Introduction
Despite recent improvements in diagnosis, lung
cancer is a major cause of death due to its high incidence,
malignant behavior and lack of major advancements in treatment
strategy (1). Lung cancer was the
leading indication for respiratory surgery (47.5%) in 2009 in Japan
(2). Over 31,000 patients
underwent surgery at Japanese institutions in 2009 (2). The clinical behavior of lung cancer
is largely associated with its stage. The cure of the disease by
surgery is only achieved in cases representing an early stage of
lung cancer (3).
NRF2 is a transcription factor belonging to the
cap'n'collar subfamily of the basic-leucine zipper (bZIP) family of
transcription factors. NRF2 plays a significant role in the
adaptive responses to oxidative stress (4). NRF2 is expressed widely in various
human tissues (5), including lung
cancer tissue (6). The
overexpression of NRF2 in premalignant cells enables the cancer
cells to survive in an oxidizing tumor environment. Subsequently,
the cancer cells alter the metabolism process and induce
mitochondrial dysfunction and the activation of oncogenic signals.
It has been shown that patients with lung tumors containing the
NRF2 gene (NFE2L2) mutation have a poorer prognosis
than patients with non-mutant tumors (7,8).
Moreover, the mutations of the NRF2 gene have been
associated with primary lung cancer (6–9). The
NRF2 gene somatic mutation is more common in lung squamous
cell carcinomas (7).
Multidrug-resistant proteins (MRPs) are members of the ATP-binding
cassette superfamily that facilitate detoxification by transporting
toxic compounds, including chemotherapeutic drugs, out of cells
(10–12). Analysis of the MRP3 promoter
revealed the presence of multiple putative electrophile responsive
element (EpRE) sequences that suggested the possible regulation of
this gene by NRF2 (13).
Although we have reported the NRF2 gene
mutation status in lung cancers (7), the association of the NRF2
gene mutation and MRP3 expression status in Japanese lung
cancer has not been previously reported. To determine the
MRP3 mRNA expression status, we used quantitative real-time
PCR (qPCR) using LightCycler. The findings were compared with the
clinicopathological features of the lung squamous carcinomas.
Patients and methods
Patients
The study group included 67 lung squamous cell
carcinoma patients who had undergone surgery at the Department of
Surgery, Nagoya City University Hospital. Tumor samples were
immediately frozen and stored at −80°C until assayed. Consent was
obtained from the patients and the study was approved by the Ethics
Committee of Nagoya City University Hospital.
The clinical and pathological characteristics of the
67 lung squamous cell carcinoma patients were as follows: 30 cases
at stage I, 17 at stage II and 20 at stage III. The mean age was
66.8 years (range, 49–79). Among the 67 lung cancer patients, 28
had lymph node metastasis, 62 were male and 14 had NRF2 gene
mutations. The samples from these patients had been sequenced for
the NRF2 gene (7).
PCR assays for NRF2
Total RNA was extracted from lung cancer tissues
using an Isogen kit (Nippon Gene, Tokyo, Japan) according to the
manufacturer's instructions. RNA concentrations were determined
using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies,
Inc., Rockland, DE, USA). Five cases were excluded from each assay,
since the tumor cells were too few to sufficiently extract tumor
RNA. The RNA (1 μg) was reverse-transcribed using a First strand
cDNA synthesis kit with 0.5 μg oligo (dT)16 (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's instructions. The reaction mixture was incubated at
25°C for 15 min, 42°C for 60 min, 99°C for 5 min and then at 4°C
for 5 min. The cDNA concentration was determined using the Nano
Drop ND-1000 Spectrophotometer. Approximately 200 ng of each cDNA
was used for PCR analysis. To ensure the fidelity of mRNA
extraction and reverse transcription, the samples were subjected to
PCR amplification with a β-actin primers kit (Nihon Gene
Laboratory, Miyagi, Japan) using a LightCycler-FastStart DNA Master
HybProbe kit (Roche Diagnostics GmbH). The PCR assay reactions were
performed using LightCycler FastStart DNA Master SYBR-Green I kit
(Roche Diagnostics GmbH) in a 20-μl reaction volume. The primer
sequences for MRP3 gene were as follows: forward:
5-ACAGGGGATGCAGTATCTGG-3 (at exon 14) and reverse:
5-CCTGGCTCCTCTTCACGTAG-3 (at exon 16). The cycling conditions were
as follows: initial denaturation at 95°C for 10 min, followed by 40
cycles at 95°C for 10 sec, annealing at 61°C for 10 sec and
extension at 72°C for 7 sec.
Statistical analysis
Statistical analysis was performed using the
Mann-Whitney U-test for unpaired samples and Wilcoxon's signed rank
test for paired samples. Linear relationships between variables
were determined by means of simple linear regression. Correlation
coefficients were determined by rank correlation using Spearman's
and χ2 tests. The overall survival of the lung cancer
patients was examined by Kaplan-Meier methods and differences were
examined by the log-rank test. Analyses were carried out using the
Stat-View software package (Abacus Concepts, Inc., Berkeley, CA,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
NRF2 gene mutation in Japanese lung
cancer patients
The NRF2 gene mutation status and N-terminal
domain were investigated by direct sequencing as previously
reported (7). A total of 291
non-small cell lung cancer (NSCLC) cases, including 148 squamous
cell carcinoma patients, were investigated. Sixteen had NRF2
gene mutations (Table I). Patients
with mutations were male with squamous cell carcinomas. Fifteen
were smokers and 4 were stage I. The NRF2 gene mutations
were clustered on exon 2 and resulted in amino acid changes in
either the DLG or the ETGE motif of the regulatory Neh2 domain
(7).
 | Table INRF2 mutations in Japanese lung
cancers. |
Table I
NRF2 mutations in Japanese lung
cancers.
| Nucleotide
mutation | Amino acid
change | Histology | Gender | Age (years) |
|---|
| 238 A-G | T80A
(Thr>Ala) | SCC | Male | 68 |
| 72G-C | W24C
(Trp>Cys) | SCC | Male | 69 |
| 95 T-G | V32G
(Val>Gly) | SCC | Male | 74 |
| 100C-G | R34G
(Arg>Gly) | SCC | Male | 74 |
| 101G-C | R34P
(Arg>Pro) | SCC | Male | 63 |
| 101G-A | R34Q
(Arg>Gln) | SCC | Male | 65 |
| 101G-A | R34Q
(Arg>Gln) | SCC | Male | 66 |
| 101G-A | R34Q
(Arg>Gln) | SCC | Male | 79 |
| 230A-G | D77G
(Asp>Gly) | SCC | Male | 76 |
| 235G-C | E79Q
(Glu>Gln) | SCC | Male | 77 |
| 85 G-T | D29Y
(Asp>Tyr) | SCC | Male | 58 |
| 235G-A | E79K
(Glu>Lys) | SCC | Male | 77 |
| 101G-A | R34Q
(Arg>Gln) | SCC | Male | 67 |
| 237G-C | E79D
(Glu>Asp) | SCC | Male | 73 |
| 101G-A | R34Q
(Arg>Gln) | SCC | Male | 64 |
| 235G-A | E79K
(Glu>Lys) | SCC | Male | 77 |
MPR3 mRNA levels in Japanese lung cancer
patients
The levels of MRP3/β-actin were
investigated in the 67 squamous cell carcinoma patients, including
14 NRF2 mutant patients. The mean MRP3/β-actin
level in the lung cancer tissue was 1.124±1.490 and did not
correlate with age (R2=0.038, p=0.1123). The
MRP3/β-actin mRNA levels did not correlate with age
(≤65 vs. >65 years; p=0.1080) or smoking status (Brinkman index
≤400 vs. >400; p=0.4741). The MRP3/β-actin mRNA
levels also did not correlate with lymph node metastasis, tumor
invasion status and pathological differentiation status. Although
the MRP3/β-actin mRNA levels did not correlate with
pathological stage, there was a tendency towards higher
MRP3/β-actin mRNA levels at higher pathological
stages (stage I, 0.824±0.887; stage II, 1.079±1.055; stage III,
1.611±2.277). MRP3/β-actin mRNA levels were
significantly higher in the male cases (1.200±1.524) than in the
female cases (0.179±0.083; p=0.0036).
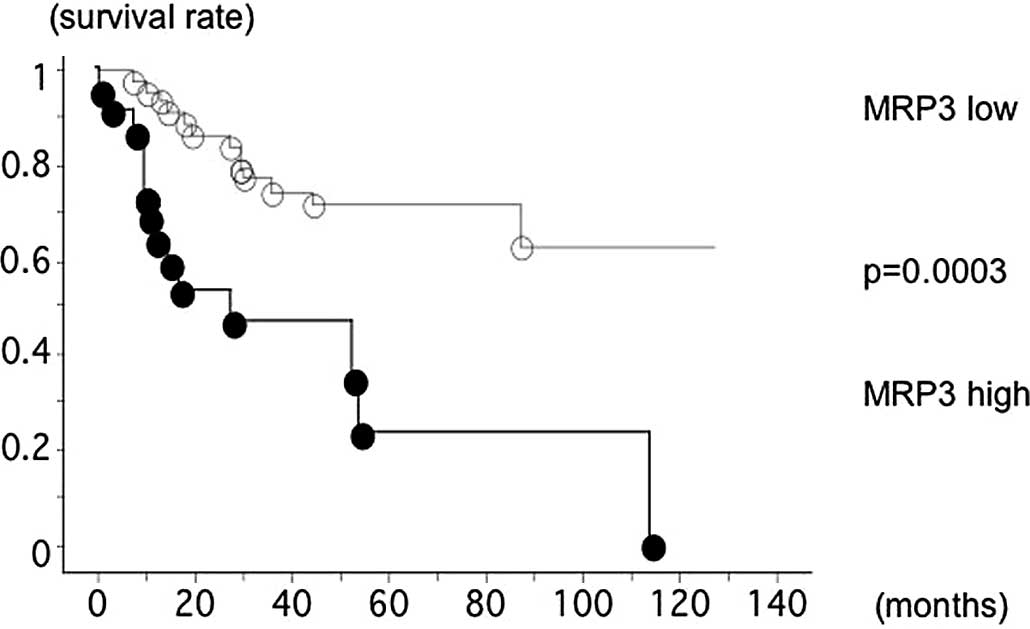
The overall survival of the 67 lung squamous cell
carcinoma patients, with follow-up through to December 31, 2010,
was studied with reference to whether the patient's
MRP3/β-actin mRNA level was >1.124 (high) or not.
The survival of patients with high levels of
MRP3/β-actin mRNA (14 out of 22 were deceased, mean
survival 45.6 months) was significantly worse than that of patients
with low MRP3/β-actin mRNA levels (13 out of 45 were
deceased, mean survival 69.0 months; log-rank test, p=0.0003;
Fig. 1).
Discussion
In this study, we found that MRP3 mRNA levels
correlated with NRF2 mutations. Although the MRP3
mRNA levels also correlated with gender, this was due to the
NRF2 mutations all being identified in male squamous cell
carcinoma patients. High MRP3 mRNA levels also correlated
with poor prognosis.
The NRF2 gene is a master transcriptional
activator of genes encoding numerous cytoprotective enzymes that
are induced in response to environmental and endogenously-derived
oxidative/electrophilic agents (14–16).
A previous study has shown that the RNAi-mediated silencing of
NRF2 gene expression in NSCLC inhibited tumor growth
(17). NRF2 gene promoter
polymorphism has been identified and was suggested to correlate
with carcinogenesis (18). The
association between NRF2 mutation and the MRP3 mRNA
levels of lung squamous cell carcinomas suggests a role for NRF2 in
chemoresistance. The constitutive expression of NRF2 has been
reported to provide a survival advantage to invasive and metastatic
cancer cells by adaptation to the microenvironment and the
evolution of chemoresistance in cancer cells under hypoxia
(19,20). The degree of CDDP-induced DNA
crosslinking and the number of apoptotic cells have been revealed
to increase significantly in A549 cells transfected with
NRF2-siRNA (21). The
expression levels of multidrug resistance-associated proteins, the
drug efflux proteins, have also been reported to be significantly
reduced in NRF2-silenced A549 cells (21). A previous study revealed that
inhibition of the NRF2 function restored CDDP sensitivity in human
ovarian cancer cells (22).
Previous analysis of the human MRP3 gene
revealed four putative NRF2-binding sites (EpREs) (12,13).
These findings suggest that the activation of NRF2
contributes to the induction of MRP3. In vitro,
ChIP-analysis demonstrated an increased NRF2 binding to the −805 bp
EpRE following treatment with a NRF2 activator. In NSCLC cell
lines, the total basal levels of MRP3 mRNA and NRF2 protein
were reported to be concordant (13). It has been shown that wild-type
NRF2 proteins decrease rapidly, whereas mutant NRF2 proteins are
degraded more slowly, having half-lives of approximately twice
those of the wild-type proteins (8). In addition, mutant NRF2 proteins have
been reported to be significantly more active than wild-type NRF2
when analyzed by luciferase activity (8).
Higher MRP3 mRNA levels correlated with poor
prognosis, however, this may be due to the correlation with
NRF2 mutations. Our previous study results revealed that
patients with NRF2 mutations had poor prognosis (7), consistent with other reports
(6,8). Since we usually perform adjuvant
chemotherapy for advanced lung cancer cases, the chemosensitivity
may affect the results. In addition, although not significant,
higher MRP3 mRNA levels were observed at higher pathological
stages in our analysis.
Acknowledgements
The authors would like to thank Mrs. Miki Mochizuki
for her technical assistance. This study was supported by
Grants-in-Aid for Scientific Research, Japan Society for the
Promotion of Science (JSPS; nos. 23659674, 21390394 and 21591820)
and a grant for cancer research of the Program for Developing the
Supporting System for Upgrading the Education and Research (2009)
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan.
References
|
1
|
Ginsberg RJ, Kris MK and Armstrong JG:
Cancer of the lung. Cancer: Principles and Practice of Oncology.
DeVita VT, Hellman S and Rosenberg SA: 4th edition. Lippincott,
Williams and Wilkins; Philadelphia: pp. 673–682. 1993
|
|
2
|
Sakata R, Fujii Y and Kuwano H: Thoracic
and cardiovascular surgery in Japan during 2009. Annual report by
the Japanese Association for Thoracic Surgery Gen Thorac Cardiovasc
Surg. 59:636–667. 2011.
|
|
3
|
Postus PE: Chemotherapy for non-small cell
lung cancer: the experience of the lung cancer cooperative group of
the European Organization for Research and Treatment of Cancer.
Chest. 113(Suppl 1): S28–S31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kensler TW and Wakabayashi N: Nrf2: friend
or foe for chemoprevention? Carcinogenesis. 31:90–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moi P, Chank K, Asunis I, et al: Isolation
of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper
transcriptional activator that binds to the tandem NF-E2/AP1 repeat
of the beta-globin locus control region. Proc Natl Acad Sci USA.
91:9926–9930. 1994. View Article : Google Scholar
|
|
6
|
Solis LM, Behrens C, Dong W, et al: Nrf2
and Keap1 abnormalities in non-small cell lung carcinoma and
association with clinicopathologic features. Clin Cancer Res.
16:3743–3753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki H, Hikosaka Y, Okuda K, et al:
NFE2L2 gene mutation in male Japanese squamous cell carcinoma of
the lung. J Thorac Oncol. 5:786–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibata T, Ohta T, Tong KI, et al: Cancer
related mutations in Nrf2 impair its recognition by Keap1-Cul3 E3
ligase and promote malignancy. Proc Natl Acad Sci USA.
105:13568–13573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Ju Y, Lin D, et al: Mutation of the
Nrf2 gene in non-small cell lung cancer. Mol Biol Rep.
39:4743–4747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young LC, Campling BG, Voskoglou-Nomikos
T, et al: Expression of multidrug resistance protein-related genes
in lung cancer: correlation with drug response. Clin Cancer Res.
5:673–680. 1999.PubMed/NCBI
|
|
11
|
Kool M, van der Linden M, de Haas M, et
al: MRP3, an organic anion transporter able to transport
anti-cancer drugs. Proc Natl Acad Sci USA. 96:6914–6919. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lockhart AC, Tirona RG and Kim RB:
Pharmacogenetics of ATP-binding cassette transporters in cancer and
chemotherapy. Mol Cancer Ther. 2:685–698. 2003.PubMed/NCBI
|
|
13
|
Mahaffey CM, Zhang H, Rinna A, et al:
Multidrug resistant protein-3 gene regulation by the transcription
factor Nrf2 in human bronchial epithelial and non-small cell lung
carcinoma. Free Radic Biol Med. 46:1650–1657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merikallio H, Pääkkö P, Kinnula VL, et al:
Nuclear factor erythroid-derived 2-like 2 (Nrf2) and DJ1 are
prognostic factors in lung cancer. Hum Pathol. 43:577–584. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Wang W, Zhang Y, et al: The role
of NF-E2-related factor 2 in predicting chemoresistance and
prognosis in advanced non-small cell lung carcinomas. Clin Lung
Cancer. 12:166–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahaffey CM, Mahaffey NC, Holland W, et
al: Aberrant regulation of the MRP3 gene in non-small cell lung
carcinoma. J Thorac Oncol. 7:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahaffey CM, Zhang H, Rinna A, et al:
Multidrug resistant protein-three gene regulation by the
transcription factor Nrf2 in human bronchial epithelial and
non-small cell lung carcinoma. Free Radiac Biol Med. 46:1650–1657.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh K, Chiba T, Takahashi S, et al: An
Nef2/small Maf heterodimer mediates the induction of phase II
detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rushmore TH and Kong AN: Pharmacogenomics,
regulation and signaling pathways of phase I and II drug
metabolizing enzymes. Curr Drug Metab. 3:481–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ngyyen T, Yang CS and Pickett CB: The
pathways and molecular mechanisms regulating Nrf2 activation in
response to chemical stress. Free Radic Biol Med. 37:433–441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arisawa T, Tahara T, Shibata T, et al:
Nrf2 gene promoter polymorphism and gastric carcinogenesis.
Hepatogastroenterology. 55:750–754. 2008.PubMed/NCBI
|
|
19
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
20
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
21
|
Homma S, Ishii Y, Morishima Y, et al: Nrf2
enhances cell proliferation and resistance to anticancer drugs in
human lung cancer. Clin Cancer Res. 15:3423–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho JM, Manandhar S, Lee HR, et al: Role
of Nrf2-antioxidant system in cytotoxicity mediated by anticancer
cisplatin: implication to cancer cell resistance. Cancer Lett.
260:96–108. 2008. View Article : Google Scholar : PubMed/NCBI
|