Introduction
Cardiovascular disease is the leading cause of
mortality in chronic renal failure (CRF), accounting for ~50% of
mortalities (1,2), wherein CRF-accelerated
atherosclerosis is an important contributor. However, the
pathogenesis is currently unclear. Therefore, analysis of the
mechanism of the occurrence of CRF-accelerated atherosclerosis may
aid reduction of cardiovascular disease incidence in patients with
CRF and improve their prognosis and quality of life.
The ubiquitin-proteasome pathway (UPP) has been
identified to be significantly activated in CRF patients. This may
be closely associated with the degradation of muscle protein, as
well as the occurrence of metabolic acidosis, malnutrition,
micro-inflammation and other complications in patients with CRF
(3–5). Thus, the UPP may be involved in the
occurrence and development of atherosclerosis (6,7)
through degradation of inhibitor of κB (IκB), leading to activation
of nuclear factor-κB (NF-κB).
Previous studies have reported that the UPP was
activated in the aorta of rabbits with CRF and that MG-132
proteasome inhibitor treatment significantly inhibited NF-κB
activity (8). However, the
mechanism of UPP activation in aortic smooth muscle cells (ASMCs)
under CRF is unclear. In the present study, rabbit ASMCs were
cultured in vitro using CRF serum and changes in the UPP
were observed to determine the mechanism of UPP expression in ASMCs
under CRF conditions.
Materials and methods
Experimental animals
Twenty adult male New Zealand white rabbits,
weighing 2.0±0.2 kg, were randomly divided into two groups (n=10).
One group was used to construct the CRF animal model for serum
collection and the other group was used for normal serum
collection. Healthy male New Zealand white rabbits, weighing
0.5±0.2 kg, were used for cell culture. These animals were provided
by the Third Military Medical University Experimental Animal Center
[license no.: SYXK (Chongqing) 2007-0012]. The study was approved
by the ethics committee of Affiliated Jiangyin Hospital of
Southeast University Medical College, Jiangyin, Jiangsu, China.
Serum collection
The rabbit CRF model was constructed as described
previously (8). Briefly, rabbits
were anesthetized with 2% pentobarbital sodium (3 mg/kg) through
ear vein injection. Rabbits were fixed on the operating table in
the lateral position. Rabbit hair on both sides of the rabbit
kidney area was removed and the area was partially disinfected with
0.5% povidone-iodine. A longitudinal incision (~3 cm long) was made
in the area of the left kidney and the abdominal cavity was opened
to expose the left kidney. The perirenal fat capsule was separated
and the renal artery and its branches were isolated, revealing
three branches of the renal artery. Two branches of arteries
supplying the lower pole received ligation with the 0 line, ~2/3 of
the left kidney immediately became pale and then turned purple. The
left kidney was then returned to its usual location after 1 min.
The incision was sutured. A longitudinal incision (~2 cm long) was
made in the right kidney area to open the abdominal cavity, the
right kidney was exposed and the right renal pedicle was isolated.
The renal pedicle was ligated with line 4 and the right kidney was
removed. When intraoperative and field bleeding ended, the incision
was layer sutured to close the abdominal cavity. Following 12
weeks, the animals were anesthetized and their abdominal cavities
were cut open. Inferior vena venous blood was drawn and the serum
was separated from the blood, mixed, filtrated using a 0.22-μm
microporous membrane and stored at −70°C.
Rabbit ASMCs primary culture and
identification
As described previously (9), the rabbit aorta was extracted under
sterile conditions, the outer membrane was carefully peeled back
and the inner membrane was removed and cut into 1×1-mm sections.
Aortic SMCs (ASMCs) were cultured in DMEM/F12 supplemented with 20%
newborn calf serum and 1% penicillin-streptomycin at 37°C in a 95%
air 5% CO2 incubator. When the cells reached 80%
confluence, they were digested using trypsin, passaged and then
inoculated into culture bottles at specific densities. Cells from
passages 3–5 were used in the present study. SMCs were identified
using morphological observations and α-SM-actin
immunofluorescence.
Reverse transcription PCR (RT-PCR)
Cells were cultured in serum-free media and
synchronized in the G0 phase. Following addition of 10%
normal or CRF-stimulated serum, total RNA was extracted according
to the manufacturer’s instructions. The RNA extraction kit was
purchased from TIANDZ (Mianyang, China). The reverse
transcriptase-polymerase chain reaction (PCR) kit was purchased
from BioDev (Beijing, China). RT-PCR conditions were as follows:
pre-denaturation at 94°C for 5 min, denaturation at 94°C for 40
sec, annealing at primer-specific temperatures for 40 sec, 72°C for
1 min and 32 cycles and termination at 72°C for 5 min. The primer
sequences are presented in Table
I. PCR products were identified using agarose electrophoresis,
staining and imaging. The density ratio of the target gene fragment
and the internal reference β-actin represented relative mRNA
expression.
 | Table IPrimer pairs. |
Table I
Primer pairs.
| Gene | Primer direction | Primer sequence | Tm (°C) | Product length
(bp) |
|---|
| Ub | Forward |
5′-TGGCCGTACTCTTTCTGA-3′ | 60 | 127 |
| Reverse |
5′-CTCCACTTCCAGGGTGAT-3′ | | |
| E1 | Forward | 5′-AGCCTA
ATGGTGAGGAGATG-3′ | 57 | 287 |
| Reverse |
5′-TCAGCGGATGGTGTATCG-3′ | | |
| β-actin | Forward |
5′-GAGCTACGAGCTGCCTGACG-3′ | 57–62 | 416 |
| Reverse |
5′-CCTAGAAGCATTTGCGGTGG-3′ | | |
Western blot analysis
Following cell culture in serum-free media and
synchronization in the G0 phase, cells were incubated in
10% normal or CRF rabbit serum for 24 h. Next, total cellular
protein was extracted and measured. Protein samples (20 μg) were
used for 10% SDS-PAGE, transferred to film and blocked in 5% nonfat
dry milk at room temperature for 2 h. Antibodies against ubiquitin
(Ub) (1:1,000), E1 (1:2,000), IκBα (1:400) and β-actin (1:1,000)
were added and the samples were incubated at 4°C overnight.
HRP-labeled IgG secondary antibodies (1:10,000) were added and the
samples were incubated at room temperature for 1 h. The membrane
was washed and color light-emitting substrate was added to the
membrane, which was then analyzed through gel imaging.
Proteasome activity assay
Cells were cultured in serum-free media and
synchronized in the G0 phase. Next, cells were
interfered by 10% normal or CRF serum for 24 h. Cells were washed
with ice-cold 0.01 mol/l PBS, scraped gently and centrifuged at
1,000 × g/min for 5 min at 4°C. The cell pellet was washed with 1X
PBS twice and transferred to an EP tube, to which 1 ml of 20 mmol/l
Tris (pH 7.6) was added. The EP tube was placed in liquid nitrogen
for 5 min and then placed at 37°C for 5 min. Protein concentration
was determined using the Bradford method. Total cellular protein
extracts (20 μg) were used for each group. The extract was
incubated with the proteasome-specific substrate S, Z and B
(substrate S: N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methyleoumarin,
molecular weight 763.9, excitation/emission wavelength 380/440 nm,
chymotrypsin-like activity; substrate Z:
Z-Leu-Leu-Glu-β-naphthylamide, molecular weight 632.8,
excitation/emission wavelength 335/410 nm, peptide-Valley
aminoacyl-peptide hydrolase activity; and substrate B:
Boc-Gln-Ala-Arg-7-amido-4-methylcoumarin hydrochloride, molecular
weight 667.2, excitation/emission wavelength 380/440 nm, trypsin
activity) at 37°C for 30 min (final substrate concentration, 10
μmol/l; pH 8.0; Hepes buffer, 500 μl). The reaction was terminated
by adding the same volume of ice-cold ethanol and 3 ml deionized
water. Finally, fluorescence was measured using a fluorescence
microplate reader and the absorbance values reflected the activity.
Samples of each group were formed of three parallel tubes.
Experiments were repeated three times.
Statistical analysis
Statistical analyses were performed using SPSS 10.0
software (SPSS, Inc., Chicago, IL, USA) and data are expressed as
mean ± SD. Groups were compared using ANOVA and further pairwise
comparisons were performed using an LSD t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell morphology and biochemical
index
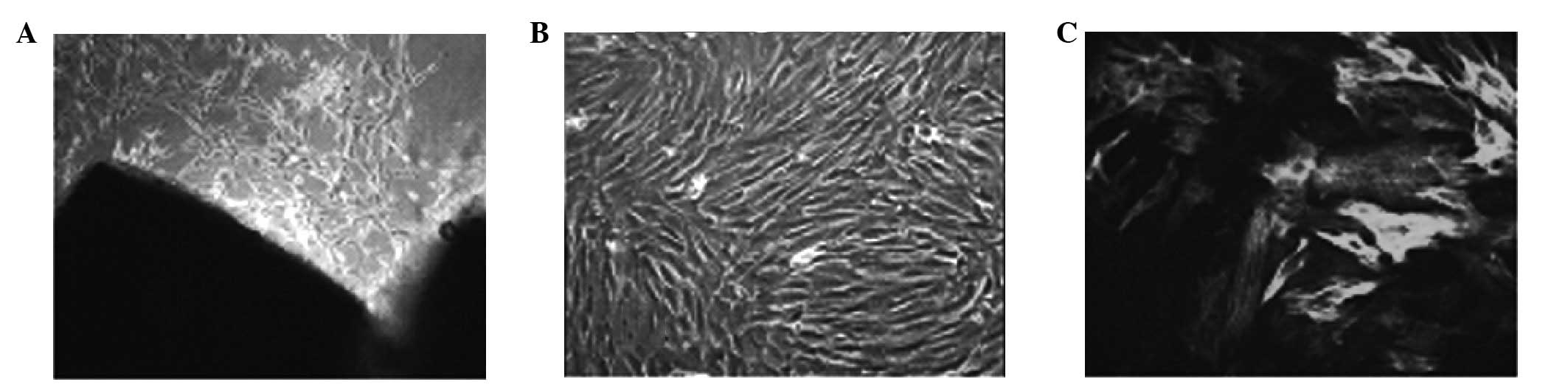
Cells were isolated and grew from homogenized aorta
following 3–5 days. The majority of the cells were elongated
spindle-shaped and fusion revealed typical ‘peak’- and
‘valley’-like growth states. α-SM immunofluorescence staining was
positive and a filament-like structure was visible in the cytoplasm
(Fig. 1). Results of the
biochemical analysis of CRF and control rabbits are presented in
Table II.
 | Table IIBiochemical index for the pooled sera
of CRF and normal rabbits. |
Table II
Biochemical index for the pooled sera
of CRF and normal rabbits.
| Rabbits | BUN (mmol/l) | SCr (μmol/l) | K+
(mmol/l) | Na+
(mmol/l) | Cl−
(mmol/l) | CO2CP
(mmol/l) | Ca2+
(mmol/l) | P3+
(mmol/l) | iPTH (pg/ml) |
|---|
| Normal | 7.65 | 76.60 | 4.80 | 135.10 | 98.32 | 24.00 | 2.07 | 1.13 | 9.8 |
| CRF | 25.75 | 350.80 | 5.80 | 145.20 | 101.25 | 22.00 | 1.62 | 1.26 | 23.2 |
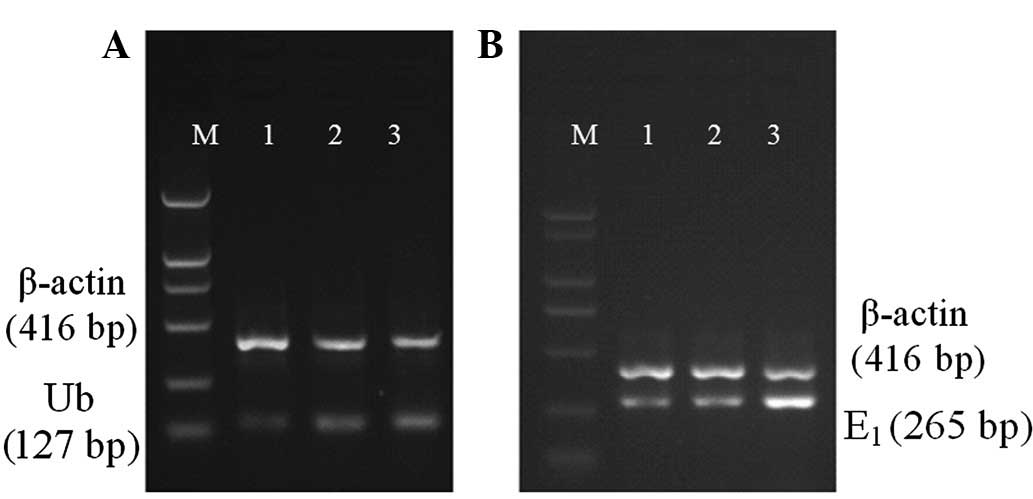
Ub and E1 mRNA expression
Compared with normal serum, 10% CRF serum was found
to increase Ub (0.16±0.03 vs. 0.26±0.02) and E1 (0.18±0.02 vs.
0.89±0.02) mRNA levels in ASMCs significantly (P<0.01; Fig. 2).
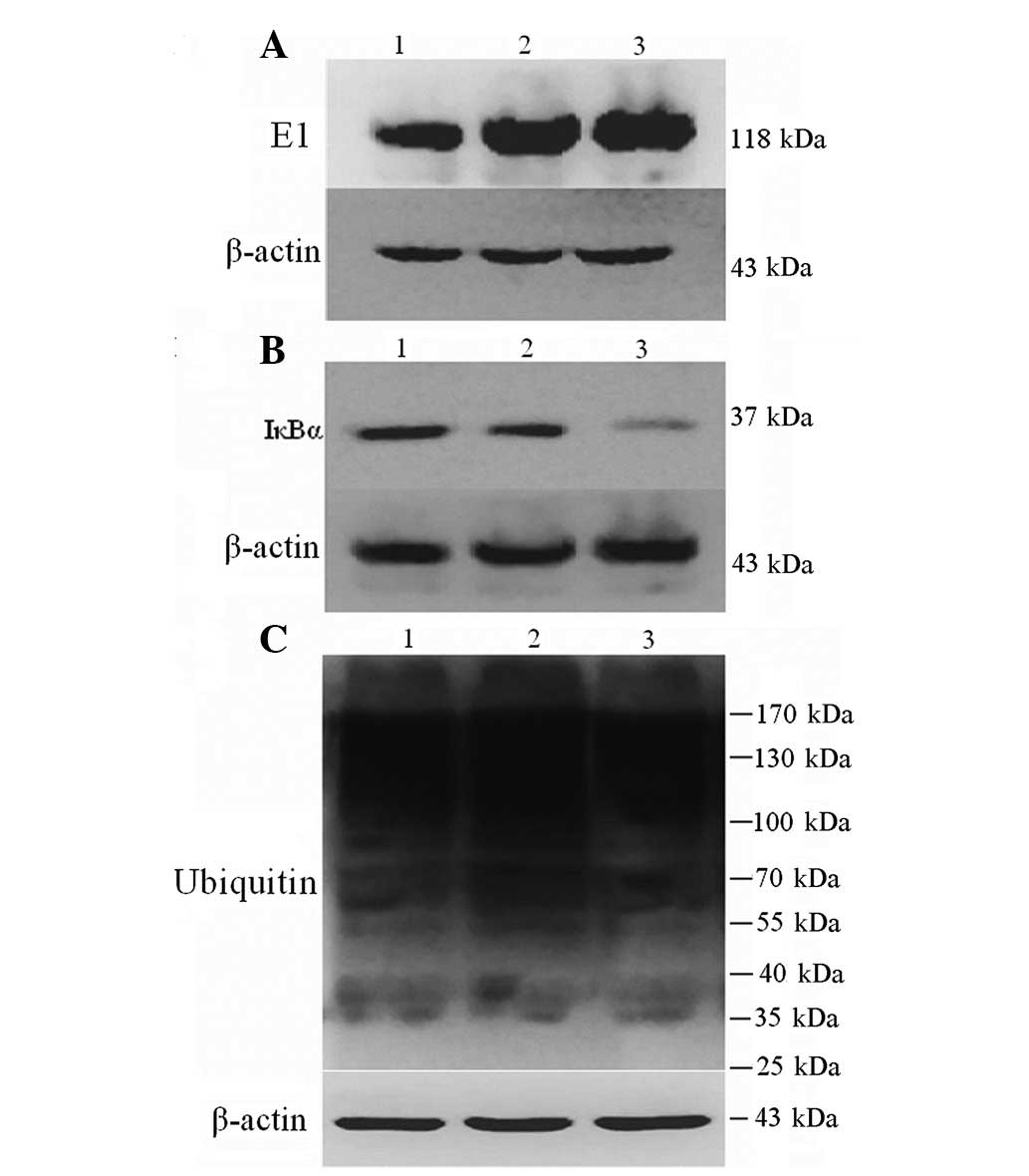
E1, IκBα and ubiquitinated protein
expression
Compared with normal serum, 10% CRF serum was
observed to significantly increase E1 (2.63±0.15 vs. 3.60±0.20) and
reduce IκBα protein expression (0.81±0.03 vs. 0.12±0.02) of ASMCs
(both P<0.01), but had no significant effect on expression of
ubiquitinated proteins (12.76±0.42 vs. 11.12±0.46; P>0.05;
Fig. 3).
Proteasome activity
Following 24 h incubation with 10% normal and CRF
serum, SMC proteasome activity increased. This effect was found to
be significantly higher in samples in CRF serum (P<0.01,
Table III).
 | Table IIIEffect of CRF serum on aortic
endothelial cells, proteasome 20S subunit activity (mean ± SD,
n=3). |
Table III
Effect of CRF serum on aortic
endothelial cells, proteasome 20S subunit activity (mean ± SD,
n=3).
| Fluorescence
absorbance value (A) |
|---|
|
|
|---|
| Group | Substrate S | Substrate Z | Substrate B |
|---|
| Serum-free | 37.47±1.14 | 90.67±2.97 | 70.10±1.77 |
| Normal serum | 50.50±2.75a | 127.13±4.18a | 101.10±5.19a |
| CRF serum | 90.57±5.85a,b | 229.30±6.93a,b | 192.73±5.61a,b |
Discussion
Atherosclerosis is a chronic inflammatory response
in vascular cells. Causative agents act on endothelial cells
repeatedly, making the cells appear dysfunctional, which triggers
inflammatory cytokine expression and consequently, the
atherosclerosis dynamic link. Abnormal proliferation of SMCs is key
to atherosclerosis development (10,11).
The middle vessel wall is composed primarily of
ASMCs, for which there are shrinkage and synthesis types. The
former stimulates chemical and mechanical contraction, whereas the
latter is primarily involved in the secretion of the extracellular
matrix and the synthesis of vasoactive substances, as well as cell
division and proliferation under growth factor stimulation involved
in the formation and damage repair of the vessel wall (11). During atherosclerosis, the
activation of endothelial cells triggers a conversion in the SMC
phenotype from contractile to synthetic and the cells migrate to
the intima under the effect of various factors. Abnormal
proliferation occurs, which involves foam cell accumulation and the
formation of fibrous and atherosclerotic plaques (12).
Previous studies on the SMC proliferation mechanism
and prevention have reported that the inhibition of only one factor
cannot suppress the formation of lesions completely. Therefore, the
final common pathway for the effective prevention of vascular
proliferative disease may be achieved by regulating cell
proliferation, differentiation and migration.
The UPP is present in all eukaryotic cells. In
addition to Ub expression, the pathway also includes the expression
of E1, ubiquitin-conjugating enzyme (E2), ubiquitin ligases (E3)
and the 26S proteasome. UPP selectively degrades intracellular
ubiquitination proteins using the proteasome. Thus, UPP not only
destroys old damaged proteins, but is also involved in regulating a
variety of important life processes (13–15),
particularly through degradation of intracellular signaling
pathways inhibitors and/or activators.
The UPP is the most important mechanism for
regulation of the IKK/NF-κB signaling pathway activation. To date,
hundreds of forms of the E3 ligase have been identified.
Recognition and binding of proteins and targets are specific and
the tissue distribution of proteins are different. Previous studies
have also found a variety of proteasome subtypes, which may play
major roles in various pathophysiologies (16,17).
Interfering with blood cell-specific UPP activation, thereby
inhibiting the proliferation of local tissue response to chronic
inflammation, may be a promising approach in the clinical
development of specific drugs.
In the present study, following CRF serum
stimulation, Ub and E1 mRNA upregulation, as well as E1 protein
expression in ASMCs, increased, but ubiquitinated protein levels
were not found to be significantly altered. Protein ubiquitination
is a prerequisite for UPP activation. Two hypotheses may account
for the insignificant change in ubiquitination: i) degradation was
not significant; or ii) the increase in proteasomal activity was
compensated by degrading the increased pathological proteins.
The 26S proteasome is the final location of target
protein degradation. This proteasome is a huge multi-subunit
protease complex, primarily composed of two ring-shaped 19S
regulatory subunits and a 20S catalytic subunit. A 20S subunit
consists of four rings, including α and β rings constituted by
seven homologous α subunits and seven homologous β subunits. Active
sites are located in the hollow center of the cylindrical 20S
structure and the N-terminal threonine of the β subunit functions
as a protease catalytic center, prominent in the barrel cavity.
Only three of seven β-subunits have catalytic activity, namely,
trypsin-, chymotrypsin- and cysteine protease-like. These
activities cleave carboxy-terminal alkaline, hydrophobic or
aromatic and acidic amino acid residues, thus degrading the
substrate protein into small peptides (18).
To identify UPP activation signaling pathways, the
activity of three 20S proteasome subunits of SMCs were examined.
The activities of these enzymes were significantly higher compared
with the control group. Carbó et al(19) studied human umbilical vein
endothelial cells stimulated by uremic serum using a proteomic
method. The authors found that levels of the proteasome subunit β
type 4 precursor and the proteasome activator complex subunit 3
expression increased, consistent with results in the present study.
These observations indicate that CRF serum induced vascular cell
UPP activation.
Degradation of ubiquitinated IκB is an important
step in regulation of NF-κB activation. The present study found
that CRF serum stimulation significantly reduced ASMC expression of
IκB mRNA and protein, implying that UPP may be involved in the
activation of the CRF serum-induced ASMC inflammatory response.
Further studies are required to determine whether regulation of the
NF-κB signaling pathway by UPP activation inhibits proliferation of
the SMC inflammatory response and improves SMC function. In
summary, CRF serum stimulation activates UPP in ASMCs, thereby
affecting regulation of the NF-κB signaling pathway. These results
are likely to provide theoretical and experimental insight to aid
research into the prevention and treatment of atherosclerosis
CRF.
References
|
1
|
Locatelli F, Pozzoni P, Tentori F and del
Vecchio L: Epidemiology of cardiovascular risk in patients with
chronic kidney disease. Nephrol Dial Transplant. 18:2–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou FF, Ma ZG, Mei CL, et al: Epidemiology
of cardiovascular risk in Chinese chronic kidney disease patients.
Zhonghua Yi Xue Za Zhi. 85:753–759. 2005.(In Chinese).
|
|
3
|
Mitch WE, Du J, Bailey JL and Price SR:
Mechanisms causing muscle proteolysis in uremia: the influence of
insulin and cytokines. Miner Electrolyte Metab. 25:216–219. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Price SR, Du JD, Bailey JL and Mitch WE:
Molecular mechanisms regulating protein turnover in muscle. Am J
Kidney Dis. 37(Suppl 2): 112–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitch WE: Robert H Herman Memorial Award
in Clinical Nutrition Lecture, 1997. Mechanisms causing loss of
lean body mass in kidney disease. Am J Clin Nutr. 67:359–366.
1998.PubMed/NCBI
|
|
6
|
Fukai T: Targeting proteasome worsens
atherosclerosis. Circ Res. 101:859–861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herrmann J, Soares SM, Lerman LO and
Lerman A: Potential role of the ubiquitin-proteasome system in
atherosclerosis: aspects of a protein quality disease. J Am Coll
Cardiol. 51:2003–2010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng B, Zhang Y, Mu J, et al: Preventive
effect of a proteasome inhibitor on the formation of accelerated
atherosclerosis in rabbits with uremia. J Cardiovasc Pharmacol.
55:129–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao R, Lu L, Zhang R, Hu J, Ni J and Shen
W: Triptolide inhibits rat vascular smooth muscle cell
proliferation and cell cycle progression via attenuation of ERK1/2
and Rb phosphorylation. Exp Mol Pathol. 90:137–142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Little PJ, Ivey ME and Osman N:
Endothelin-1 actions on vascular smooth muscle cell functions as a
target for the prevention of atherosclerosis. Curr Vasc Pharmacol.
6:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung SW, Park JW, Lee SA, Eo SK and Kim
K: Thrombin promotes proinflammatory phenotype in human vascular
smooth muscle cell. Biochem Biophys Res Commun. 396:748–754. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newby AC: Matrix metalloproteinases
regulate migration, proliferation and death of vascular smooth
muscle cells by degrading matrix and non-matrix substrates.
Cardiovasc Res. 69:614–624. 2006. View Article : Google Scholar
|
|
13
|
Murata T and Shimotohno K: Ubiquitination
and proteasome-dependent degradation of human eukaryotic
translation initiation factor 4E. J Biol Chem. 281:20788–20800.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciechanover A and Iwai K: The ubiquitin
proteolytic system: from an idea to the patient bed. Proc Am Thorac
Soc. 3:21–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gomes AV, Young GW, Wang Y, et al:
Contrasting proteome biology and functional heterogeneity of the 20
S proteasome complexes in mammalian tissues. Mol Cell Proteomics.
8:302–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drews O, Wildgruber R and Zong C:
Mammalian proteasome subpopulations with distinct molecular
compositions and proteolytic activities. Mol Cell Proteomics.
6:2021–2031. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Groll M, Ditzel L, Löwe J, et al:
Structure of the 20S proteasome from yeast at 2.4 A resolution.
Nature. 386:463–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carbó C, Arderiu G, Escolar G, et al:
Differential expression of proteins from cultured endothelial cells
exposed to uremic versus normal serum. Am J Kidney Dis. 51:603–612.
2008.PubMed/NCBI
|