Introduction
Gastric cancer is one of the most common
malignancies in the world, and ~800,000 people are diagnosed with
this disease each year (1).
Gastric cancer has a high mortality rate and is the second leading
cause of cancer mortality (2). The
average five-year survival rate for gastric cancer is <10%
(3). Neoadjuvant therapy,
radiotherapy and additional drug therapies coupled with surgery
have greatly improved the survival rate of gastric cancer survival
rate (4). In Western countries,
preoperative and postoperative adjuvant chemotherapies have become
the standard regimen for gastric cancer treatment and in Asia,
postoperative conventional chemotherapy is also performed for
gastric cancer treatment, indicating the importance of
postoperative drug treatment for gastric cancer (5). Conventional chemotherapeutic
treatments include 5-fluorouracil-based combination regimens
containing mitomycin C or anthracycline antibiotics. Although this
treatment regimen has been demonstrated to significantly reduce the
tumour recurrence rate, a number of disadvantages, including
expensive treatment costs and serious side effects, have prevented
many patients from benefiting from this chemotherapy. Therefore,
the identification of a more economical drug associated with fewer
toxic side effects for gastric cancer treatment has become the
focus of research in recent years.
Flavonoids have long been utilised in traditional
medicine and are associated with low toxicity. In addition,
previous studies have identified these compounts to exhibit
significant antitumour effects (6–8).
Alpinetin is a natural flavonoid, primarily found in
Zingiberaceae, including turmeric, cardamom and radix
curcumae(9). A number of
studies have demonstrated that alpinetin has marked antitumour and
inhibitory effects on tumour cell proliferation and inhibits the
growth of numerous types of tumour cells, including breast, colon,
lung, cervical and liver cancer cells (10–12).
However, the inhibitory effects of alpinetin on human gastric
cancer cell growth has not been investigated. In addition, the
mechanisms by which alpinetin mediates its antitumourigenic effects
remain poorly understood.
In the present study, the cytotoxic and
pro-apoptotic effects of alpinetin in human gastric cancer cells
were investigated. Furthermore, the relationship between
alpinetin-induced apoptosis in human gastric cancer cells and the
mitochondrial apoptosis pathway was investigated.
Materials and methods
Materials and chemicals
Alpinetin (≤98% purity) was obtained from the
National Institute for Food and Drug control (Beijing, China).
Propidium iodide (PI), 5,5′,6,6′-tetrachloro-1,
1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) and
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). The Annexin
V-FITC/PI kit was purchased from BD Biosciences (Franklin Lakes,
NJ, USA). All antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Cell culture
Human gastric cell lines AGS (gastric
adenocarcinoma) and N87 (gastric cancer) were purchased from
American Type Culture Collection (Manassas, VA, USA). Following
passaging, AGS cells were cultured in F-12K medium containing 10%
foetal bovine serum (FBS; Hyclone Laboratories, Inc., Logan UT,
USA), 100 U/ml penicillin and streptomycin (Gibco-BRL, Carlsbad,
CA, USA) and N87 cells were cultured in RPMI-1640 medium containing
10% FBS, 100 U/ml penicillin and streptomycin. Cells were cultured
in incubators containing 5% CO2 and 95% O2 at
37°C.
Cell viability
Cells (1×105 cells/ml) were seeded in
96-well plates. Following adherence, cells were treated with
various doses of alpinetin with six replicates for each
concentration. A negative control group without drugs was also
established. Cells were placed in an incubator with a 5%
CO2 atmosphere and incubated for 24, 48 and 72 h prior
to the colorimetric reaction. MTT solution (20 μl; 5 mg/ml) was
added to each well and the plate was incubated for 4 h in a
CO2 atmosphere incubator. Following incubation, culture
medium was removed and 150 μl dimethylsulfoxide (DMSO) was added to
each well and mixed by agitation at room temperature for 10 min.
The optical density (OD; A570 nm) of each well was
measured using a microplate reader.
Detection of apoptosis by Annexin
V-FITC/PI double staining
During the early stages of apoptosis, the cell
membrane loses its symmetry, and phosphatidylserine, which is
normally located in the inner leaflet of the plasma membrane,
becomes exposed on the outer leaflet of the plasma membrane. Cells
were collected using the trypsin digestion method and cell density
was adjusted to 1×106 cells/ml. Annexin V-FITC (5 μl)
and PI (5 μl) were then added. Cells were stained in the dark at
4°C for 30 min, followed by flow cytometry analysis.
Cell cycle analysis
Cells in each experimental group were collected
using the trypsin digestion method, washed with phosphate-buffered
saline (PBS) and fixed overnight in 70% cold ethanol at 4°C. The
ethanol was then discarded followed by a PBS wash. Cell density was
adjusted to 1×106 cells/ml and the final volume was 100
μl. DNAStain comprehensive dye solution (500 μl) containing 50 mg/l
RNase, 100 mg/l PI and 1 ml/l Triton X-100 was added to the cells
which were then placed them in the dark at room temperature for 30
min prior to flow cytometry.
Mitochondrial membrane potential
detection
Cell density was adjusted to 1×106
cells/ml. JC-1 dye (10 μg/ml), which was dissolved in DMSO, was
added to the cells, mixed thoroughly and the cells were incubated
in the dark for 30 min in an incubator at 37°C with 5%
CO2 atmosphere followed by three PBS washes. A flow
cytometer (BD Biosciences) was used for analysis. FL1-H and FL2-H
represented the green and red fluorescence intensity, respectively.
CellQuest analysis software was used for the quantitative
analysis.
Detection of caspase activity
Detection of caspase-3 and -9 activities was
performed as described previously (13). The Perkin-Elmer LS-50B fluorescence
spectrophotometer (Waltham, MA, USA) was used to measure changes in
fluorescence intensity at excitation and emission wavelengths of
380 and 460 nm, respectively. Alterations in caspase activity were
determined by comparing caspase expression levels in the
alpinetin-treated and control groups.
Mitochondria isolation, protein
extraction and western blot analysis
Mitochondria separation, purification and protein
extraction was performed as described previously (14). Following quantification using the
bicinchoninic acid method, samples were loaded into an SDS-PAGE gel
and separated. Proteins were transferred to a polyvinylidene
fluoride membrane using the semi-dry method and the membrane was
blocked overnight in 5% non-fat dry milk at 4°C. Following this,
the membrane was washed in Tris-buffered saline with Tween (TBST),
primary antibodies were added followed by 1 h hybridisation at 37°C
and TBST washes. Secondary antibodies were then added followed by a
1 h hybridisation at 37°C, a TBST wash, 5 min of the chromogenic
reaction and autoradiography. OD values were analysed and
determined using Quantity One software and the results were
expressed as the ratio of the sample OD value to the OD value of
the internal reference.
Statistical analysis
SPSS 16.0 software was used for statistical
analysis. Values are presented as the mean ± SD. Statistical
analysis was performed using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
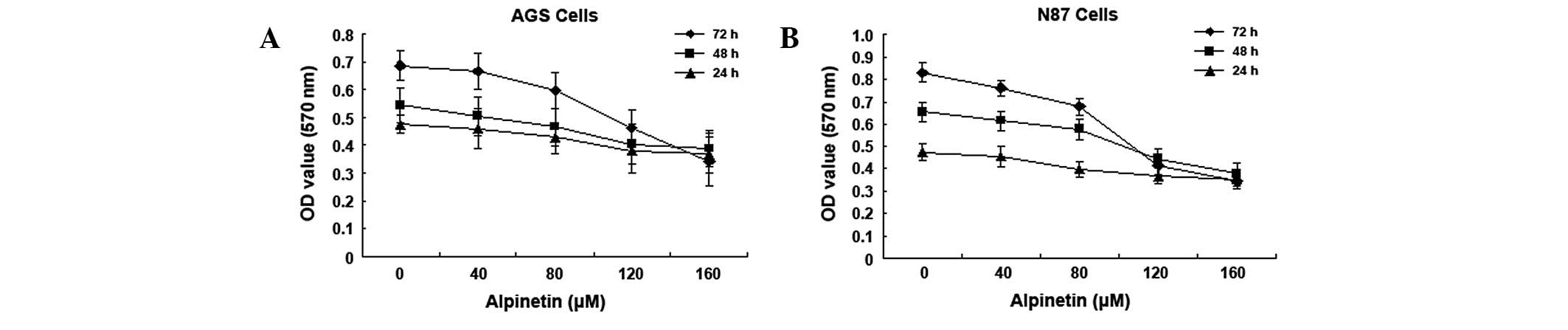
Alpinetin inhibits human gastric cancer
cell proliferation
Various doses of alpinetin (0, 40, 80, 120 and 160
μM) were used to treat human gastric cancer cell lines, AGS and
N87, for 24, 48 and 72 h. Cell viability was measured by MTT assay.
Results demonstrate that alpinetin inhibits human gastric cancer
cell proliferation in a time- and dose-dependent manner (Fig. 1). As the alpinetin concentration
was increased from 0 to 160 μM, the A570 nm value
measured for the human gastric cancer cells gradually decreased and
this decrease was found to be most significant at 120 μM
(IC50).
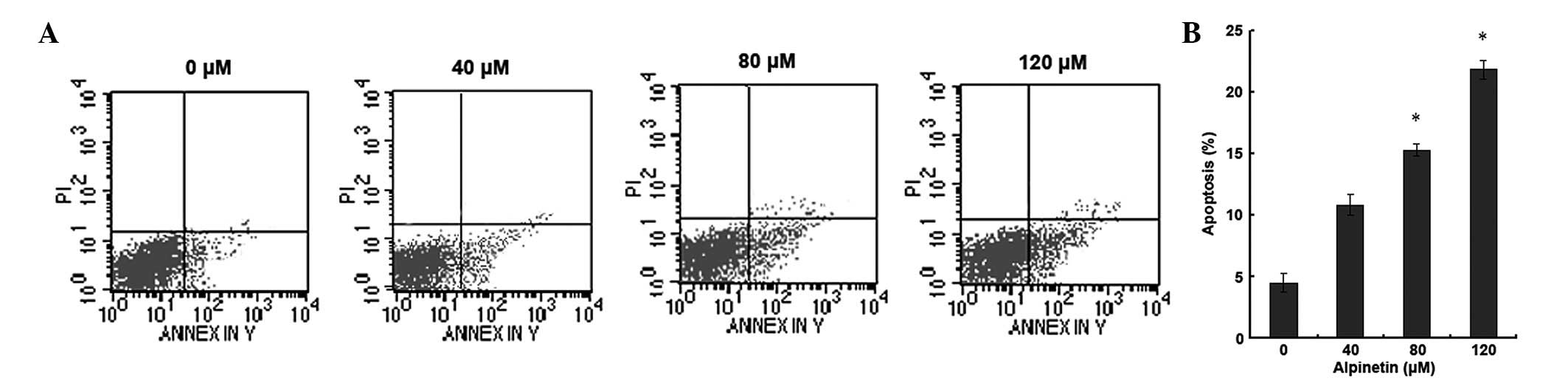
Alpinetin induces apoptosis in human
gastric cancer cells
To investigate whether alpinetin induces apoptosis
in human gastric cancer cells, AGS cells were treated with various
doses of alpinetin for 48 h and flow cytometry was used to detect
apoptosis (Fig. 2). Compared with
the control group, as the alpinetin dose increased, the number of
apoptotic gastric cancer cells significantly increased. These
results indicate that alpinetin induces apoptosis in a
dose-dependent manner and inhibits proliferation of gastric cancer
cells.
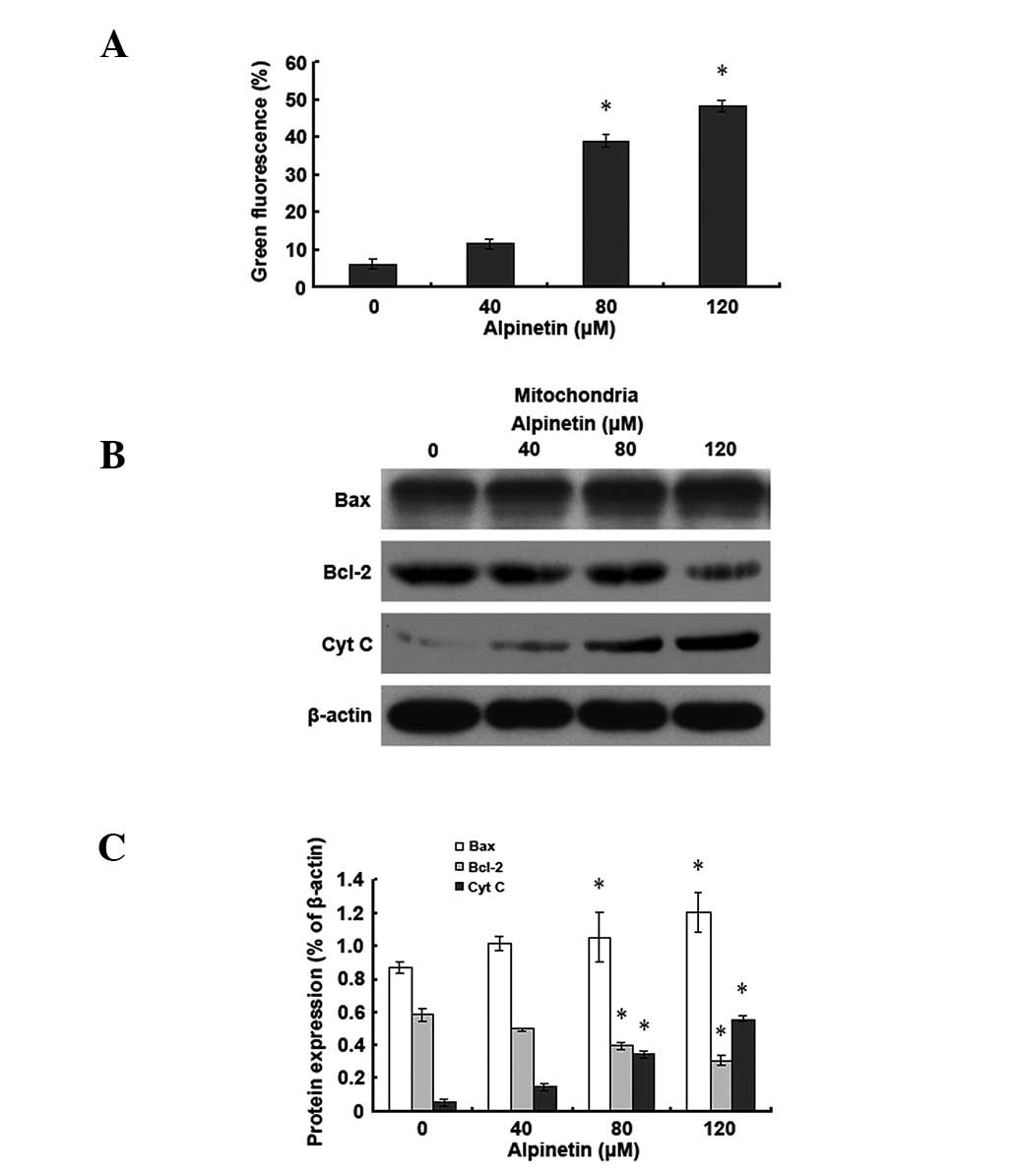
Alpinetin induces apoptosis in human
gastric cancer cells via the mitochondrial pathway
To further understand the molecular mechanisms of
alpinetin-induced apoptosis in human gastric cancer cells, AGS
cells were treated with various doses of alpinetin for 48 h.
Western blot analysis was used to detect changes in levels of
Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2) and
cytochrome C (Cyt C) and JC-1 staining was applied to detect
changes in mitochondrial membrane potential (Fig. 3). Results demonstrated that,
compared with the control group, as the alpinetin dose increased,
mitochondrial Bax levels increased and Bcl-2 levels decreased in
the alpinetin treatment group. Mitochondrial membrane potential was
subsequently decreased and cytosolic Cyt C levels increased
gradually. Observations indicate that alpinetin promotes Bax and
Bcl-2 translocation, leading to reduced mitochondrial membrane
potential and release of Cyt C into the cytoplasm, ultimately
leading to apoptosis of human gastric cancer cells.
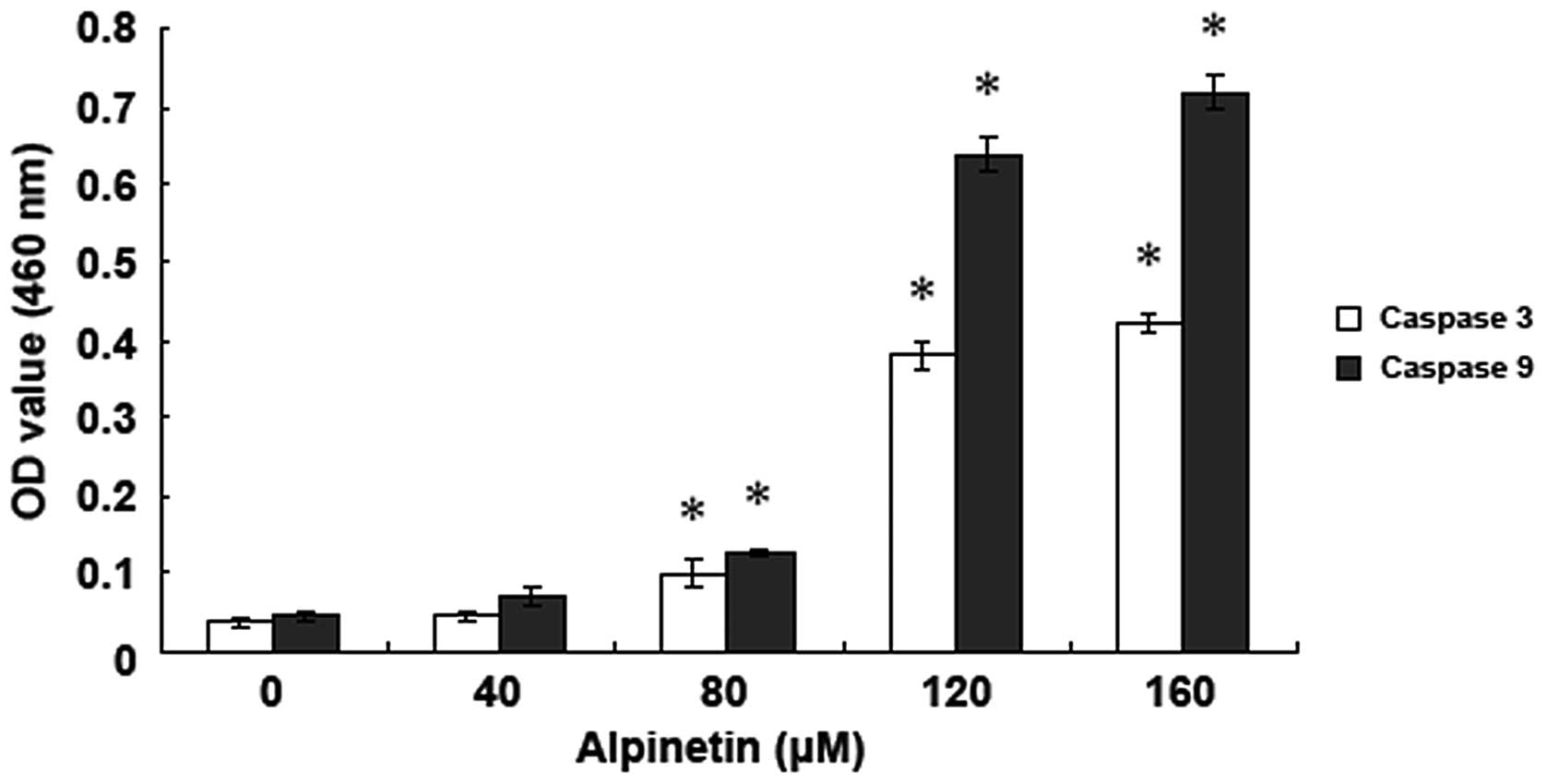
Alpinetin activated caspase in human
gastric cancer cells
To detect the effect of alpinetin on the caspase
family of apoptotic proteins, AGS cells were treated with various
doses of alpinetin for 48 h and changes in caspase-3 and -9
activity were detected using a fluorescence spectrophotometer
(Fig. 4). Data demonstrate that,
following treatment of AGS cells with various doses of alpinetin,
caspase-3 and -9 activities were significantly increased and peaked
at 120 μM, indicating that alpinetin induces activation of caspase
family members and caspase-dependent apoptosis in AGS cells.
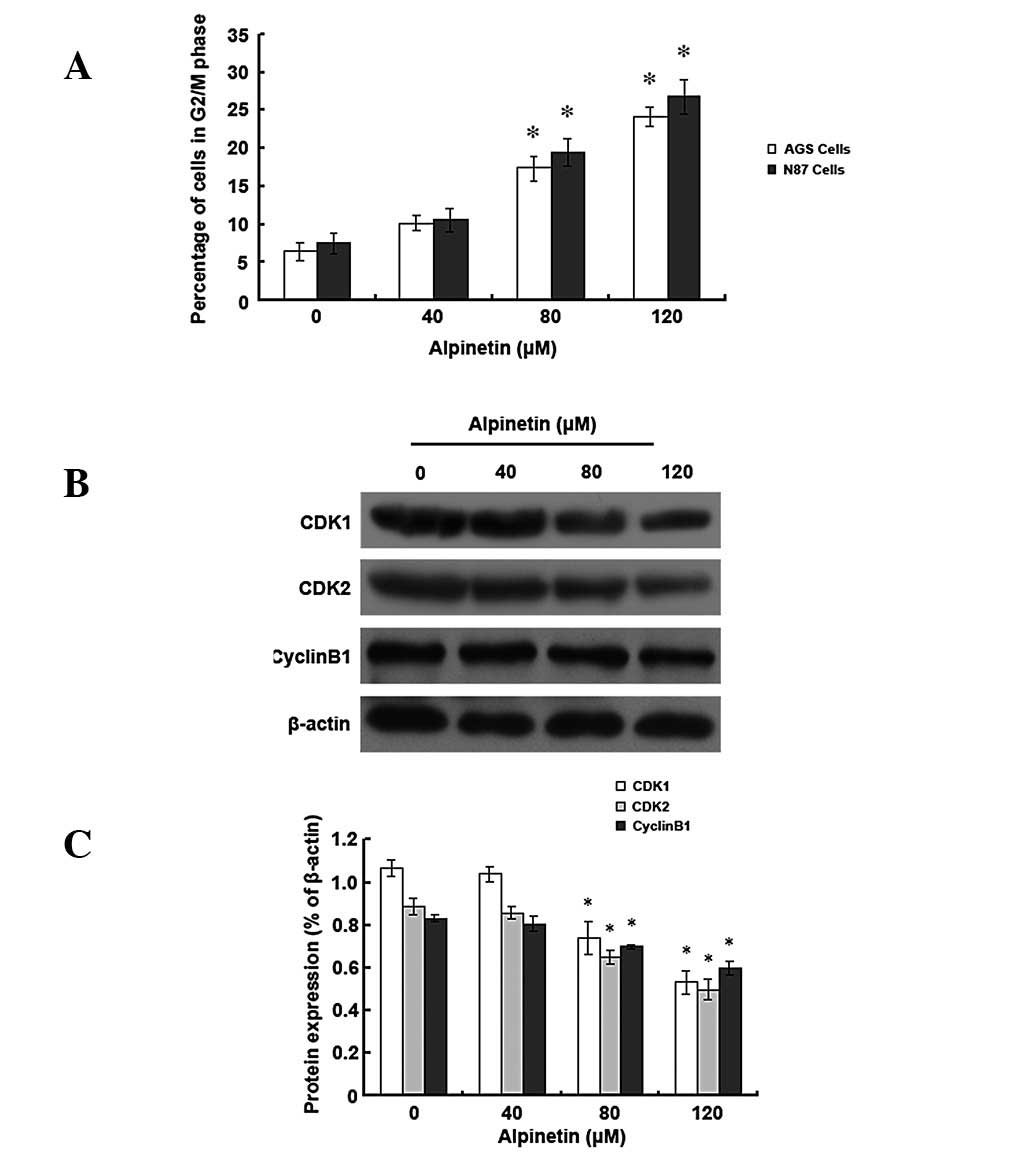
Alpinetin induces cell cycle arrest at
the G2/M phase in human gastric cancer cells
To investigate whether alpinetin regulates cell
cycle distribution of human gastric cancer cells, AGS and N87 cells
were treated with various alpinetin doses for 48 h and flow
cytometry was performed to detect cell cycle progression (Fig. 5). Results revealed that, as the
alpinetin dose increased, the number of AGS and N87 cells entering
the G2/M phase increased and the majority of the cells
were blocked at the G2/M phase in the
alpinetin-treatment group compared with the control group.
Furthermore, we found that protein expression levels of
cyclin-dependent kinase (CDK) 1 and 2 and cyclin B1 were
significantly reduced. Our data indicate that the alpinetin-induced
anti-proliferative effect may also be mediated by G2/M
phase arrest.
Discussion
Gastric cancer is a common malignancy. Each year,
~800,000 individuals are diagnosed with gastric malignancies
worldwide, accounting for 9% of newly diagnosed malignancies
(15). The gastric cancer
mortality rate ranks second among all malignant tumours. Advances
in research over the past 10 years have led to a gradual
improvement in diagnosis of gastric cancer; however, the efficacy
of gastric cancer treatments remains poor (16). Although surgical resection is the
primary gastric cancer treatment method, it has long been
recognised that malignant tumours are systemic diseases. Surgery
only removes the tumour and the post-operative recurrence rate
remains high. Therefore, drug therapies specifically targeting
gastric cancer which aim to decrease the gastric cancer recurrence
rate have become an important area of research. Although
chemotherapeutic drugs have been demonstrated to reduce the
recurrence rate of gastric cancer, adverse reactions in patients
caused by chemotherapeutic drugs, including bone marrow
suppression, cannot be ignored.
Previous studies have identified a number of
features in plant-derived antitumour drugs, including diversity,
fewer side effects and adverse reactions. Flavonoids have been
demonstrated to exhibit anti-inflammatory, -allergic, -oxidative,
-damage and -tumour effects (17,18).
In recent years, studies on flavonoids have entered a new phase
with these compounds revealed to inhibit proliferation of cancer
cells, including breast, colon, lung, cervical and liver cancers
and lymphoma cells (19–23). In addition, alpinetin has also been
identified to inhibit proliferation of leukemic cells (24). However, few studies have reported
the effects of alpinetin on gastric cancer cells and its underlying
mechanism. In the present study, we found that alpinetin inhibits
proliferation and induces apoptosis of gastric cancer cells.
At present, the occurrence of malignant tumours is
considered to be associated with abnormal proliferation and
decreased apoptosis of tumour cells. Therefore, it has been
proposed that the growth of tumour cells may be reduced or
inhibited by promoting apoptosis using a variety of methods
(25,26). Previously, we found that alpinetin
regulates expression of Bcl-2 family members and X-linked inhibitor
of apoptosis protein, which promotes release of Cyt C and further
activates apoptotic proteases that eventually affect proliferation
of pancreatic cancer cells and induce apoptosis. In this study,
alpinetin was identified to have significant inhibitory effects on
human gastric cancer cell proliferation. Using flow cytometry, we
demonstrated that alpinetin induces apoptosis in human gastric
cancer cells. Apoptosis is often closely linked with the cell
cycle. Previous studies have revealed that blocking tumour cells at
a certain cell cycle phase using various methods induces apoptosis
of tumour cells or terminates tumour cell growth (27,28).
In the current study alpinetin was identified to significantly
inhibit cell cycle alterations in human gastric cancer cells,
arresting the cell cycle at the G2/M phase, thereby
inhibiting cell proliferation.
The induction of apoptosis is considered to be an
effective antitumour method. There are two major signal
transduction pathways that trigger apoptosis, the endogenous
mitochondrial and the exogenous death receptor pathway. This study
found that human gastric cancer cells treated for 48 h with various
alpinetin doses caused Bax and Bcl-2 translocation followed by Cyt
C release. These results indicate that alpinetin-induced apoptosis
in human gastric cancer cells may be mediated by the mitochondrial
pathway. Previous studies have demonstrated that high expression
levels of Bax in patients with gastric cancer improve chemotherapy
efficacy (29). Bcl-2/Bax family
members are key regulatory factors in the endogenous mitochondrial
apoptotic pathway (30,31). Upon stimulation with pro-apoptotic
factors, Bax translocates from the cytoplasm to the mitochondrial
membrane, which alters the permeability of the mitochondrial
membrane and promotes release of Cyt C from the mitochondria into
the cytoplasm (32). The apoptotic
cascade pathway is subsequently initiated, eventually leading to
apoptosis.
Due to its ability to activate apoptosis-related
proteases during the apoptotic process, the activation of caspase
family members is an important prerequisite for apoptosis (33). Previous studies have found that Bax
translocation leads to alterations in mitochondrial membrane
potential, triggering release of Cyt C and further activating
caspase-9 to promote apoptosis (34). The present study demonstrates that
following alpinetin treatment of human gastric cancer cells,
activated caspase-3 and -9 were enhanced in a dose-dependent manner
and release of Cyt C from the mitochondria to the cytoplasm was
correspondingly increased. Release of Cyt C from the mitochondria
into the cytoplasm activates caspase-3 and -9 and therefore is key
to the apoptosis pathway (35).
In summary, results of the present study demonstrate
that alpinetin inhibits human gastric cancer cell proliferation and
induces apoptosis in these cells via the mitochondrial pathway in a
dose-dependent manner. In addition, alpinetin arrests the cell
cycle at the G2/M phase. Therefore, alpinetin may be a
potential compound for the treatment of gastric cancer in the
future.
Acknowledgements
The authors thank Professor Songqing He from the
Affiliated Hospital of Guilin Medical University for guidance
during this study.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Mackenzie M, Spithoff K and Jonker D:
Systemic therapy for advanced gastric cancer: a clinical practice
guideline. Curr Oncol. 18:e202–e209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ngeow J, Tan IB and Choo SP: Targeted
therapies in the treatment of gastric cancer. Asia Pac J Clin
Oncol. 7:224–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dikken JL, van de Velde CJ, Coit DG, Shah
MA, Verheij M and Cats A: Treatment of resectable gastric cancer.
Therap Adv Gastroenterol. 5:49–69. 2012. View Article : Google Scholar
|
|
6
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines
OJ, Gukovskaya AS, Go VL and Eibl G: Baicalein, a component of
Scutellaria baicalensis, induces apoptosis by Mcl-1
down-regulation in human pancreatic cancer cells. Biochim Biophys
Acta. 1813:1465–1474. 2011.
|
|
7
|
Gao J, Morgan WA, Sanchez-Medina A and
Corcoran O: The ethanol extract of Scutellaria baicalensis
and the active compounds induce cell cycle arrest and apoptosis
including upregulation of p53 and Bax in human lung cancer cells.
Toxicol Appl Pharmacol. 254:221–228. 2011.PubMed/NCBI
|
|
8
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He W, Li Y, Tang J, Luan F, Jin J and Hu
Z: Comparison of the characterization on binding of alpinetin and
cardamonin to lysozyme by spectroscopic methods. Int J Biol
Macromol. 39:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
In LL, Azmi MN, Ibrahim H, Awang K and
Nagoor NH: 1′S-1′-acetoxyeugenol acetate: a novel phenylpropanoid
from Alpinia conchigera enhances the apoptotic effects of
paclitaxel in MCF-7 cells through NF-κB inactivation. Anticancer
Drugs. 22:424–434. 2011.
|
|
11
|
Malek SN, Phang CW, Ibrahim H, Norhanom AW
and Sim KS: Phytochemical and cytotoxic investigations of
Alpinia mutica rhizomes. Molecules. 16:583–589. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He ZH, Ge W, Yue GG, Lau CB, He MF and But
PP: Anti-angiogenic effects of the fruit of Alpinia
oxyphylla. J Ethnopharmacol. 132:443–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du J, Tang B, Wang J, Sui H, Jin X, Wang L
and Wang Z: Antiproliferative effect of alpinetin in BxPC-3
pancreatic cancer cells. Int J Mol Med. 29:607–612. 2012.PubMed/NCBI
|
|
14
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
15
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar
|
|
16
|
Lee JH, Kim KM, Cheong JH and Noh SH:
Current management and future strategies of gastric cancer. Yonsei
Med J. 53:248–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Li Y, Xue C, Hu Z, Chen X and Sheng
F: Effect of Chinese medicine alpinetin on the structure of human
serum albumin. Bioorg Med Chem. 13:1837–1845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh R, Singh S, Kumar S and Arora S:
Evaluation of antioxidant potential of ethyl acetate
extract/fractions of Acacia auriculiformis A. Cunn. Food
Chem Toxicol. 45:1216–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Li C, Zhang H, Lu Z, Li Z, You Q, Lu
N and Guo Q: VI-14, a novel flavonoid derivative, inhibits
migration and invasion of human breast cancer cells. Toxicol Appl
Pharmacol. 261:217–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White JB, Beckford J, Yadegarynia S, Ngo
N, Lialiutska T and d’Alarcao M: Some natural flavonoids are
competitive inhibitors of Caspase-1, -3 and -7 despite their
cellular toxicity. Food Chem. 131:1453–1459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salmela AL, Pouwels J, Kukkonen-Macchi A,
Waris S, Toivonen P, Jaakkola K, Mäki-Jouppila J, Kallio L and
Kallio MJ: The flavonoid eupatorin inactivates the mitotic
checkpoint leading to polyploidy and apoptosis. Exp Cell Res.
318:578–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang B and Zhang X: Inhibitory effects of
Broccolini leaf flavonoids on human cancer cells. Scanning. 34:1–5.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Konan NA, Lincopan N, Collantes Díaz IE,
de Fátima Jacysyn J, Tanae Tiba MM, Amarante Mendes JG, Bacchi EM
and Spira B: Cytotoxicity of cashew flavonoids towards malignant
cell lines. Exp Toxicol Pathol. 64:435–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang J, Li N, Dai H and Wang K: Chemical
constituents from seeds of Alpinia katsumadai, inhibition on
NF-kappaB activation and anti-tumor effect. Zhongguo Zhong Yao Za
Zhi. 35:1710–1714. 2010.(In Chinese).
|
|
25
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams GT: Programmed cell death:
apoptosis and oncogenesis. Cell. 65:1097–1098. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Powathil GG, Gordon KE, Hill LA and
Chaplain MA: Modelling the effects of cell-cycle heterogeneity on
the response of a solid tumour to chemotherapy: Biological insights
from a hybrid multiscale cellular automaton model. J Theor Biol.
308:1–19. 2012. View Article : Google Scholar
|
|
28
|
Wang XM, Cui JW, Li W, Cai L, Song W and
Wang GJ: Silencing of the COPS3 gene by siRNA reduces proliferation
of lung cancer cells most likely via induction of cell cycle arrest
and apoptosis. Asian Pac J Cancer Prev. 13:1043–1048. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pietrantonio F, Biondani P, de Braud F,
Pellegrinelli A, Bianchini G, Perrone F, Formisano B and Di
Bartolomeo M: Bax expression is predictive of favorable clinical
outcome in chemonaive advanced gastric cancer patients treated with
capecitabine, oxaliplatin and irinotecan regimen. Transl Oncol.
5:155–159. 2012. View Article : Google Scholar
|
|
30
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szabò I, Soddemann M, Leanza L, Zoratti M
and Gulbins E: Single-point mutations of a lysine residue change
function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse
embryonic fibroblasts: novel insights into the molecular mechanisms
of Bax-induced apoptosis. Cell Death Differ. 18:427–438. 2011.
|
|
32
|
Zeng L, Li T, Xu DC, Liu J, Mao G, Cui MZ,
Fu X and Xu X: Death receptor 6 induces apoptosis not through type
I or type II pathways, but via a unique mitochondria-dependent
pathway by interacting with bax protein. J Biol Chem.
287:29125–29133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eldering E, Mackus WJ, Derks IA, Evers LM,
Beuling E, Teeling P, Lens SM, van Oers MH and van Lier RA:
Apoptosis via the B cell antigen receptor requires Bax
translocation and involves mitochondrial depolarization, cytochrome
C release and caspase-9 activation. Eur J Immunol. 34:1950–1960.
2004. View Article : Google Scholar
|
|
35
|
Chen M and Wang J: Initiator caspases in
apoptosis signaling pathways. Apoptosis. 7:313–319. 2002.
View Article : Google Scholar
|