Introduction
Traditional Chinese medicine is one of the important
resources for the development of modern drugs. Artemisinin
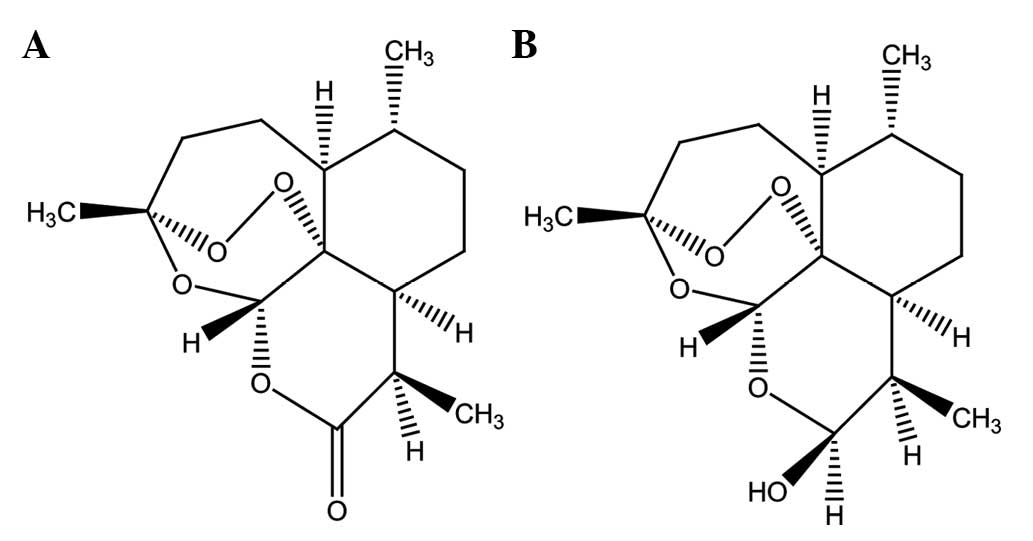
(Fig. 1A), the active component of
the Chinese medicinal herb Artemisia annua L., and its
derivatives (ARTs) are used extensively as anti-malarial drugs
worldwide (1). ARTs have also been
studied as candidates for cancer therapy, as they effectively
inhibit proliferation in various cancer cells (2–8). It
appears that iron-mediated cleavages of the endoperoxide bridge
play a vital role in achieving their anti-cancer effects (9,10).
Pre-treatment with an iron chelator reverses the proliferative
inhibition of ARTs, while pre-loading cancer cells with iron or
holotransferrin enhances the cytotoxicity (2,11,12).
ARTs lead to cell cycle arrest (3,6,8,13),
apoptosis (2,8,14–16)
or oncosis-like cell death (17)
in various cell types. Recently, proteomics were used to further
elucidate the molecular mechanisms of ARTs, and the investigators
found that the endoplastic reticulum stress induced by
dihydroartemisinin (DHA, Fig. 1B),
one of the main active metabolites of ARTs, partially contributed
to its anti-cancer activity (13).
However, as the authors proposed, a sub-cellular proteomics study
is required to avoid the biases of whole cellular proteomics, such
as the neglect of low-expressing proteins (13).
Mitochondria generate the majority of the chemical
energy in eukaryotic cells and are involved in a series of
physiological processes, such as signaling transduction and cell
cycle processes, as well as the control of cell death. Previous
studies indicated that mitochondria are important in the
pharmacological activities of ARTs (3,18).
The aim of this study was to detect the differentially expressed
mitochondrial proteins using proteomics to recognize the possible
mechanisms involved.
In this study, we found that DHA effectively
triggered cell proliferative inhibition in human hepatocellular
carcinoma BEL-7402 cells. By profiling the altered protein
expression pattern following DHA treatment, seven differentially
expressed mitochondrial proteins were identified between the
control and DHA-treated groups.
Materials and methods
Materials
DHA was kindly provided by Professor Ying Li,
Shanghai Institute of Materia Medica, Chinese Academy of
Sciences (Shanghai, China). Sulforhodamine B (SRB) was obtained
from Sigma Chemical Co. (St. Louis, MO, USA).
Cell line and culture
The human hepatocellular carcinoma BEL-7402 cell
line was provided by Professor Jian Ding, Shanghai Institute of
Materia Medica, Chinese Academy of Sciences (Shanghai,
China) and maintained in RPMI-1640 (Sigma) supplemented with 10%
heat-inactivated fetal bovine serum (Sijiqing, Hangzhou, China).
Cells were incubated in a humidified atmosphere of 95% air plus 5%
CO2 at 37°C.
SRB assay
The effect of DHA on cell proliferation was
determined by a SRB assay as previously described (13). Briefly, BEL-7402 cells were seeded
into 96-well plates and treated with the indicated concentrations
of DHA for 48 h. The medium was discarded immediately following the
drug treatment, and the cells were fixed with TCA. After the plate
was washed and dried, SRB was added. The plate was washed, dried
and the SRB retained in the cells was dissolved in 150 μl of 10 mM
Tris-HCl. The OD values at 515 nm were then measured using a
multi-well plate reader (SpectraMax Microplate Reader Series 2,
Molecular Devices, Sunnyvale, CA, USA).
Observation of morphologic changes
BEL-7402 cells were seeded into 96-well plates and
treated with the indicated concentration of DHA for 48 h. The cell
morphology was observed using a microscope (NIB-100, NOVEL,
China).
Protein sample preparation
BEL-7402 cells were treated with 50 μM DHA for 24 h
and the mitochondria were extracted according to the manufacturer’s
instructions (C3601, Beyotime, China). The mitochondria component
was lysed in 200 μl lysis solution (30 mM Tris-HCl, 8 M urea, 4%
CHAPS, pH 8.5) for 30 min at 4°C and centrifuged at 12,000 g for 20
min at 4°C. Finally, the protein concentrations in the suspended
solutions were determined by the Amersham 2D QuantKit (GE
Healthcare).
Two-dimensional gel electrophoresis
(2-DE)
A total of 300 μg protein was resolved in 450 μl
rehydration solution (8 M Urea, 2% CHAPS, 20 mM DTT, 0.5% IPG
buffer, trace amount of bromophenol blue), loaded onto the 24 cm
immobiline dry strips (pH 3–10, NL, GE Healthcare), and incubated
for 12 h. Isoelectric focusing (IEF) was performed on the Ettan™
IPGPhor (Amersham) and focused for 12 h at 30 V, 1 h at 500 V, 1 h
at 1,000 V, and 8,000 V for 64,000 Vhr. The strips were immediately
equilibrated twice for 15 min each using a solution containing 50
mM Tris-HCL, pH 8.8, 6 M Urea, 30% glycerol, 2% SDS and 0.002%
bromophenol blue. Subsequently, 1% DTT and 2.5% iodoacetamide were
added into the first and second equilibration steps, respectively.
Following equilibration, the strips were loaded onto a 12.5%
homogeneous SDS polyacrylamide gel and resolved in the second
dimension in the Ettan™ DALT Six (Amersham). The proteins on the
gels were visualized by the silver stain method. All 2-DE images
were scanned with the Typhoon 9400 (Amersham). Image Master 2D 5.0
software was used for matching and analysis of the protein spots.
The spots with an average increase or decrease of 1.8-fold compared
with that of the control group were used.
In-gel digestion and matrix-assisted
laser desorption/ionization time-of-flight mass spectrometry
(MALDI-TOF MS)
For identification, each protein spot of interest
was excised from the gels, and destained by washing with 50 μl
destaining solution [50 mM
Na2S2O3, 15 mM
K3Fe(CN)6] for 10 min. The spots were then
washed with 200 μl NH4HCO3 (200 mM) for 10
min. The gel spots were dehydrated by acetonitrile for 8 min.
Subsequently, they were enzymolysed using 12.5 ng/μl trypsin at
37°C overnight. The peptides were extracted twice using extract
liquor (H2O:TFA:acetonitrile=45:5:50) and freeze dried.
In total, 1.2 μl eluent containing 0.1% TFA was added into the
peptides and 0.3 μl sample solution was analyzed by MALDI-TOF MS
(ABI 4800 Plus). The protein identification was performed by
searching the NCBI database using the MASCOT search engine.
Results
Effects of DHA on the proliferation of
BEL-7402 cells
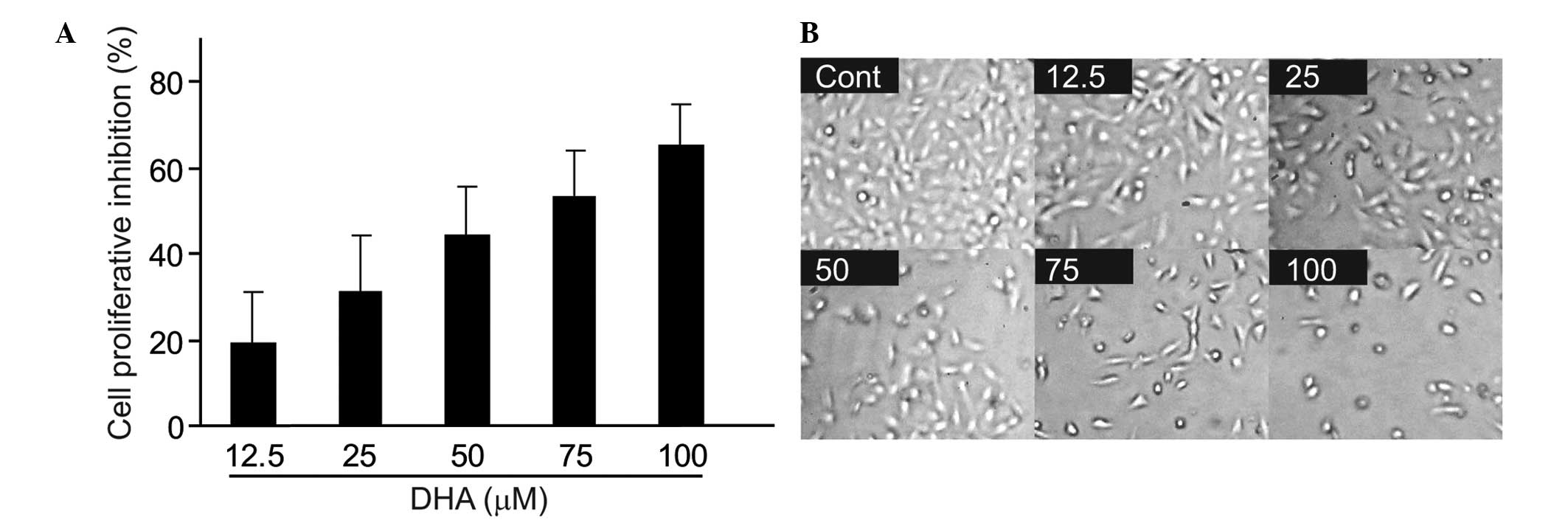
Human hepatocarcinoma BEL-7402 cells were treated
with various concentrations of DHA for 48 h and the cell
proliferation was evaluated by SRB assay. DHA was found to inhibit
cell proliferation in a concentration-dependent manner (Fig. 2A). Morphological changes were also
observed following DHA treatment. DHA decreased the percentage of
adherent cells in a concentration-dependent manner in BEL-7402
cells (Fig. 2B).
Mitochondria proteomics analysis
Since mitochondria may be involved in the
pharmacological activity of ARTs as previously reported (3,18),
the mitochondrial proteomics were used to explore the molecular
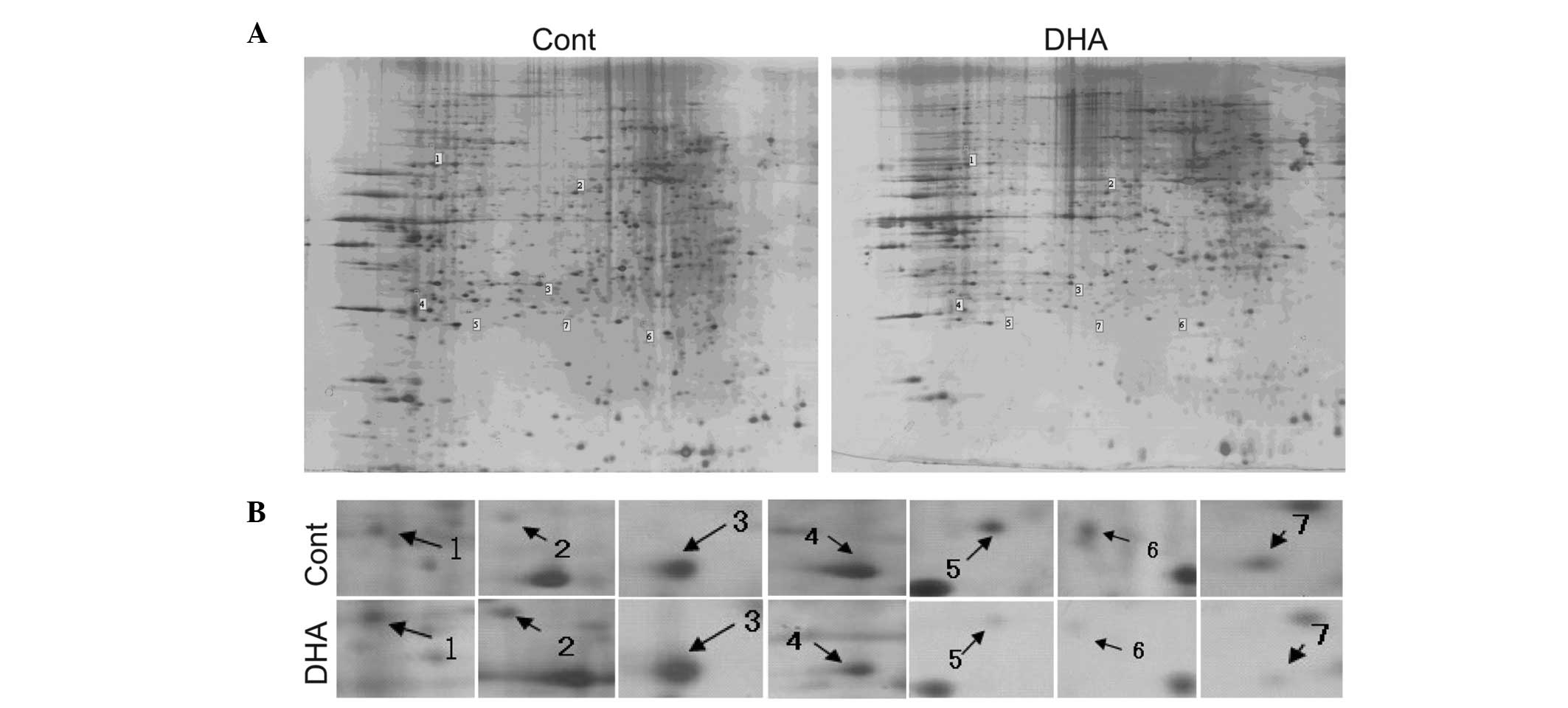
mechanisms of action of DHA in BEL-7402 cells. 2-DE plus MALDI-TOF
MS analysis were conducted to compare the mitochondrial protein
expression profiles between control and DHA-treated cells. BEL-7402
cells were treated with 50 μM DHA for 24 h to trigger a cell
response and the mitochondrial proteins were extracted and
separated with 2-D gel as described in Materials and methods
(Fig. 3A). The magnified
differentially expressed protein spots are shown in Fig. 3B. According to the results analyzed
by Image Master 2D 5.0 software, several differentially expressed
proteins (>1.8-fold) were further identified by MALDI-TOF MS
analysis following DHA treatment. Three mitochondrial proteins
[fumarate hydratase (FH), 60 kDa heat shock protein (HSP60),
enoyl-CoA hydratase (ECAH)] were upregulated and four
[3-hydroxyacyl-CoA dehydrogenase (HCAD), two subunits of ATP
synthase and NADPH:adrenodoxin oxidoreductase (NAO)] were
downregulated following DHA treatment (Table I). The majority of the proteins
were correlated with energy metabolism, indicating the vital role
of mitochondria in the anti-cancer activities of DHA. The
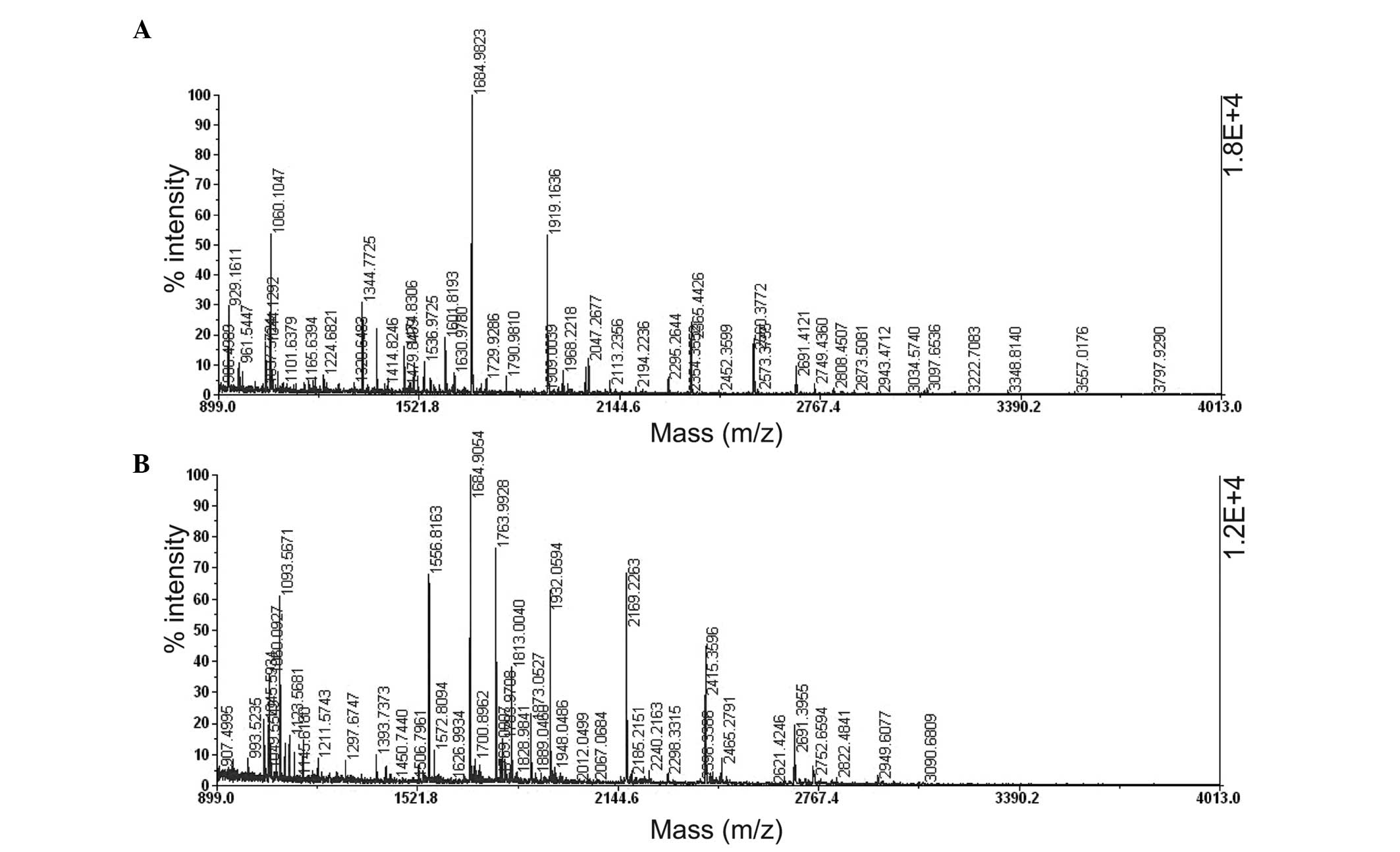
MALDI-TOF-MS peptide maps of in-gel tryptic digest resulting from
HSP60 (spot 2) and ATP synthase subunit d (spot 6) are shown in
Fig. 4.
 | Table IThe mitochondrial proteins with
altered expression in BEL-7402 cells after DHA treatment. |
Table I
The mitochondrial proteins with
altered expression in BEL-7402 cells after DHA treatment.
| Spot | Acc. no. | Protein name | Change | Function | Score |
|---|
| 1 | 19743875 | Fumarate
hydratase | ↑2.93 | Catalyzes the
formation of L-malate from fumarate | 98 |
| 2 | 31542947 | 60 kDa heat shock
protein | ↑5.19 | Essential for the
folding and assembly of newly imported proteins in the
mitochondria | 138 |
| 3 | 194097323 | Enoyl-CoA
hydratase | ↑1.83 | Catalyzes the
hydration of 2-trans-enoyl-CoA intermediates to
L-3-hydroxyacyl-CoAs | 169 |
| 4 | 83715985 | 3-hydroxyacyl-CoA
dehydrogenase type-2 isoform 2 | ↓1.82 | Catalyzes the
oxidation of a wide variety of fatty acids, alcohols and
steroids | 131 |
| 5 | 13111901 | ATP5A1 protein | ↓2.34 | Catalyzes ATP
synthesis | 55 |
| 6 | 51479152 | ATP synthase subunit
d, mitochondrial isoform b | ↓3.79 | Catalyzes ATP
synthesis | 142 |
| 7 | 111118981 | NADPH: adrenodoxin
oxidoreductase | ↓3.89 | Initiates electron
transport for cytochromes P450 receiving electrons from NADPH | 66 |
Discussion
A previous study reported that endoplasmic reticulum
stress is important in DHA-induced cell proliferative inhibition in
colorectal carcinoma HCT116 cells by using proteomics (13). To the best of our knowledge, this
is the first time that mitochondrial proteomics has been performed
to search for differentially expressed proteins in human
hepatocellular carcinoma BEL-7402 cells following DHA
treatment.
In the present study, seven mitochondrial proteins
differentially expressed in the control and DHA-treated groups were
detected. Among them, three proteins were upregulated in the
DHA-treated group while four were downregulated. Notably, six of
the seven proteins are enzymes that are important in energy
metabolism, with the exception of one molecular chaperone known as
HSP60. FH is an important tricarboxylic acid cycle enzyme that
catalyzes the hydration of fumarate to form malate and ECAH,
located in the matrix of mitochondria, and is a key enzyme that
catalyzes the second step in the β-oxidation pathway of
fatty acid metabolism. Both of these enzymes were upregulated
following DHA treatment. By contrast, HCAD, which catalyzes the
third step of β-oxidation, two subunits of ATP synthase and
NAO, which initiates electron transport for the cytochrome P450
receiving electrons from NADPH, were downregulated in the
DHA-treated group. ATP synthase and NAO are involved in the later
steps of ATP production compared with that of FH and ECAH,
indicating that the upregulation of FH and ECAH may be a stimulated
response following DHA treatment, while the downregulation of the
enzymes of the final ATP production may actually contribute to the
anti-cancer potential of DHA. Our hypothesis is further supported
by the fact that ARTs, such as artemisinin, artesunate and DHA
showed a decrease in intracellular ATP concentration in K562/adr
and GLC4/adr cancer cells (19).
Another concern is that the ATP synthase is slightly upregulated in
HCT116/R cells which are resistant to DHA-induced cell
proliferative inhibition (20).
All of these findings suggest that energy metabolism is crucial for
realizing the anti-cancer effects of DHA. Besides, the biological
roles in the signal transduction of these proteins should not be
ignored. For example, defects in FH result in hypoxia-inducible
transcription factor (HIF) expression, which leads to cancer cell
resistance to apoptosis (21).
Therefore, the upregulation of FH following DHA treatment may
inhibit the expression of HIF, and thus partially contribute to
DHA-induced proliferative inhibition in BEL-7402 cells. However,
these proteins require further validation with other methods in
future studies.
HSP60, a mitochondrial protein that is important for
folding key proteins after importing into the mitochondria, was
significantly increased in DHA-treated BEL-7402 cells. Similarly,
the level of HSP60 was notably increased following exposure of
human cancer cells to cisplatin, a chemotherapy drug that causes
cross-linking of DNA (22,23). This phenomenon was also
demonstrated in human lung non-small carcinoma H460 cells following
aloe-emodin treatment (24).
However, not all the results are the same. For example,
doxorubicin, a topoisomerase II inhibitor, does not cause marked
alteration of HSP60 levels in human breast cancer MCF-7 cells
(25). Moreover, HSP60 is
overexpressed in a number of tumors and plays a pro-survival role
in certain cases, suggesting that it is a target for cancer therapy
(26). The function of HSP60 in
cancer therapy is complicated and the exact role of the
upregulation of HSP60 in DHA-treated BEL-7402 cells should be
investigated.
It should be mentioned that glucose-regulated
protein 78 (GRP78), a protein closely correlated with endoplasmic
reticulum stress, was also upregulated, which is consistent with
the result obtained from the human colorectal carcinoma HCT116
cells (13). This phenomenon is
also observed in human breast cancer MCF-7 cells (data not shown),
indicating that DHA-induced endoplasmic reticulum stress may be a
universal mechanism in DHA-induced cancer cell proliferative
inhibition. However, GRP78 does not transfer into the mitochondria.
Several other proteins, such as 70 kDa heat shock protein, protein
disulfide isomerase, moesin and maspin (data not shown), were also
detected with different expression manners in our experiment,
indicating that this method should be further improved for the much
more pure mitochondria to be received.
In conclusion, the expression levels of several
mitochondrial proteins were changed following DHA treatment and the
imbalance of energy metabolism may contribute, at least in part, to
the anti-cancer potential of DHA in BEL-7402 cells which may help
to shed a new light into DHA-driven events.
Acknowledgements
We thank Professors Jian Ding and Ying Li for
providing the BEL-7402 cell line and DHA, respectively. We also
thank Ms. Yu-Fei Chen and Mr. Jun-Ren Zhang for their help with the
cell culture and 2-DE assay. This study was supported by the
Zhejiang Provincial Education Department (No. Y201016139), National
Natural Science Foundation of China (No. 81001450) and Zhejiang
Chinese Medicine University (No. 2009ZZ04).
Abbreviations:
|
ARTs
|
artemisinin and its derivatives
|
|
2-DE
|
two-dimensional gel
electrophoresis
|
|
DHA
|
dihydroartemisinin
|
|
ECAH
|
enoyl-CoA hydratase
|
|
FH
|
fumarate hydratase
|
|
GRP78
|
glucose-regulated protein 78
|
|
HCAD
|
3-hydroxyacyl-CoA dehydrogenase
|
|
IEF
|
isoelectric focusing
|
|
HIF
|
hypoxia-inducible transcription
factor
|
|
HSP60
|
60 kDa heat shock protein
|
|
MALDI-TOF MS
|
matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry
|
|
NAO
|
NADPH:adrenodoxin oxidoreductase
|
|
SRB
|
sulforhodamine B
|
References
|
1
|
White NJ: Qinghaosu (artemisinin): the
price of success. Science. 320:330–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu JJ, Meng LH, Cai YJ, et al:
Dihydroartemisinin induces apoptosis in HL-60 leukemia cells
dependent of iron and p38 mitogen-activated protein kinase
activation but independent of reactive oxygen species. Cancer Biol
Ther. 7:1017–1023. 2008. View Article : Google Scholar
|
|
3
|
Efferth T, Sauerbrey A, Olbrich A, et al:
Molecular modes of action of artesunate in tumor cell lines. Mol
Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Efferth T, Olbrich A and Bauer R: mRNA
expression profiles for the response of human tumor cell lines to
the antimalarial drugs artesunate, arteether, and artemether.
Biochem Pharmacol. 64:617–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L
and Sun ZX: Artesunate attenuates the growth of human colorectal
carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int J
Cancer. 121:1360–1365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J and
Fan SJ: Dihydroartemisinin is an inhibitor of ovarian cancer cell
growth. Acta Pharmacol Sin. 28:1045–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen T, Li M, Zhang R and Wang H:
Dihydroartemisinin induces apoptosis and sensitizes human ovarian
cancer cells to carboplatin therapy. J Cell Mol Med. 13:1358–1370.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan W, Lu J, Huang M, et al: Anti-cancer
natural products isolated from chinese medicinal herbs. Chin Med.
6:272011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercer AE, Maggs JL, Sun XM, et al:
Evidence for the involvement of carbon-centered radicals in the
induction of apoptotic cell death by artemisinin compounds. J Biol
Chem. 282:9372–9382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Efferth T, Benakis A, Romero MR, et al:
Enhancement of cytotoxicity of artemisinins toward cancer cells by
ferrous iron. Free Radic Biol Med. 37:998–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Efferth T: Molecular pharmacology and
pharmacogenomics of artemisinin and its derivatives in cancer
cells. Curr Drug Targets. 7:407–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu JJ, Chen SM, Zhang XW, Ding J and Meng
LH: The anti-cancer activity of dihydroartemisinin is associated
with induction of iron-dependent endoplasmic reticulum stress in
colorectal carcinoma HCT116 cells. Invest New Drugs. 29:1276–1283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu YY, Chen TS, Qu JL, Pan WL, Sun L and
Wei XB: Dihydroartemisinin (DHA) induces caspase-3-dependent
apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed
Sci. 16:162009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Sun B, Pan S, Jiang H and Sun X:
Dihydroartemisinin inhibits growth of pancreatic cancer cells in
vitro and in vivo. Anticancer Drugs. 20:131–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu JJ, Meng LH, Shankavaram UT, et al:
Dihydroartemisinin accelerates c-MYC oncoprotein degradation and
induces apoptosis in c-MYC-overexpressing tumor cells. Biochem
Pharmacol. 80:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du JH, Zhang HD, Ma ZJ and Ji KM:
Artesunate induces oncosis-like cell death in vitro and has
antitumor activity against pancreatic cancer xenografts in vivo.
Cancer Chemother Pharmacol. 65:895–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Mo W, Shen D, et al: Yeast model
uncovers dual roles of mitochondria in action of artemisinin. PLoS
Genet. 1:e362005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reungpatthanaphong P and Mankhetkorn S:
Modulation of multidrug resistance by artemisinin, artesunate and
dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines.
Biol Pharm Bull. 25:1555–1561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu JJ, Chen SM, Ding J and Meng LH:
Characterization of dihydroartemisinin-resistant colon carcinoma
HCT116/R cell line. Mol Cell Biochem. 360:329–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King A, Selak MA and Gottlieb E: Succinate
dehydrogenase and fumarate hydratase: linking mitochondrial
dysfunction and cancer. Oncogene. 25:4675–4682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhao X, Yang J and Wei Y: Proteomics
profile changes in cisplatin-treated human ovarian cancer cell
strain. Sci China C Life Sci. 48:648–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castagna A, Antonioli P, Astner H, et al:
A proteomic approach to cisplatin resistance in the cervix squamous
cell carcinoma cell line A431. Proteomics. 4:3246–3267. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai MY, Hour MJ, Wing-Cheung Leung H, Yang
WH and Lee HZ: Chaperones are the target in aloe-emodin-induced
human lung nonsmall carcinoma H460 cell apoptosis. Eur J Pharmacol.
573:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen ST, Pan TL, Tsai YC and Huang CM:
Proteomics reveals protein profile changes in doxorubicin--treated
MCF-7 human breast cancer cells. Cancer Lett. 181:95–107. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cappello F, Conway de Macario E, Marasa L,
Zummo G and Macario AJ: Hsp60 expression, new locations, functions
and perspectives for cancer diagnosis and therapy. Cancer Biol
Ther. 7:801–809. 2008. View Article : Google Scholar : PubMed/NCBI
|