Introduction
Pancreatic cancer remains a significant, unresolved
therapeutic challenge with similar incidence and mortality rates
(1,2). Complete resection remains the only
therapeutic option. Thus, the overall 5-year survival of the
patients has not been improved over the last 2 decades (3,4).
Therefore, it is necessary to develop novel therapeutic approaches
to treat pancreatic cancer. Over the past decade, improved
understanding and knowledge of the immune system have generated
novel strategies for immunotherapy (5). While the success of tumor
immunotherapy primarily relies on the identification of tumor
antigens, the expression of transformation-associated stress genes
commonly provokes innate immune reactions. These responses may be
exploited to develop immunotherapeutic approaches to treat cancer
(6).
MHC class I polypeptide-related sequence B (MICB) is
a glycosylated, polymorphic and membrane-anchored non-classical MHC
class I molecule, and is a stress-induced antigen. In normal
tissue, MICB has been shown to be mostly restricted to the
gastrointestinal tract, but was also shown to be stress inducible
in a series of cell lines. However, MICB is upregulated by a range
of primary tumors, including lung, kidney, prostate, breast and
colon cancers (7). Immune response
induced by MICB-NKG2D has been well documented to play an important
role in the eradiation of tumors by T and/or NK cells (8,9).
A previous study suggested that MICB was induced and
expressed widely in pancreatic cancer cell lines (10). However, MICB can be cleaved by
proteolytic shedding and released into the bloodstream as soluble
MICB (sMICB). sMICB has been correlated with poor differentiation
and high tumor stage of pancreatic cancer (11). Two mechanisms have been proposed,
whereby release of sMICB may reduce immunogenicity of tumor cells:
the shedding of MICB leads to decreased MICB expression levels on
the tumor cell surface, which directly affects tumor cell lysis
(12). Secondly, it is reported
that sMICB shedding from membrane-binding MICB on tumor cells
impaired NKG2D-mediated cytotoxicity by reducing the expression of
NKG2D receptor through internalization and degradation (13). Therefore, elucidating the molecular
mechanisms of MICB shedding from the pancreatic cancer cell
membrane and, subsequently, developing therapeutic strategies to
suppress MICB shedding may be of substantial benefit for pancreatic
cancer treatment.
Previously, members of the metzincin superfamily,
such as ADAMs (a disintegrin and metalloproteinase), have been
reported to play crucial roles in the proteolytic release of the
ectodomain of transmembranous proteins, including MICB, from the
cell surface (14). MICB shedding
of CV1 cells and human glioma U373 cells was found to be inhibited
through the silencing of the ADAM17 proteases. This suggests that
ADAM17 is involved in the shedding of membrane-bound MICB (15). However, it remains to be determined
wherther other ADAM proteases are able to affect MICB shedding.
Gemcitabine is a first-line chemotherapy drug for
pancreatic cancer. Gemcitabine alone or in combination with
5-fluorouracil or radiation treatment may prolong the survival of
pancreatic cancer patients (16).
Plate et al(17)
characterized the change of immune cells in pancreatic cancer
patients treated with gemcitabine. The data suggest that
gemcitabine therapy may increase memory T cells and promote naïve
T-cell activation, and that gemcitabine therapy is not
immunosuppressive, but may enhance antitumor immunity induced by
tumor vaccine. To develop further uses for gemcitabine in
pancreatic cancer treatment, its immunological impact needs to be
evaluated.
In the present study, we investigated the expression
of MICB and ADAM15 in pancreatic ductal adenocarcinoma (PDAC), and
studied the correlation between the clinicopathological
characteristics and the expression of MICB and ADAM15, further
showing the relevance of MICB and ADAM15 in PDAC. Of note, ADAM15
knockdown experiments demonstrated the essential roles of ADAM15
protease in the shedding of MICB molecules. Gemcitabine, a
nucleoside analog that has been approved as an antipancreatic
cancer molecularly targeted chemotherapy, effectively downregulated
sMICB while upregulating membrane-bound MICB via inhibition of
ADAM15.
Materials and methods
Subjects and samples
Ninety-three patients with PDAC [56 males and 37
females; age, 40–83 years (mean age, 67.3 years)] were enrolled
between January 2004 and June 2009. The patients were surgically
treated in the Xiangya Hospital affiliated to the Central South
University (Hunan, China). Fifteen normal pancreatic tissues were
collected through an organ donor procurement program, whenever
there was no suitable recipient for pancreas transplantation. The
samples of cancer tissue and normal pancreatic tissues were
obtained during surgery. The samples were then fixed in 10%
formalin solution and embedded in paraffin. The diagnosis of the
samples was confirmed histopathologically. The use of the clinical
samples for analysis was approved by the Ethics Committee of the
Central South University.
Immunohistochemistry
Immunohistochemical staining was performed using the
streptavidin-biotin peroxidase method as previously described
(18). MICB monoclonal antibodies
(sc-80527, dilution 1:100; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and mouse anti-ADAM15 monoclonal antibodies
(sc-365752, dilution 1:100; Santa Cruz Biotechnology, Inc.) were
used in this study.
Evaluation of MICB and ADAM15
immunohistochemical staining
Two independent investigators unaware of patient
outcomes assessed the sections. This was a double-blind study. MICB
expression was evaluated according to staining intensity and scored
(18): negative (−), no staining
in cancer cells (same or weaker compared with the cancer stroma);
weak expression (+), the staining of the cancer cells is a little
stronger compared with that of the cancer stroma in the whole area,
or much stronger in a limited (<20%) area; strong expression
(++), the staining of the cancer cells is much stronger compared
with that of the cancer stroma in the whole section. In these
groups, the weak (+) and strong (++) cases were considered as
positive results for statistical analysis. Cases with negative (−)
and weak expression (+) were defined as the low-expression group,
and cases with strong expression (++) were defined as the
high-expression group.
The total immunohistochemical staining score for
ADAM15 staining of each section was calculated according to the
percentage of positive-staining tumor cells and the staining
intensity (19). The percentage of
positive cells was judged ranging from 1 to 4 (1, <10%; 2,
10–50%; 3, 51–80%; 4, >80% positive tumor cells), while the
level of staining intensity was classified between 0 and 3 (0,
negative; 1, weak; 2, moderate; 3, strong staining). Multiplying
positive-cell numbers by staining intensity yielded a score of
0–12. We considered samples as positive where the score was >2.
A score of 3–6 was regarded as moderate (+) and >6 as strong
(++).
Tumor cell lines and culture
The PANC-1 cell line was previously established in
our laboratory. The cells were cultured in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS; Sigma-Aldrich), 100 U/ml penicillin and 100
μg/ml streptomycin, and were maintained at 37°C with 5%
CO2 in a humidified atmosphere. The experiments were
carried out on logarithmically growing cells.
RNA silencing
The small interfering RNA (siRNA) method was used to
knockdown ADAM15. Stealth RNAi oligonucleotide targeting ADAM15 and
scrambled oligonucleotides as the control were purchased from
Invitrogen (Carlsbad, CA, USA). Cells were transfected by RNAiMAX
Transfection reagent (Invitrogen) with 50 nmol/l siRNA. The siRNAs
used were: ADAM15, 5′-AACCCAGCTGTCACCCTCGAA-3′; scramble control,
5′-TTCGAGGGTGACAGCTGGGTT-3′.
Real-time reverse-transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol RNA extraction
reagent (Sigma-Aldrich), according to the manufacturer’s
instructions. cDNA was synthesized with the SuperScript III
First-Strand Synthesis kit (Invitrogen) and kept frozen at −20°C
until analysis. cDNA was amplified (40 cycles, 95°C for 15 sec,
60°C for 1 min) using ABI PRISM® 7700 Sequence Detection
system (Applied Biosystems, Foster City, CA, USA) in a final volume
of 20 μl containing 1 μl of cDNA, 12.5 μl of SYBR-Green Master mix
(Applied Biosystems) and 0.5 μM forward and reverse primers in
DNAse-free water. The ΔCt method was used for relative
quantifications. The primer sequences used in this study were:
ADAM15 forward, 5′-AGC CTCAAAAAGGTGCTTCA-3′ and reverse,
5′-CCCTGGTAG CAGCAGTTCTC-3′; MICB forward, 5′-CTTCGTTACAAC
CTCATGGT-3′ and reverse, 5′-ATATGAGTCAGGGTCCTC CT-3′; 18S rRNA
forward, 5′-CCATCCAATCGGTAGTAG CG-3′ and reverse,
5′-GTAACCCGTTGAACCCCATT-3′.
Western blotting
Western blot analysis was carried out as previously
described (20). PANC-1 cells were
lysed by the addition of lysis buffer containing 0.5% sodium
deoxycholate, 1% Nonidet P-40, 50 mM Tris-HCl and 150 mM NaCl. Cell
lysates were cleared by centrifugation at 16,000 rpm, 4°C for 10
min. Cleared lysates were boiled for 5 min at 100°C after the
addition of 5X sample loading buffer containing 1 M Tris-HCl,
sodium dodesylsulphate, glycerol and bromphenolblue. The samples
were electrophoresed at 200 V on 12.5% polyacrylamide gels and
transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA,
USA), blocked with 5% non-fat dry milk and incubated with primary
antibody. Anti-ADAM15 (sc-365752, dilution 1:1,000; Santa Cruz
Biotechnology, Inc.) and anti-GAPDH (sc-137179, dilution 1:1,000;
Santa Cruz Biotechnology, Inc.) were used as the primary
antibodies.
Determination of MICB levels in the
culture supernatant
Secretion of MICB by PANC-1 cells cultured under
various conditions was assessed by ELISA using commercially
available kits (R&D Systems, Minneapolis, MN, USA) and
following the manufacturer’s instructions.
Assessment of surface MICB
expression
For the detection of membrane-bound MICB, cells were
incubated with an anti-MICB-specific antibody (sc-80527, Santa Cruz
Biotechnology, Inc.) and stained with phycoerythrin (PE)-goat
anti-mouse immunoglobulin (BD Biosciences, Franklin Lakes, NJ, USA)
as a secondary reagent and then subjected to flow cytometry. Flow
cytometry was performed using a FACScan flow cytometer
(Becton-Dickinson, San Jose, CA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The viability of the PANC-1 cells was determined by
MTT assay. Cells were stained with MTT (Amresco, Solon, OH, USA).
Following 4 h of additional incubation, the medium was discarded,
100 μl of acidic iso-propanol (0.1 M HCl in absolute isopropanol)
was added and the plate was agitated gently. Absorbance was
measured on an ELISA reader at a test wavelength of 540 nm.
Statistical analysis
Data are reported as the means ± standard deviation
(SD), range and frequencies. MICB and ADAM15 expression for
pancreatic cancer tissues and normal pancreatic tissue groups were
compared using the Wilcoxon rank sum test. The correlation between
the clinicopathological characteristics and the expressions of MICB
and ADAM15 were analyzed using the Chi-square test. The
correlations between MICB and ADAM15 were determined using the
Spearman’s rank correlation coefficient. Continuous variables were
compared using the Student’s t-test or ANOVA if normally
distributed and the Wilcoxon rank sum test, if distributions were
non-parametric. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference. The calculations were
performed using the SPSS 13.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Expression levels of MICB and ADAM15 in
pancreatic cancer and normal pancreatic tissues
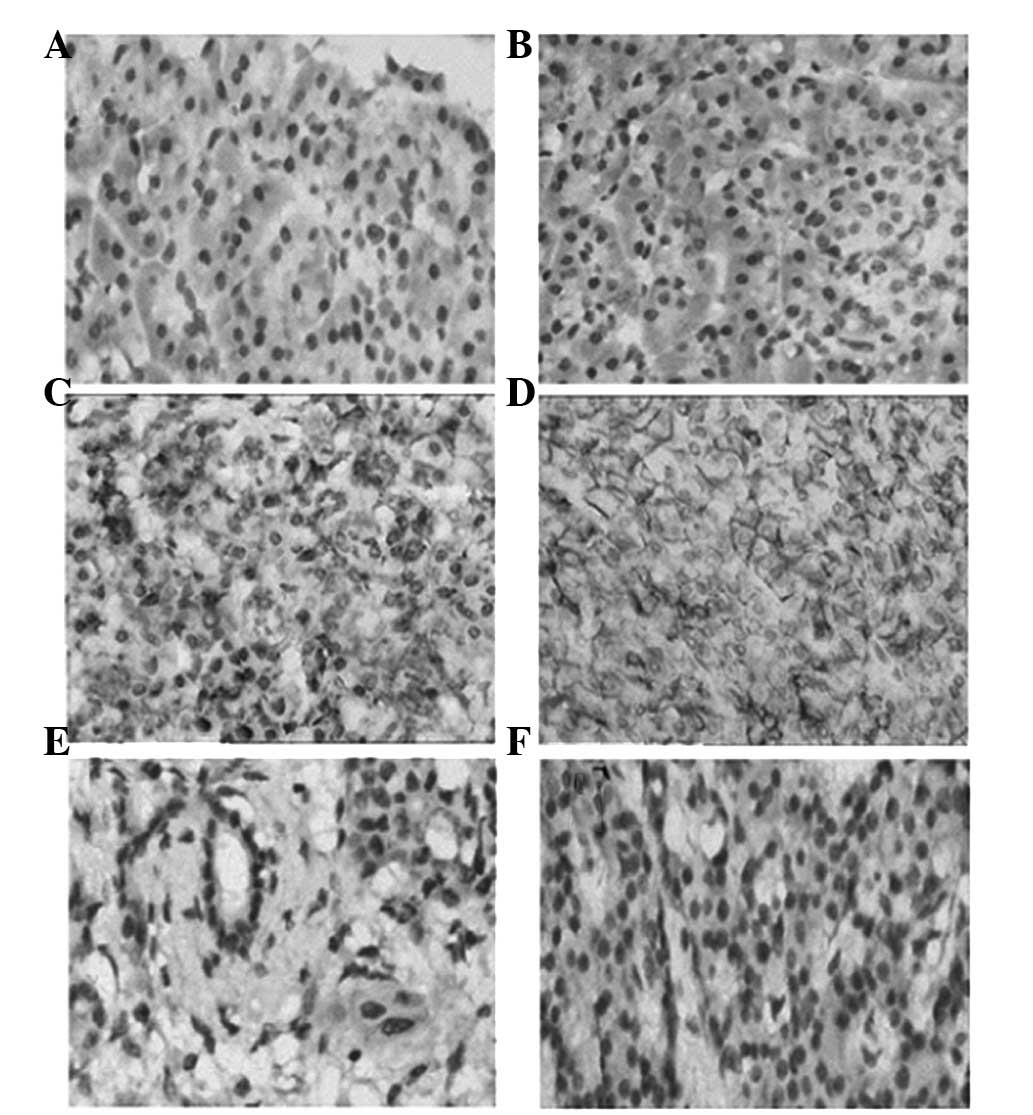
The MICB expression was predominantly distributed on
the membrane of the cells. The positive rates of MICB expression
were 69.9% (65/93) in pancreatic cancer tissues and 6.7% (1/15) in
normal pancreatic tissues. The Wilcoxon rank sum test indicated
that the expression of MICB in pancreatic cancer was significantly
higher compared with that in normal pancreatic tissues (P<0.01).
Positive ADAM15 immunostaining was intensely located in cytoplasm
and on the cell membrane. The positive rates of ADAM15 expression
were 78.5% (73/93) in pancreatic cancer tissues and 13.3% (2/15) in
normal tissues of the pancreas. The expression of ADAM15 in normal
pancreatic tissues was significantly lower compared with that in
pancreatic cancer tissues (P<0.01). The immunohistochemical
staining results of MICB and ADAM15 are shown in Table I. The immunohistochemical staining
is shown in Fig. 1.
 | Table IExpression of MICB and ADAM15 in
pancreatic cancer and normal pancreatic tissues. |
Table I
Expression of MICB and ADAM15 in
pancreatic cancer and normal pancreatic tissues.
| | MICB | ADAM15 |
|---|
| |
|
|
|---|
| Groups | No. | − | + | ++ | − | + | ++ |
|---|
| Pancreatic cancer
tissues | 93 | 28 | 39 | 26 | 20 | 28 | 45 |
| Normal pancreatic
tissues | 15 | 14 | 1 | 0 | 13 | 2 | 0 |
Correlation between MICB, ADAM15
expression and clinicopathological characteristics
The correlation between the clinicopathological
characteristics of pancreatic cancer patients and expression levels
of MICB and ADAM15 is summarized in Table II. A significant difference was
observed in the MICB expression with regard to the histological
grade (P=0.033) and TNM stages (P=0.016). There was no significant
correlation between the MICB expression and the clinicopathological
characteristics with regard to age, gender, tumor size and distant
or lymph node metastases (P>0.05, respectively). The ADAM15
expression was found to correlate with lymph node metastasis
(P=0.022) and TNM stages (P=0.039). However, the ADAM15 expression
was not associated with age, gender, tumor size, histological grade
or distant metastasis (P>0.05).
 | Table IICorrelation between MICB and ADAM15
expression and clinicopathological characteristics. |
Table II
Correlation between MICB and ADAM15
expression and clinicopathological characteristics.
| | MICB | ADAM15 |
|---|
| |
|
|
|---|
| Characteristics | No. | High | Low | P-value | Positive | Negative | P-value |
|---|
| Age (years) |
| ≤65 | 50 | 18 | 32 | 0.062 | 41 | 9 | 0.375 |
| >65 | 43 | 8 | 35 | | 32 | 11 | |
| Gender |
| Male | 56 | 15 | 41 | 0.757 | 42 | 14 | 0.313 |
| Female | 37 | 11 | 26 | | 31 | 6 | |
| Tumor size (cm) |
| ≤3 | 45 | 14 | 31 | 0.512 | 33 | 12 | 0.241 |
| >3 | 48 | 12 | 36 | | 40 | 8 | |
| Histological
grade |
| Well | 28 | 13 | 15 | 0.033 | 21 | 7 | 0.658 |
| Moderately | 39 | 8 | 31 | | 30 | 9 | |
| Poorly | 26 | 5 | 21 | | 22 | 4 | |
| Lymph node
metastasis |
| Positive | 39 | 9 | 30 | 0.373 | 34 | 5 | 0.022 |
| Negative | 24 | 8 | 16 | | 15 | 9 | |
| Distant
metastasis |
| Present | 14 | 6 | 8 | 0.305 | 9 | 5 | 0.293 |
| Absent | 79 | 20 | 59 | | 64 | 15 | |
| TNM stage |
| I+II | 61 | 22 | 39 | 0.016 | 44 | 17 | 0.039 |
| III+IV | 32 | 4 | 28 | | 29 | 3 | |
Correlation between MICB and ADAM15
expression in pancreatic cancer tissues
The Spearman’s rank test suggested that the
expression of MICB was correlated inversely with that of ADAM15 in
pancreatic cancer tissues (r=−0.253, P=0.014, Table III).
 | Table IIICorrelation between MICB and ADAM15
expression in pancreatic cancer. |
Table III
Correlation between MICB and ADAM15
expression in pancreatic cancer.
| ADAM15 | |
|---|
|
| |
|---|
| MICB | − | + | ++ | Total |
|---|
| − | 5 | 7 | 16 | 28 |
| + | 4 | 13 | 22 | 39 |
| ++ | 11 | 8 | 7 | 26 |
| Total | 20 | 28 | 45 | 93 |
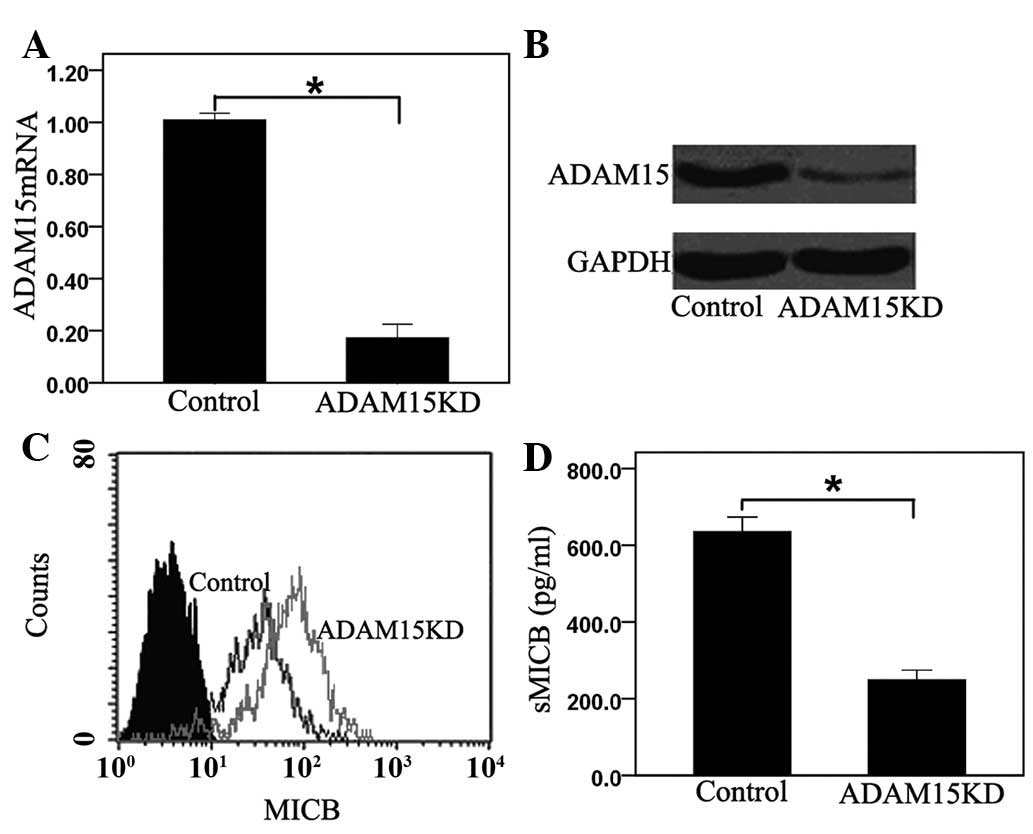
ADAM15 mediates the shedding of MICB in
PANC-1 cells
To directly verify if ADAM15 is involved in MICB
ectodomain shedding of PANC-1 cells, PANC-1 cells were transfected
with siRNA targeted against endogenous human ADAM15. No difference
was noted in proliferation between the control and ADAM15 knockdown
cells (data not shown). The downregulation of ADAM15 transcripts
and ADAM15 protein was monitored by real-time RT-PCR and by western
blotting, respectively. The siRNA duplexes targeted against ADAM15
depleted endogenous levels of the mRNA by 85.88±2.73% (Fig. 2A) and the protein by 74.39±3.26%,
when compared with cells transfected with control siRNA (Fig. 2B). Knockdown of ADAM15 for PANC-1
cells resulted in a 40.74±3.15% increase in membrane-bound MICB
(Fig. 2C) and in a 59.58±4.65%
reduction of sMICB levels in the culture medium (Fig. 2D). Taken together, the results of
the current study make a strong case that ADAM15 is a principal
sheddase for membrane-bound MICB in PANC-1 cells.
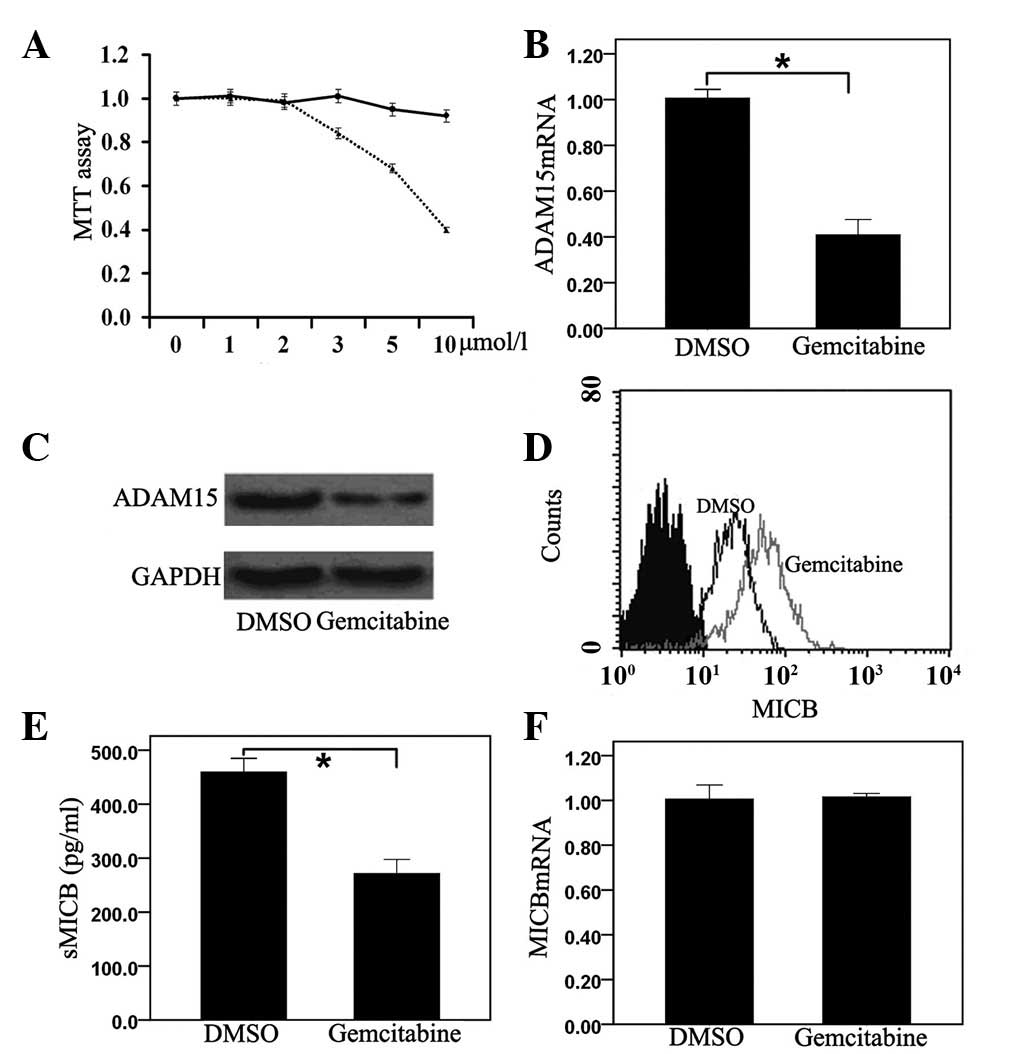
Gemcitabine suppresses ADAM15 expression
and inhibits MICB shedding of PANC-1 cells
The cytotoxicity of gemcitabine to PANC-1 cells was
evaluated using the MTT assay. Gemcitabine was found to have little
effect on the growth of PANC-1 cells at concentrations of ≤2 μmol/l
for 24 h (Fig. 3A). Based on this
finding, gemcitabine with concentrations of 0.5 μmol/l was used to
examine the biological effect on PANC-1 cells. PANC-1 cells were
cultured for 24 h with gemcitabine and ADAM15 expression was
evaluated at the mRNA and protein levels. Gemcitabine suppressed
ADAM15 expression in PANC-1 cells (Fig. 3B and C). Treatment with gemcitabine
was also observed to have markedly augmented membrane-bound MICB
expression (Fig. 3D) and
significantly decreased sMICB in PANC-1 cells (Fig. 3E). However, the mRNA levels of MICB
did not change in gemcitabine-treated PANC-1 cells (Fig. 3F).
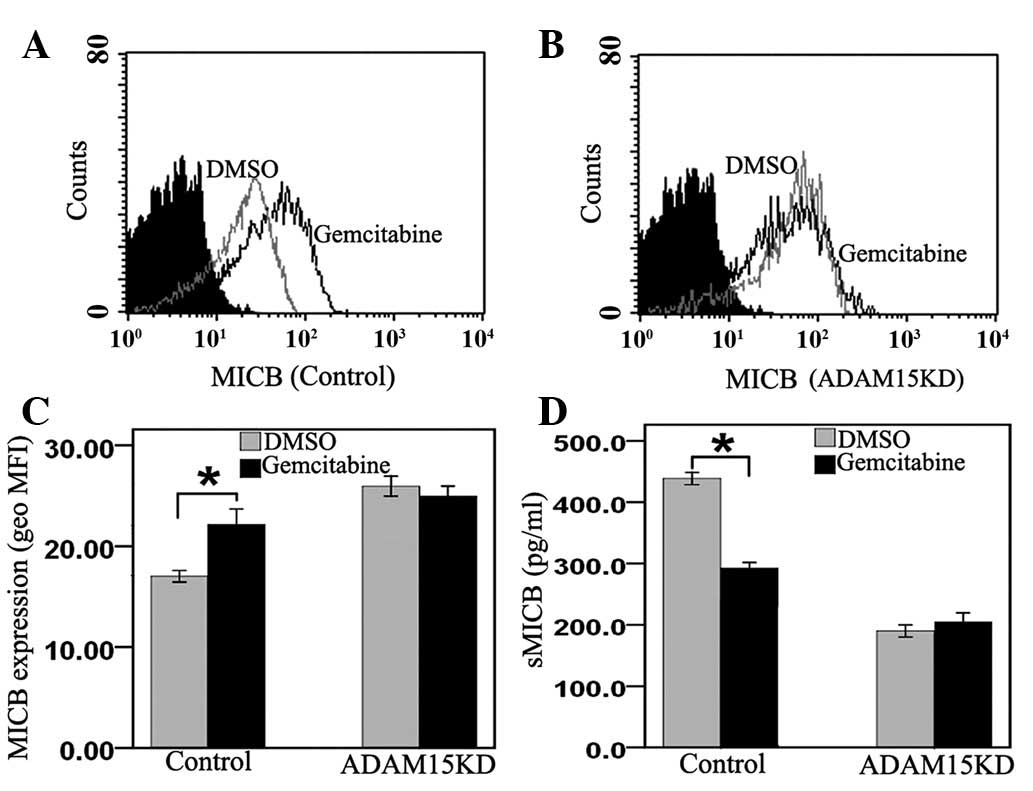
Gemcitabine inhibits MICB shedding of
PANC-1 cells by suppressing ADAM15
To verify whether gemcitabine inhibits MICB
ectodomain shedding through the suppression of ADAM15, PANC-1 cells
were transfected with ADAM15 siRNA or control siRNA and then
subjected to treatment with gemcitabine or DMSO. The data showed
that gemcitabine upregulated MICB surface expression (Fig. 4A and C) and downregulated the
levels of sMICB in control cells (Fig.
4D). Nevertheless, membrane-bound MICB (Fig. 4B and C) and sMICB levels (Fig. 4D) did not change in ADAM15
knockdown PANC-1 cells. These results suggest that gemcitabine
inhibits MICB shedding in PANC-1 cells by downregulating the
expression of ADAM15.
Discussion
Although upregulation of MICB had been observed in
pancreatic cancer cell lines, information on MICB expression on
human pancreatic cancer is scarce (10). In the present study, the expression
of MICB was examined in 93 pancreatic cancer patients using
immunohistochemistry. MICB was found to be overexpressed in PDAC
compared with normal pancreatic tissues. Thus, MICB is hypothesized
to be associated with the malignant transformation of pancreatic
cancer. Moreover, a significant difference was noted in the MICB
expression with regard to the histological grade and TNM stages.
The data also showed that although it was highly expressed in
pancreatic cancer, membrane-bound MICB was prevalent only in
low-grade cancers and positive staining for MICB was diffusely
distributed in the stroma in high-grade cancers. In particular, in
carcinomas with TNM stage I, most tumor cells showed negative cell
surface MICB immunoreactivity and diffuse MICB staining was shown
in the stroma. To the best of our knowledge, this is the first
study demonstrating the correlation between MICB expression and
clinicopathological characteristics in pancreatic cancer. MICB
expressed on the surface of pancreatic cancer cells may be released
into the tumor stroma and circulation in high-grade cancers
(21). Serum levels of sMICB in
pancreatic cancer patients were found to correlate significantly
with high tumor stage and poor differentiation (11). MICB shedding is thought to be a
principal mechanism by which tumor cells escape from NKG2D-mediated
immunosurveillance in pancreatic cancer (22). Therefore, it is necessary to
elucidate the molecular mechanisms of MICB ectodomain shedding in
pancreatic cancer.
ADAM15, cloned from mammary epithelial cells, is a
catalytically active member of the ADAM family of disintegrin
proteinases (23). One of the
major function of ADAM15 is the proteolytic release of ectodomains
of transmembranous proteins, including cytokines, growth factors
and cell adhesion molecules, from the cell surface (22). ADAM15 has been reported to be
frequently overexpressed in tumors and is thought to play key roles
in various steps of tumorigenesis (19). To date, limited data have been
published on the expression of ADAM15 in pancreatic cancer. The
present study demonstrated that ADAM15 expression is significantly
higher in PDAC compared with normal pancreatic tissues. The results
are consistent with findings in various additional malignant
tumors, including stomach and colorectal tumors (24). In this study, ADAM15 expression was
closely correlated with lymph node metastasis and TNM stages,
suggesting that ADAM15 may contribute to the invasive growth and
progression of pancreatic cancer.
A positive correlation between MICB and ADAM15
expression has also been confirmed in the present study. Of note,
the expression of membrane-bound MICB was inversely correlated with
that of ADAM15 in pancreatic cancer tissues. With the progression
of pancreatic cancer, the expression of ADAM15 increased, but MICB
was released into the tumor stroma and bloodstream as sMICB
(21). To the best of our
knowledge, this is the first study to report a large number of
clinical samples showing the exact correlation between MICB and
ADAM15.
The finding that ADAM15 was essential for the
cleavage of MICB was supported by testing the effect of the
siRNA-mediated knockdown of ADAM15 on the release of sMICB. In this
study, we demonstrated that ADAM15 knockdown resulted in increased
membrane-bound MICB and decreased sMICB. The present study is the
first to demonstrate that ADAM15 is involved in the shedding of the
MICB in PANC-1 cells. The observations in this study suggest that
ADAM15 may be a potential therapeutic target for inhibiting MICB
shedding.
Gemcitabine is currently the standard chemotherapy
for unresectable pancreatic cancer (25). Gemcitabine is a nucleoside analog
that exerts its antitumor activity via multiple mechanisms of
action. These include i) incorporation of gemcitabine into
replicating DNA, which inhibits DNA replication and cell growth;
ii) masked DNA chain termination; iii) several self-potentiation
mechanisms that serve to increase intracellular levels of the
active compound. It thus halts DNA synthesis and is invisible to
DNA repair systems, leading the cells into the apoptotic pathway
(26). Despite the promising
results associated with gemcitabine, its mode of action and of the
enzymes it interacts with have yet to be fully documented (27). Our results demonstrated that
gemcitabine downregulated ADAM15 expression, thereby resulting in
the increased expression of membrane-bound MICB and the decreased
production of sMICB. However, the data showed that the mRNA levels
of MICB did not change following exposure to gemcitabine in PANC-1
cells. To the best of our knowledge, we are the first to report
that gemcitabine is able to modulate PANC-1 cells by downregulating
ADAM15 expression, thereby inhibiting MICB ectodomain shedding. The
combination of molecularly targeted therapy and immunotherapy
targeting activation of NK cells may improve the antitumor effect
against unresectable pancreatic cancer and the prognosis of
patients with pancreatic cancer (28). Further studies (e.g., animal
models) are required to establish whether gemcitabine results in
reduced sMICB levels and an enhanced NKG2D-mediated tumor
elimination.
In conclusion, although the expression of surface
MICB at an early stage of pancreatic cancer is thought to lead to
the activation of effector cells and the destruction of the tumor
cells, the development of pancreatic cancer may lead to the
shedding of MICB. The present study has demonstrated unequivocally
for the first time that MICB is constitutively shed by ADAM15. We
have also shown that gemcitabine suppressed MICB ectodomain
shedding from PANC-1 cells through the inhibition of ADAM15. The
present study sheds light on previously unrecognized effects of
gemcitabine on modulating ADAM15 and MICB shedding, thus suggesting
its use in chemoimmunotherapy against human pancreatic cancer.
References
|
1
|
Sargent M, Boeck S, Heinemann V, Jauch KW,
Seufferlein T and Bruns CJ: Surgical treatment concepts for
patients with pancreatic cancer in Germany - results from a
national survey conducted among members of the ‘Chirurgische
Arbeitsgemeinschaft Onkologie’ (CAO) and the ‘Arbeitsgemeinschaft
Internistische Onkologie’ (AIO) of the Germany Cancer Society
(DKG). Langenbecks Arch Surg. 396:223–229. 2011.PubMed/NCBI
|
|
2
|
Hackert T, Büchler MW and Werner J:
Surgical options in the management of pancreatic cancer. Minerva
Chir. 64:465–476. 2009.PubMed/NCBI
|
|
3
|
Siegel R, Desantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levi F, Lucchini F, Negri E and La Vecchia
C: Pancreatic cancer mortality in Europe: the leveling of an
epidemic. Pancreas. 27:139–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldmann TA: Immunotherapy: past, present
and future. Nat Med. 9:269–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dranoff G: Coordinated tumor immunity. J
Clin Invest. 111:1116–1118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groh V, Rhinehart R, Secrist H, Bauer S,
Grabstein KH and Spies T: Broad tumor-associated expression and
recognition by tumor-derived gamma delta T cells of MICB and MICB.
Proc Natl Acad Sci USA. 96:6879–6884. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
González S, Groh V and Spies T:
Immunobiology of human NKG2D and its ligands. Curr Top Microbiol
Immunol. 298:121–138. 2006.
|
|
9
|
Wu JD, Higgins LM, Steinle A, Cosman D,
Haugk K and Plymate SR: Prevalent expression of the
immunostimulatory MHC class I chain-related molecule is
counteracted by shedding in prostate cancer. J Clin Invest.
114:560–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohashi M, Yoshida K, Kushida M, et al:
Adenovirus-mediated interferon gene transfer induces regional
direct cytotoxicity and possible systemic immunity against
pancreatic cancer. Br J Cancer. 93:441–449. 2005. View Article : Google Scholar
|
|
11
|
Märten A, von Lilienfeld-Toal M, Büchler
MW and Schmidt J: Soluble MIC is elevated in the serum of patients
with pancreatic carcinoma diminishing gammadelta T cell
cytotoxicity. Int J Cancer. 119:2359–2365. 2006.PubMed/NCBI
|
|
12
|
Diefenbach A, Jensen ER, Jamieson AM and
Raulet DH: Rae1 and H60 ligands of the NKG2D receptor stimulate
tumour immunity. Nature. 413:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:734–738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salih HR, Goehlsdorf D and Steinle A:
Release of MICB molecules by tumor cells: mechanism and soluble
MICB in sera of cancer patients. Hum Immunol. 67:188–195. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boutet P, Agüera-González S, Atkinson S,
et al: Cutting edge: the metalloproteinase
ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding
of the MHC class I-related chain B protein. J Immunol. 182:49–53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
17
|
Plate JM, Plate AE, Shott S, Bograd S and
Harris JE: Effect of gemcitabine on immune cells in subjects with
adenocarcinoma of the pancreas. Cancer Immunol Immunother.
54:915–925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Mandai M, Hamanishi J, et al:
Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in
ovarian cancer: high expression of ULBP2 is an indicator of poor
prognosis. Cancer Immunol Immunother. 58:641–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schütz A, Härtig W, Wobus M, Grosche J,
Wittekind Ch and Aust G: Expression of ADAM15 in lung carcinomas.
Virchows Arch. 446:421–429. 2005.
|
|
20
|
Chen X, Liao J, Lu Y, Duan X and Sun W:
Activation of the PI3K/Akt pathway mediates bone morphogenetic
protein 2-induced invasion of pancreatic cancer cells PANC-1.
Pathol Oncol Res. 17:257–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holdenrieder S, Stieber P, Peterfi A,
Nagel D, Steinle A and Salih HR: Soluble MICB in malignant
diseases: analysis of diagnostic significance and correlation with
soluble MICA. Cancer Immunol Immunother. 55:1584–1589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaiser BK, Yim D, Chow IT, et al:
Disulphide-isomerase-enabled shedding of tumour-associated NKG2D
ligands. Nature. 447:482–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Najy AJ, Day KC and Day ML: The ectodomain
shedding of E-cadherin by ADAM15 supports ErbB receptor activation.
J Biol Chem. 283:18393–18401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lucas N, Najy AJ and Day ML: The
therapeutic potential of ADAM15. Curr Pharm Des. 15:2311–2318.
2009. View Article : Google Scholar
|
|
25
|
Heinemann V, Boeck S, Hinke A, Labianca R
and Louvet C: Meta-analysis of randomized trials: evaluation of
benefit from gemcitabine-based combination chemotherapy applied in
advanced pancreatic cancer. BMC Cancer. 8:822008. View Article : Google Scholar
|
|
26
|
Abou-Alfa GK, Letourneau R, Harker G, et
al: Randomized phase III study of exatecan and gemcitabine compared
with gemcitabine alone in untreated advanced pancreatic cancer. J
Clin Oncol. 24:4441–4447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanagimoto H, Shiomi H, Satoi S, et al: A
phase II study of personalized peptide vaccination combined with
gemcitabine for non-resectable pancreatic cancer patients. Oncol
Rep. 24:795–801. 2010.PubMed/NCBI
|
|
28
|
Koido S, Homma S, Takahara A, et al:
Current immunotherapeutic approaches in pancreatic cancer. Clin Dev
Immunol. 2011:2675392011. View Article : Google Scholar : PubMed/NCBI
|