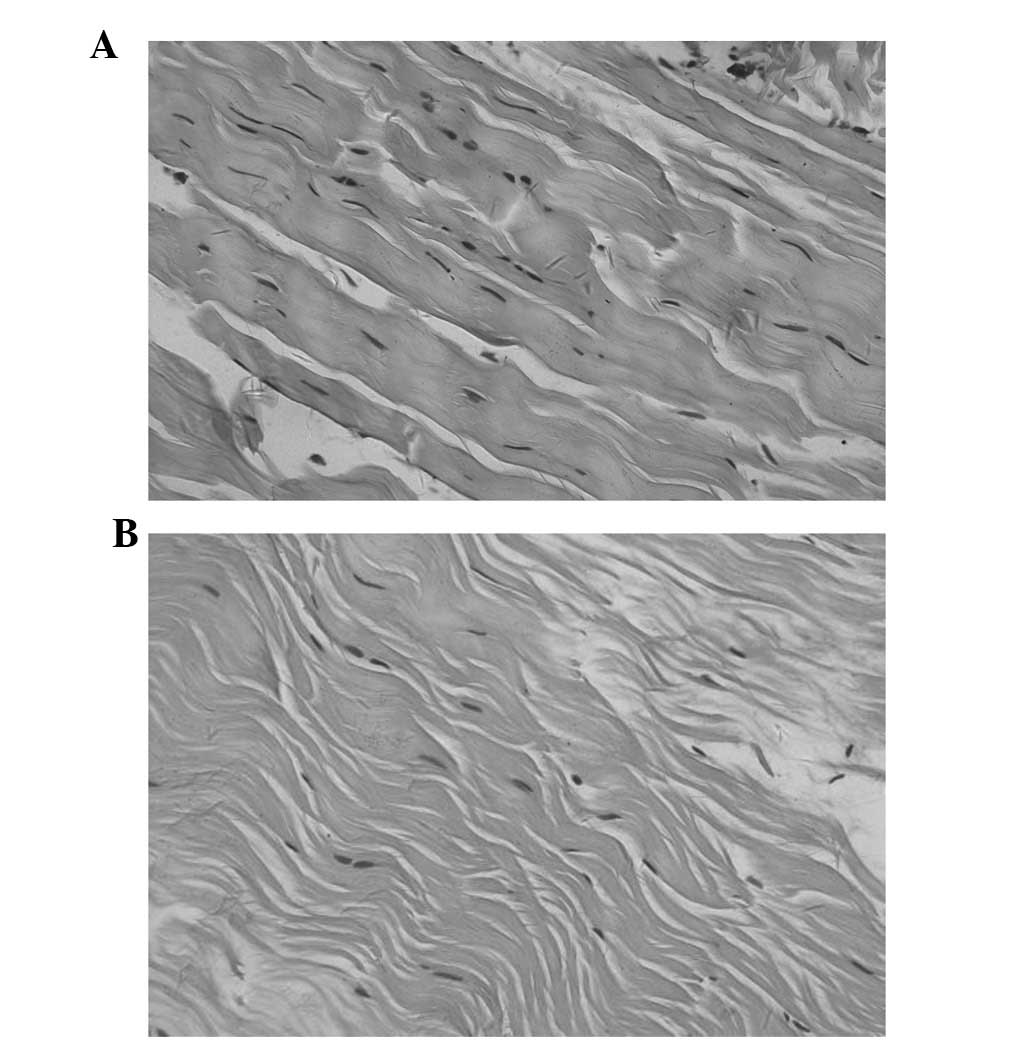
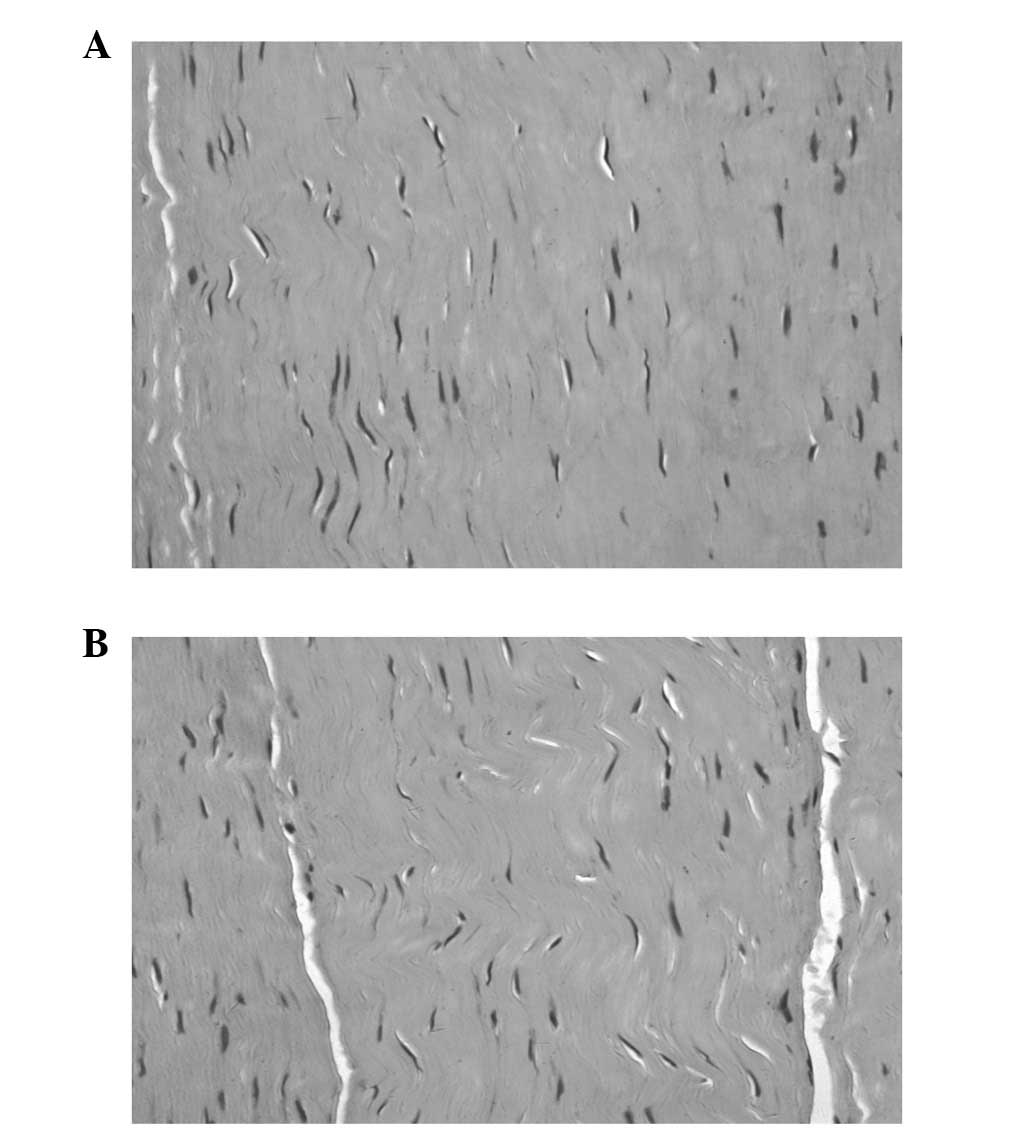
Optical microscopy was used to observe the 4
morphological stages, including tissue necrosis, inflammatory
infiltration, cell regeneration and tissue molding. In group B, a
reduced number of fibroblasts and collagen fibers was observed at
each time-point. In group A, there were more fibroblasts and
collagen fibers and postoperative month 6 tissue structures were
similar to normal tissue (Figs. 1
and 2).
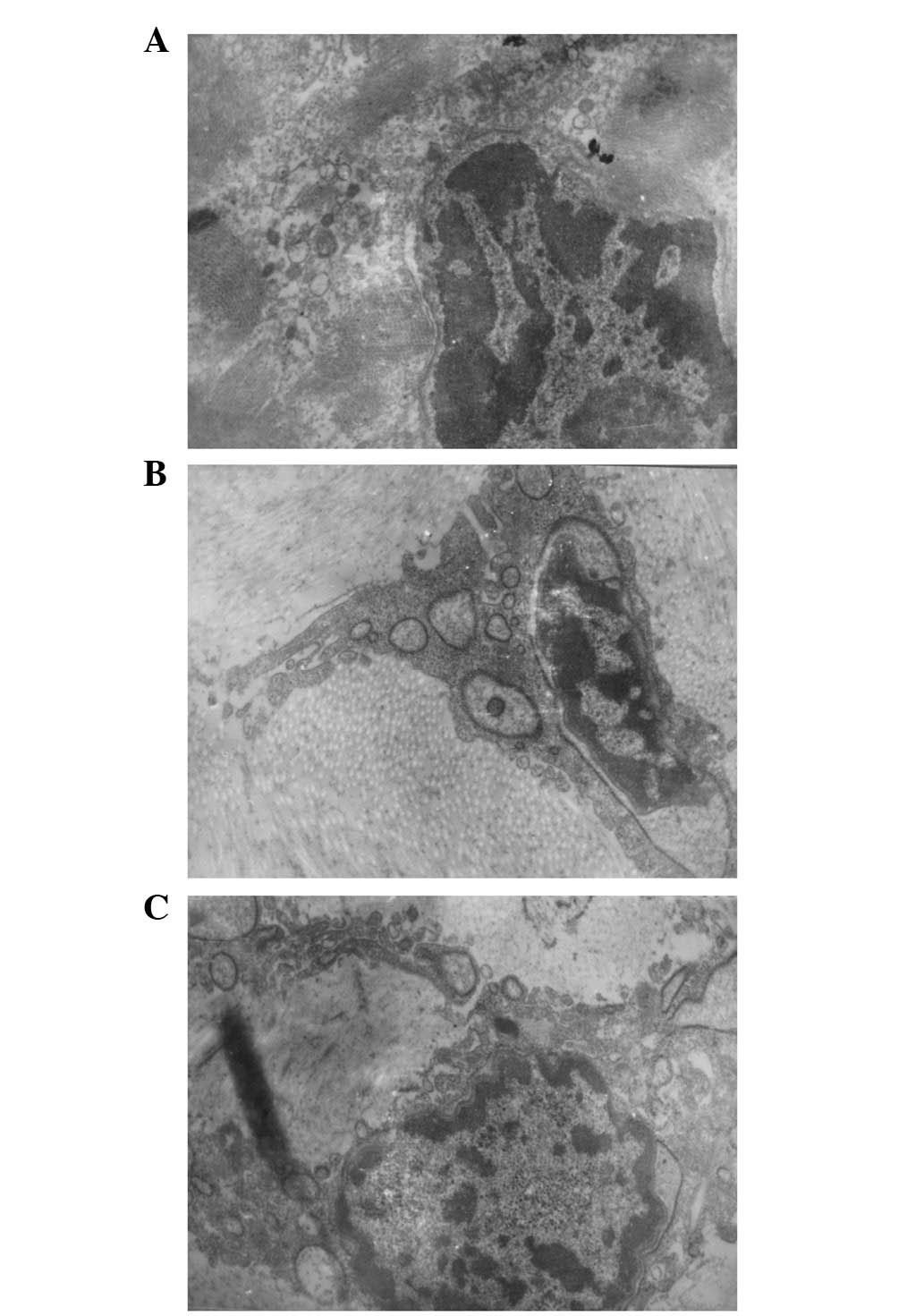
At postoperative month 1, mainly reticular fibers
were observed by electron microscopy, collagen fibers were reduced,
liposomes and necrotic tissue were present, nuclear shrinkage,
accumulation of abnormal chromatin and mitosis were noted and
cellular proliferation was active. At postoperative month 3,
collagen fibers were markedly increased and active mitosis and cell
proliferation were present. Electron microscopy identified
increased levels of mitosis and an abundance of cellular matrix,
endoplasmic reticulum and mitochondria in group A at each
time-point compared with B (Figs.
3 and 4).
Biomechanical results
No statistical difference in each biomechanical
parameter between the 2 groups at postoperative month 1 was
identified. However, at postoperative months 3 and 6, maximum force
and elastic modulus were found to be significantly greater in group
A than in B (P<0.0.5), however, other biochemical parameters
were not identified to be significantly different between the 2
groups (Tables I–III).
 | Table IPostoperative month 1 biomechanical
results in the groups (n=8). |
Table I
Postoperative month 1 biomechanical
results in the groups (n=8).
| Group | MBL (N) | DML (mm) | SFML
(N/mm2) | Strain (%) | EM
(N/mm2) |
|---|
| A | 21.77±2.56 | 13.16±2.45 | 1.288±0.37 | 62.37±8.67 | 2.064±0.215 |
| B | 20.26±2.31 | 13.42±2.61 | 1.199±0.32 | 63.61±7.94 | 1.885±0.223 |
 | Table IIIPostoperative month 6 biomechanical
results in the groups (n=8). |
Table III
Postoperative month 6 biomechanical
results in the groups (n=8).
| Group | MBL (N) | DML (mm) | SFML
(N/mm2) | Strain (%) | EM
(N/mm2) |
|---|
| A | 54.87±3.26a | 11.97±2.52 | 2.23±0.27 | 55.88±7.86 | 3.990±0.156a |
| B | 47.15±4.29 | 12.15±2.38 | 2.14±0.30 | 57.58±7.94 | 3.715±0.144 |
Discussion
A variety of autologous tendon grafts are currently
used to reconstruct the anterior cruciate ligament, however,
complete graft-host healing is difficult and a number of severe
complications, including relaxation and rupture of the autologous
tendon graft, are associated with the proceedure.
TGF-β1 possesses a number of biological functions.
The growth factor promotes the synthesis of I, III, V and VI type
collagen and glycoproteins and improves the differentiation
potential of mesenchymal stem cells into chondrocytes and
osteocytes. TGF-β1 promotes tissue repair by increasing fibroblast
and extracellular matrix synthesis and inhibiting the degradation
of newly synthesized matrix (3).
TGF-β1 increases angiopoiesis and induces fibroblast, monocyte and
macrophage migration to sites of injury, promoting ligament
healing. A previous study reported TGF-β1 expression in the rabbit
anterior cruciate and medial collateral ligaments 10 days prior to
healing (4), and local application
of exogenous TGF-β1 has been hypothesized to increase ligamentous
strength, stiffness and anti-drag force (5).
However, TGF-β1 possesses low bioavailability due to
fast metabolism in vivo and immunogenicity which affects
in vivo application (6).
Gene plasmids store cytokines in vectors in DNA form, delivering a
more stable cytokine with lower immunogenicity and higher
expression. In traditional gene transfection techniques, host cells
are removed and incubated in vitro, transfected with the
target gene and then re-transplanted into the host. This method is
complex, expensive and requires an unsuitable time-frame for repair
of acute ligament injury.
In previous years, in situ tissue engineering
has been researched and developed and has demonstrated promise for
use in a number of acute injury repair procedures (7). In situ tissue engineering
involves the combination of biological materials with DNA plasmids
to develop a GAM gene delivery system. The GAM system is then
transplanted into the defective tissue where endogenous cells enter
the matrix and are transfected with the plasmids. The transfected
cells secrete plasmid-encoded protein products functioning as local
in vivo bio-reactors. This system is known as a local gene
delivery carrier (8). In
situ gene engineering is associated with a number of
advantages, including simple procedures, high bioavailability and
reduced immunogenicity.
The traditional viral vector possesses marked
transfection efficiency. However, it is expensive and easy to
trigger a severe immune response and is therefore difficult to use
in clinical practice (9).
Non-viral vectors include liposome and polymeric vectors and are
considered cheap, simple, safe, biodegradable and slow-release.
Previously, biomaterials, including collagen, hyaluronic acid,
alginates, chitosan and polylactide-co-glycotide, have been used as
vectors for repair of bone or cartilage defects. Promising results
have been reported, however, limited studies have been performed on
their use in ligament repair (10–13).
Ligament restoration following injury is mediated by
a non-specific pathological process associated with filling and
organization of granulation and fibrous tissue. At present,
repaired ligaments take an extremely long time to return to the
strength levels of normal ligaments due to decreased collagen and
disorderly arrangement. A previous study demonstrated that
cytokines increased type I and III collagen expression (mainly type
I collagen) during the ligament repair process and strength
associated with normal ligaments was obtained within a shorter
healing period (14). Therefore,
promoting collagen secretion is an effective means to improve the
quality of ligament repair. However, in vitro proliferation
of ligament cells is slow and cells reach quiescence following
several passages due to ligament cell differentiation, rendering
them unsuitable for in vitro culture as seed cells for
tissue engineering (15).
Based on the non-specific pathological process of
post-injury ligament, we hypothesized that local application of GAM
to the interface of ligament graft and bone tunnel may be an
important step in in situ tissue engineering. GAM grafts
release TGF-β1 slowly and maintain higher levels of TGF-β1 in the
local environment in vivo, enabling fibroblasts to enter the
GAM within 3–4 weeks and secrete large quantities of type I and III
collagen to promote healing of the ligament graft. In the present
study, an increased number of fibroblasts and collagen fibers was
identified by optical microscopy in group A at each time-point
compared with B. Electron microscopy indicated increased levels of
mitosis and an abundance of matrix, endoplasmic reticulum and
mitochondria in group A at each time-point compared with B.
Histological results indicate that the post-healing ligament graft
transfected by TGF-β1 in situ represents a normal ligament
more closely and demonstrates that TGF-β1 promotes cell
proliferation, differentiation and synthesis of collagen, processes
vital to ligament healing.
In the current study, the maximum force was observed
as significantly greater in group A compared with B (P<0.0.5) at
postoperative months 3 and 6, demonstrating the efficacy of in
situ transfection.
In summary, results indicate that GAM promotes
healing of rabbit anterior cruciate ligament grafts and provide a
theoretical basis for GAM application in ligament repair.
Acknowledgements
The present study was supported by the Key Project
of Medical Science of Guangzhou City (2009-ZDi-20) and the Science
and Technology Project of Guangdong Province (2007B031001003).
References
|
1
|
Duthon VB, Messerli G and Menetrey J:
Anterior cruciate ligament reconstruction: indications and
techniques. Rev Med Suisse. 4:2744–2748. 2008.(In French).
|
|
2
|
Chen NC, Brand JC and Brown CH:
Biomechanics of intratunnel anterior cruciate ligament graft
fixation. Clin Sports Med. 26:695–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sporn MB and Roberts AB: Peptide growth
factors and inflammation, tissue repair and cancer. J Clin Invest.
78:329–332. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Chamberlin TA, Schreck PJ, et al:
In situ localization of growth factors during the early healing of
knee ligaments. Trans Orthop Res Soc. 20:158–162. 1995.
|
|
5
|
Conti NA and Dahners LE: The effect of
exogenous growth factors on the healing of ligaments. Trans Orthop
Res Soc. 18:601993.
|
|
6
|
Shimomura T, Jia F, Niyibizi C and Woo SL:
Antisense oligonucleotides reduce synthesis of procollagen alpha1
(V) chain in human patellar tendon fibroblasts: potential
application in healing ligaments and tendons. Connect Tissue Res.
44:167–172. 2003. View Article : Google Scholar
|
|
7
|
Bonadio J: Tissue engineering via local
gene delivery. Mol Med (Berl). 78:303–311. 2000. View Article : Google Scholar
|
|
8
|
Bonadio J: Tissue engineering via local
gene delivery: update and future prospects for enhancing the
technology. Adv Drug Deliv Rev. 44:185–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans CH and Robbins PT: Genetically
augmented tissue engineering of the musculoskeletal system. Clin
Orthop Relat Res. 367(Suppl): S410–S418. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andree C, Voigt M, Wenger A, et al:
Plasmid gene delivery to human keratinocytes through a
fibrin-mediated transfection system. Tissue Eng. 7:757–766. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anderson DG, Peng W, Akinc A, et al: A
polymer library approach to suicide gene therapy for cancer. Proc
Natl Acad Sci USA. 101:16028–16033. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pascher A, Steinert AF, Palmer GD, et al:
Enhanced repair of the anterior cruciate ligament by in situ gene
transfer: evaluation in an in vitro model. Mol Ther. 10:327–336.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geiger F, Bertram H, Berger I, et al:
Vascular endothelial growth factor gene-activated matrix
(VEGF165-GAM) enhances osteogenesis and angiogenesis in large
segmental bone defect. J Bone Miner Res. 20:2028–2035. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cappelli E, Taylor R, Cevasco M, et al:
Involvement of XRCCI and DNA ligase III gene products in DNA base
excision repair. J Biol Chem. 272:23970–23975. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shea LD, Smiley E, Bonadio J and Mooney
DJ: DNA delivery from polymer matrices for tissue engineering. Nat
Biolechnol. 17:551–554. 1999. View
Article : Google Scholar : PubMed/NCBI
|