Introduction
Recombinant Ganoderma lucidum
immunomodulatory protein (rLZ-8), expressed using a Pichia yeast
eukaryotic expression system (1–3), was
the first member of the fungal immunomodulatory protein (FIP)
family discovered in mushrooms. It has been shown that rLZ-8 enjoys
a promising prospect as a new class I drug, since it induces
apoptosis in K562 and HL60 cells and acts as an immunomodulating
protein in a variety diseases, including in the non-obese diabetes
mouse model and in immunomodulation of food-induced allergic
reactions (4–14). However, rLZ-8 has a MW of only 13
kDa and its dynamic properties, such as the half-life, are not
satisfactory, which enormously limits its clinical application
(15–19).
In this study, we explored a strategy to overcome
the natural shortcomings of rLZ-8 using conventional PEGylation
technology to prolong its half-life and to enhance its stability
(20–27). We optimized the modification and
purification process, successfully prepared the single-point
modification product and carried out an initial characterization of
the association between the modification sites and the activity of
the modified product. This study has provided insights into the
structure-activity relationship of rLZ-8 and has also laid a
foundation for the development of long-acting formulations of
rLZ-8.
Materials and methods
Instruments and reagents
The following instruments/instrument systems were
used in this study: HPLC System (Shimadzu, Japan); AKTA Explorer
100 protein purification workstation (GE Healthcare, Franklin
Lakes, NJ, USA); ultrafiltration cup (GE Healthcare); gel imager
(Tanon, Shanghai, China); SDS-PAGE electrophoresis system (GE
Healthcare); electrospray ionization source high-resolution tandem
mass spectrometer (ESI-Q/TOF, Applied Biosciences, Carlsbad, CA,
USA) and laser desorption time-of-flight mass spectrometry
(MALDI-TOF/TOF, Applied Biosciences); Hiload 16/70 Superdex™ 75 gel
column (GE Healthcare). Methoxy-PEG-succinimidyl propionate
mPEG-SPA (5 kDa) was purchased from YareBio (Shanghai, China), and
the recombinant Ganoderma lucidum immunomodulatory protein
(rLZ-8) was obtained from our laboratory.
Modification optimization
Method
Optimization of the output of the single-point
modification reaction was carried out by altering one single effect
factor and keeping other factors the same in multi-level repeated
trials. The effect of each factor on the conformation of reaction
products was analyzed using SDS-PAGE and high-performance liquid
chromatography (HPLC).
Effect of pH and ionic strength
The modification of rLZ-8 with mPEG-SPA (1:12,
mol:mol) was carried out in a series of buffer systems at varying
pH, namely pH 4.0, 5.0, 6.0, 7.0 or 8.0, and then in pH 8.0
phosphate buffer with varying ionic strengths, at 0.025, 0.05, 0.1
or 0.2 M, at room temperature for 2 h, away from light. The results
were analyzed by SDS-PAGE followed by gel imaging after staining
with barium iodide and Coomassie Brilliant Blue.
Effect of reaction time and
temperature
In order to address the effect of time and
temperature on the modification reaction, rLZ-8 and mPEG-SPA (1:12,
mol:mol) were incubated together in a phosphate buffer system (0.1
M, pH 8.0) at 4, 16, 25 and 37°C, and sampled every 0.5 h for
analysis by HPLC, up to 2.5 h.
Effect of molar ratio of rLZ-8 and
mPEG-SPA
rLZ-8 and mPEG-SPA in a phosphate buffer system (0.1
M, pH 8.0) at molar ratios of 1:1, 1:3, 1:6, 1:12 and 1:24, were
mixed at room temperature for 2 h, away from light. HPLC and
SDS-PAGE with barium iodide or Coomassie Brilliant Blue staining
were used to monitor the reaction products.
Purification and identification of the
desired target product
The target product was purified using a Hiload 16/70
Superdex 75 gel chromatographic column with 0.05 M phosphate buffer
containing 0.15 M NaCl (pH 7.0) and a flow rate of 1 ml/min. After
equilibrating the column with the phosphate buffer for 2 column
volumes (120 ml), iso-concentration elution was applied for another
1.5 column volumes, with peak collection at every 2 ml and
detection wavelengths of 280, 254 and 215 nm. Every peak was
detected using SDS-PAGE and HPLC and the retention times of the
confirmed peaks were recorded.
Measurement of the biological activity
of the target product
A lymphocyte transformation test (BrdU reagent box)
was performed according to the manufacturer's instructions in order
to determine the biological activity of rLZ-8 and mPEG-SPA-rLZ-8.
The lymphocyte transformation test is based on the fact that
lymphocytes stimulated by certain antigens transform and
proliferate when re-exposed to the same antigens. The biological
activity of antigens is measured by monitoring the proliferation of
stimulated cells, and by measuring the incorporation of BrdU into
replicating DNA. A chromophore coupled to an anti-BrdU antibody
enables spectrophotometric detection of proliferating cells.
Analysis of the initial
characteristics of the target product Detection of protein
content
We used the Lowry method to measure the protein
concentration of the reaction product according to the guidelines
of the Chinese Pharmacopoeia 2010 Appendix VIB.
Validation of molecular weight and
modification site
Firstly, the sample (in the form of dry powder) was
placed into 50 mM NH4HCO to prepare a 1 mg/ml sample
solution. A 20-μl aliquot of the sample solution was then diluted
to obtain a 10-mM solution by adding 100 mM dithiothreitol (DTT)
and heating at 56°C for 1 h. After cooling the mixture to room
temperature, 250 mM indole-3-acetic acid (IAA) was added to obtain
a 25-mM sample solution and this mixture was protected from light
for 1 h. After completion of this step, 0.5 μg Trypsin was added
and the sample was digested at 37°C for 12 h. Finally, the reaction
was ended by adding 1 μl of 10% trifluoroacetic acid (TFA). The
digestion products and matrix were mixed at a proportion of 1:3 and
dried atmospherically. The peptide mass was detected by PMF
analysis in order to identify the PEGylated site on rLZ-8 in the
following modes: the mass spectra for the 500–4000 m/z range were
determined in the reflection positive ion mode and the 1000–10000
m/z range was determined in the linear positive ion mode.
Results
Modification optimization
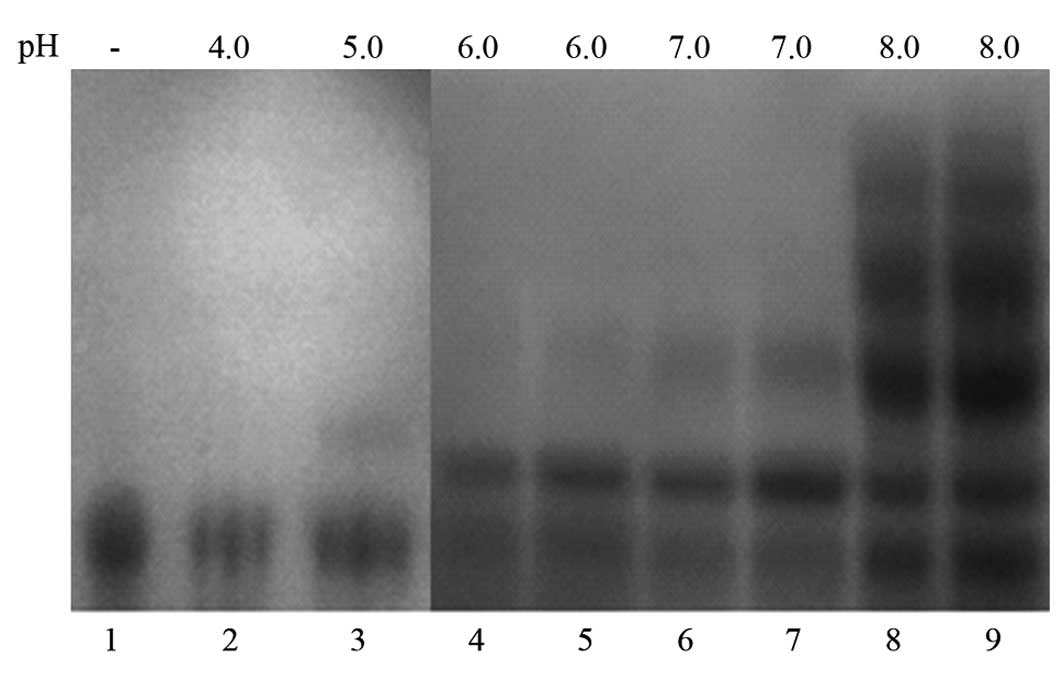
Effect of pH and ionic strength
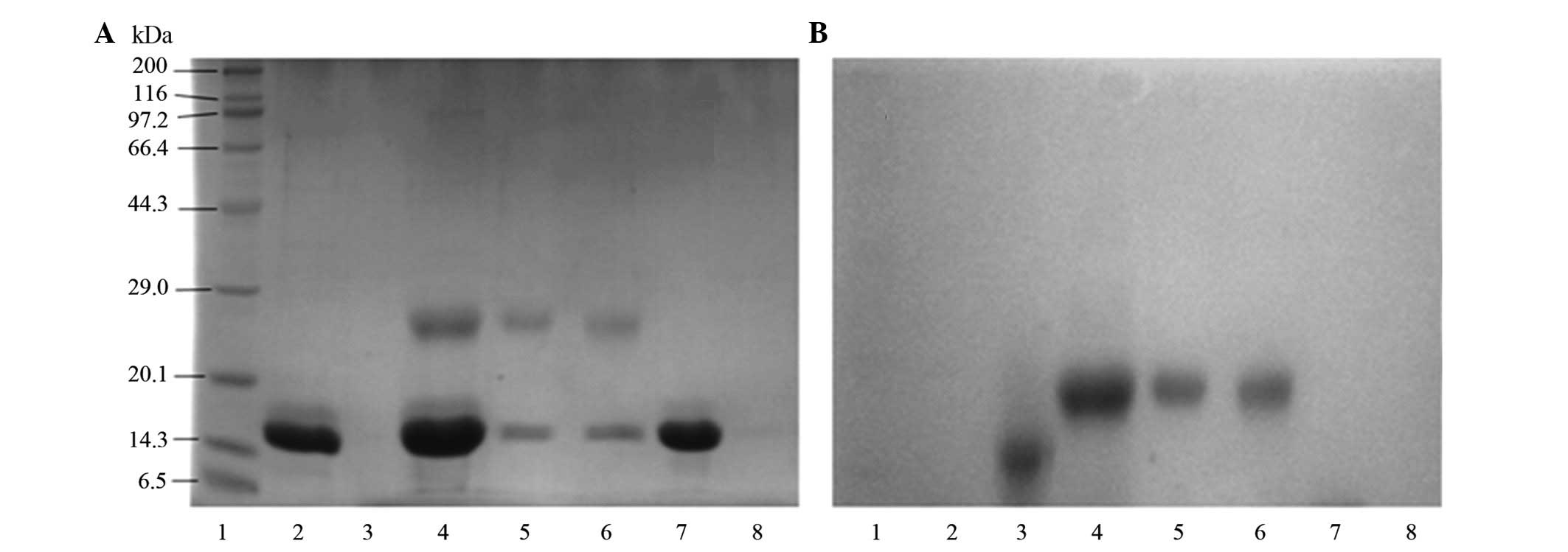
The results of SDS-PAGE analysis of the PEGylation
reaction mixture following barium iodide staining are shown in
Fig. 1. As the figure indicates,
no modification reaction (indicated by the appearance of higher-MW
bands) occurred until pH 5.0, and a weak reaction occurred between
pH 5.0 and 7.0. When the pH was >8, the reaction was more
efficient and numerous modification products were detected. By
contrast, variation of ionic strength hardly affected the
modification reaction. Therefore, we chose pH 8.0 as the reaction
pH in further studies.
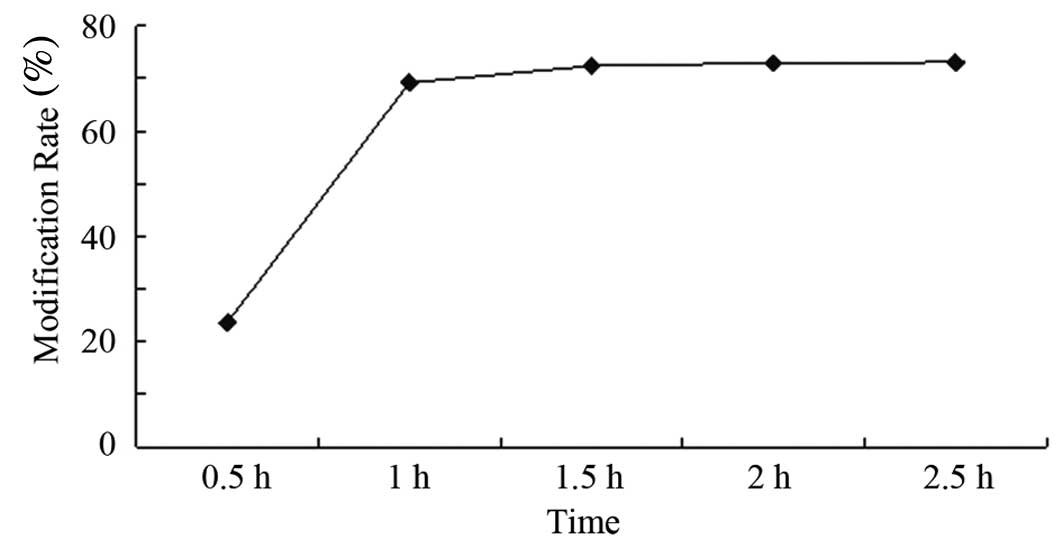
Effect of reaction time and
temperature
The reaction products were single and relatively
unstable at 0.5 h, which indicates the reaction was still in
progress (Fig. 2A). Between 1 and
2.5 h, the reaction results appeared to be similar (as shown in
Fig. 2B-E) and the reaction
products did not change significantly (Fig. 3), indicating that the reaction had
reached equilibrium after 1 h. Additionally, the experiment was
undertaken at room temperature, since temperature did not have a
significant effect on the reaction products (data not shown). As a
result, we chose room temperature and 2 h as the reaction
conditions.
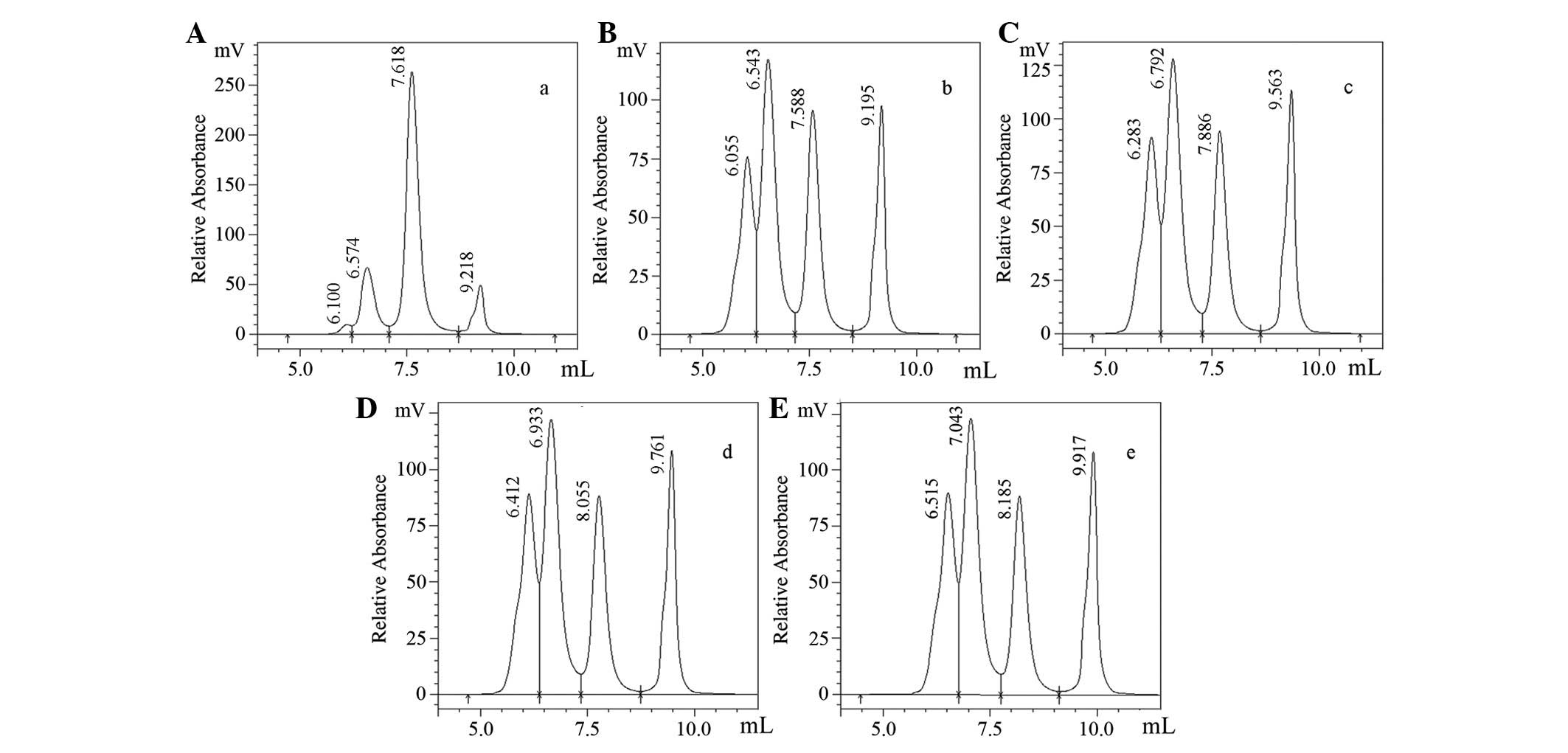
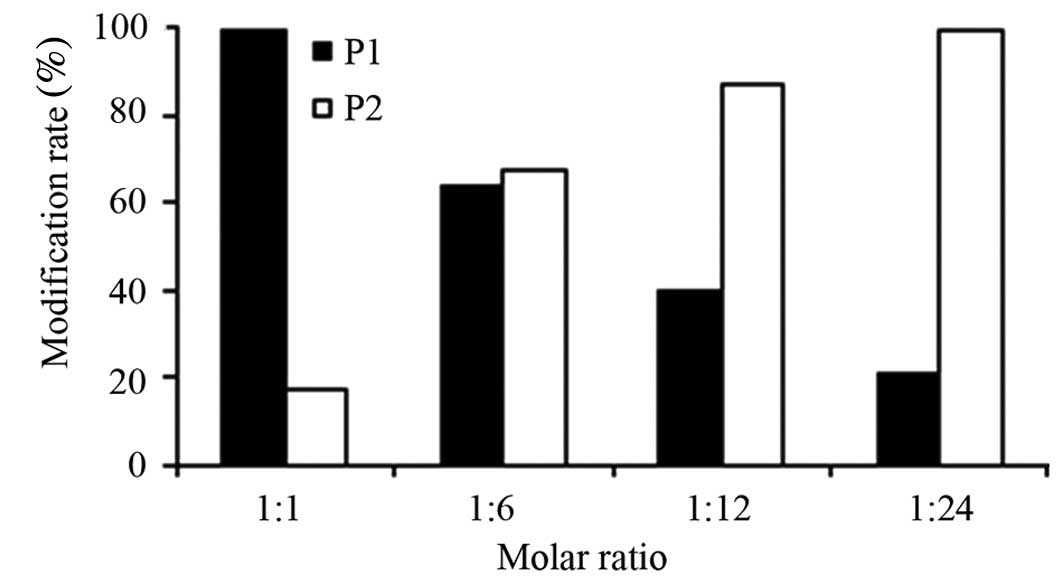
Effect of molar ratio of rLZ-8 and
mPEG-SPA
Fig. 4 illustrates
how the molar ratios of rLZ-8 to mPEG-SPA influenced the
modification reaction. The modification products became
increasingly varied (as indicated by the less discrete appearance
of the higher-MW bands) when the molar ratio of rLZ-8 to mPEG-SPA
was decreased. Only one type of modification product occurred at
the molar ratio of 1:1, so each modification product occupies 100%
of the product, with the exception of the remaining unmodified
rLZ-8. When the molar ratio was 1:24, there were four modification
products and each one contributed to 18.57% of the total mixture
(Figs. 4 and 5). For ease of purification, a molar
ratio of 1:1 was chosen for single-point modification.
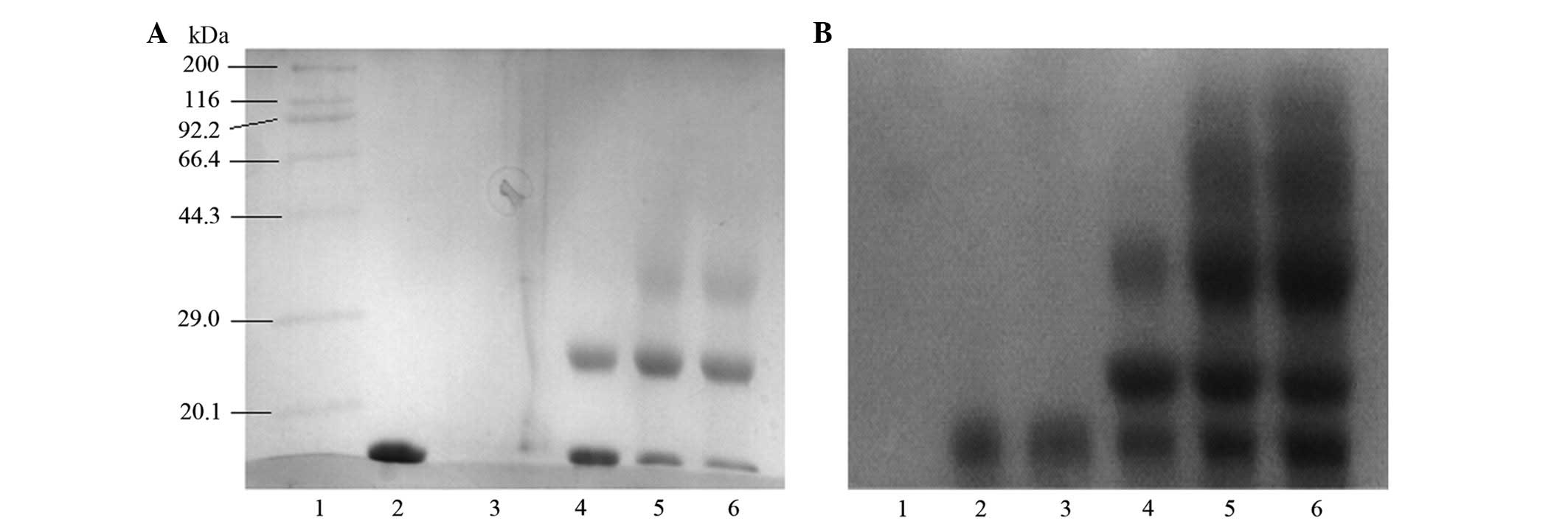
Purification and identification of the
target product
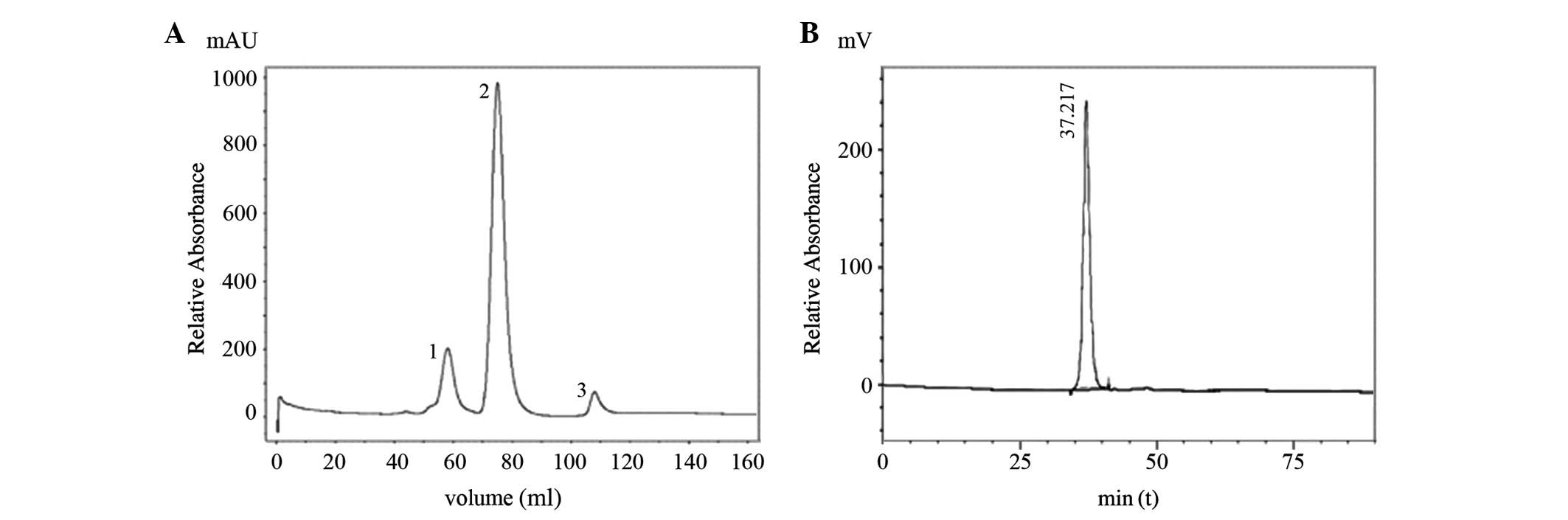
As shown in Fig. 6A
and Fig. 7, only one type of
modification product occurred at a molar ratio of 1:1. According to
the molecular sieve principle, the purity of the purification
product may reach 99% after being purified using a Superdex 75 gel
chromatographic column, a result that we also observed when using
this chromatographic purification method (Fig. 6B).
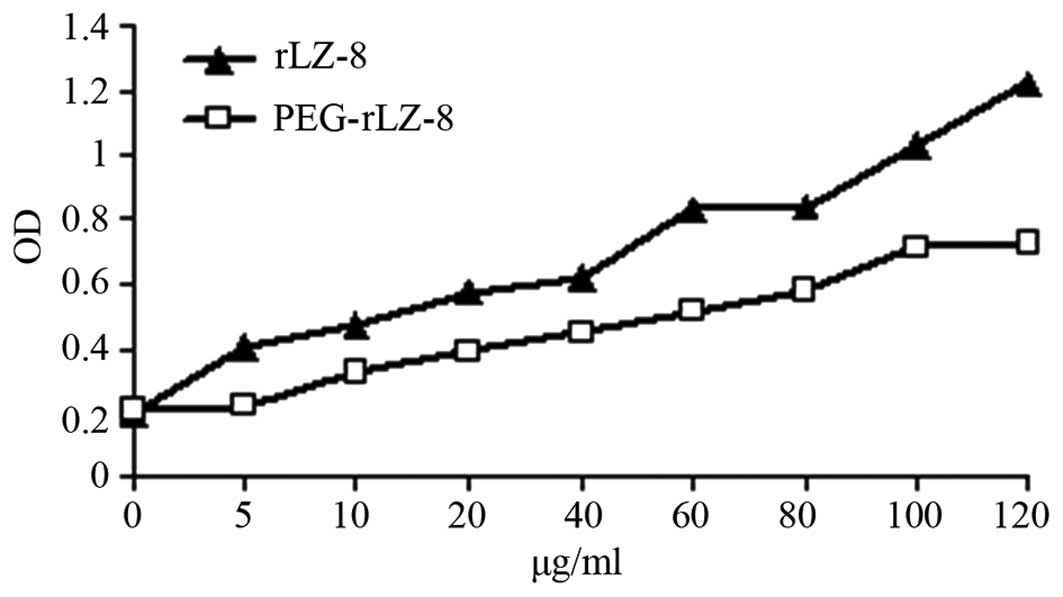
Biological activity of the purified
target product
The biological activities of rLZ-8 and PEG-rLZ-8 (as
assessed using a BrdU incorporation assay) are shown in Fig. 8. When modified by PEG, the activity
of the modification products of rLZ-8 decreased as expected.
Preliminary analysis of the
characteristics of target products
Detection of protein content
The Lowry method was used to determine the
concentration of protein in the modification product due to its
outstanding linear correlation, both before and after modification.
The amount of rLZ-8 decreased from 5 to 0.96 mg following the
modification and purification process.
Validation of molecular weight and
modification site
Several points were considered in order to correctly
identify the modification site. Firstly, the PEG-peptide may not
match the expected peptide mass fingerprint (PMF) and those that
match usually represent unPEGylated peptides in the modification
sample. Secondly, the PEGylated site is always located at the
N-terminus or on a Lys side chain. Thirdly, PEGylated Lys is
usually intractable to enzymatic cleavage, in which case, when Lys
is modified at least one uncleaved site is always included, and the
margin between the PEG-peptide and PEG should be close to the
theoretical MW of the peptide, while the MS/MS peak shape of the
PEG-peptide should be consistent with that of PEG.
From the data, we conclude that Lys is not the
PEGylated site, as all Lys in the PEG-protein matched the PMF and
the molecular weight of the PEG-peptides were close to that of PEG.
Therefore, we concluded that the PEGylated site did not fall within
the 75–111 amino acid fragment of rLZ-8 (this fragment is 4205.9858
Da). In the determination of the PMF of the PEG-protein, N-terminal
peptides with and without the M fragment (SDTALIFR) were observed,
so the PEGylated site is estimated to be at the N-terminus, as the
falling off of M in the process is not expected to affect the
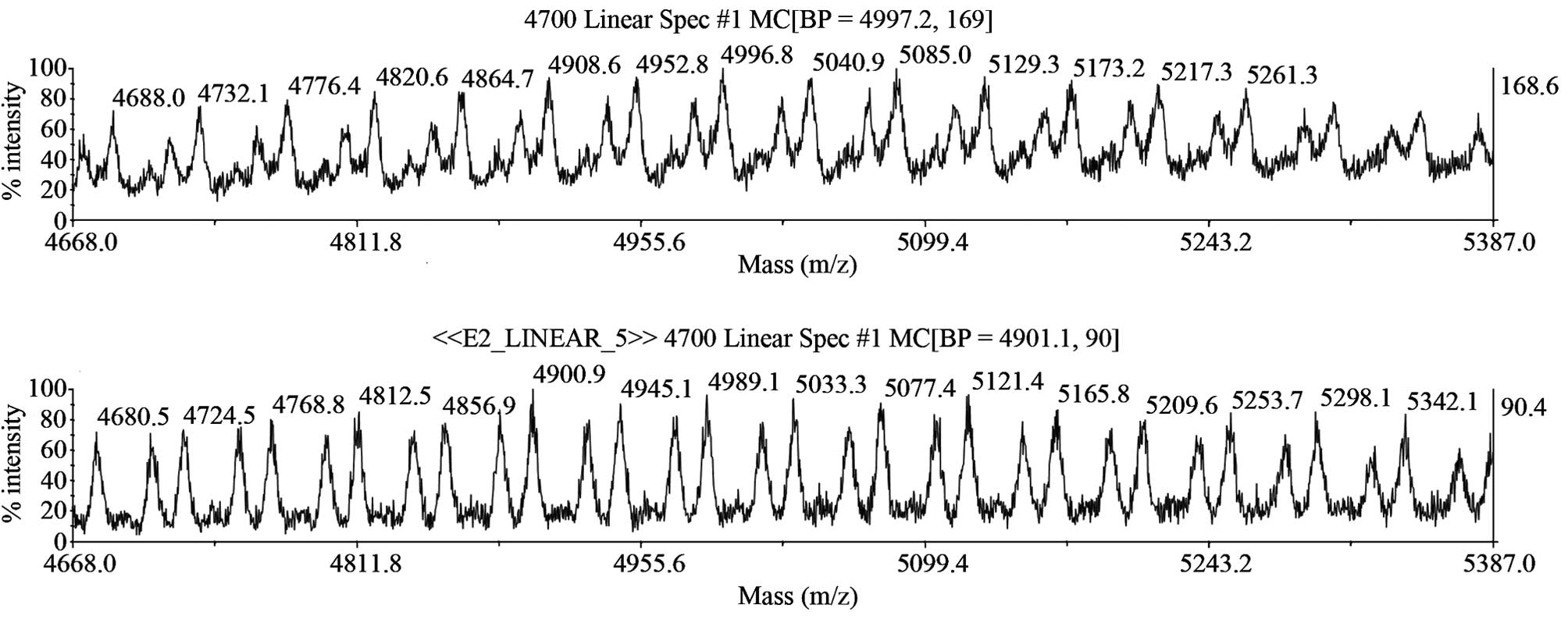
correspondence in MW between the peptides and the PMF (Fig. 9). Furthermore, the margin of the MW
between PEG-peptides and PEG are relatively close to M, which
provides further evidence that the PEGylated site is located at the
N-terminus.
Discussion
In this study, we established a strategy for
modifying rLZ-8 using mPEG-SPA and evaluated the effect of various
reaction parameters on the modification reaction. The pH of the
reaction buffer was the determining factor in the modification
reaction, and the modification reaction could not be carried out
under acidic conditions. The desired reaction products were
recovered in pH 8.0 phosphate buffer solution; however, the molar
ratio of rLZ-8 to mPEG-SPA was also an important factor affecting
the reaction products. A molar ratio of 1:1 yielded a single-site
modification product, and the composition of the reaction products
became more and more complex as the amount of mPEG-SPA was
increased. When the molar ratio was >1:12, there were four
species of products, of ~66, 44, 30 and 17.9 kDa. The reaction
temperature had almost no effect on the modification reaction, and
the reaction reached equilibrium after 1 h. From these data, we
concluded that the best conditions to obtain a single-site modified
product are: 0.05 M, pH 8.0 phosphate buffer solution, a 1:1 molar
ratio of rLZ to mPEG-SPA, and stirring at room temperature for 2 h,
protected from light.
PEG modification sites are generally at the
N-terminus or on Lys side chains. The structural analysis of rLZ-8
(17) revealed that the active
form of rLZ-8 is a non-covalently bonded dimer and that each
monomer contains 6 Lys. Therefore, in theory, one rLZ-8 molecule
may combine with 1–7 mPEG-SPA molecules, but the mPEG can only
modify the N-terminus of the protein. Since mPEG-SPA has long-chain
branches and is sticky, the greater the amount of mPEG-SPA that
combines with the protein, the greater the reduction in activity,
and the more complex the product composition is likely to be. In
this study, we focused on optimizing reaction conditions to obtain
a single-site modified product.
The MW of the single-site modified product is ~17.9
kDa, so we selected the Superdex 75 separation column to purify the
modification reaction mixture. Purification results revealed that
the modified products can be separated completely from the
unreacted rLZ-8 and that the small molecule byproducts can be
removed. Moreover, the single-site modified products were of high
purity and the excess rLZ-8 could be recycled and reused in the
experiment. Separation and purification of a variety of modified
products in excess of mPEG-SPA is more difficult, and further
studies are required to optimize recovery of other modification
products.
An in vitro assay for assessing the
biological activity of the single-site modified product indicated
that mPEG-SPA altered the efficacy of the protein in a lymphocyte
transformation assay. Additional in vivo experiments should
be carried out to determine the pharmacokinetic properties, such as
the half-life and effective dose, of the modified rLZ-8. Such
detailed analysis is likely to facilitate the clinical application
of modified products such as PEGylated rLZ-8.
Acknowledgements
This study was supported by the Science and
Technology Development Program of Jilin Province (Grant No.
20090941).
References
|
1
|
Liang C, Zhang S, Liu Z and Sun F:
Ganoderma lucidum immunomodulatory protein(Lz-8) expressed
in Pichia pastoris and the identification of
immunocompetence. Sheng Wu Gong Cheng Xue Bao. 25:441–447.
2009.
|
|
2
|
Lin JW, Hao LX, Xu GX, Sun F, Gao F, Zhang
R and Liu LX: Molecular cloning and recombinant expression of a
gene encoding a fungal immunomodulatory protein from Ganoderma
lucidum in Pichia pastoris. World J Microbiol
Biotechnol. 25:383–390. 2009. View Article : Google Scholar
|
|
3
|
Murasugi A, Tanaka S, Komiyama N, Iwata N,
Kino K, Tsunoo H and Sakuma S: Molecular cloning of a cDNA and a
gene encoding an immunomodulatory protein, Ling Zhi-8, from a
fungus, Ganoderma lucidum. J Biol Chem. 266:2486–2493.
1991.PubMed/NCBI
|
|
4
|
Wang XL, Liang CY, Li HR, Li BZ and Sun F:
Recombinant Ganoderma lucidum immunoregulatory protein
(rLZ-8) induces nuclear-stress apoptosis in K562 cells. Zhongguo
Mian Yi Xue Za Zhi. 26:616–618. 2010.(In Chinese).
|
|
5
|
Guo Q, Sun H, Liang CY, Zhang SQ, Liu ZY
and Sun F: Inhibiting and apoptosis-inducing effects of recombinant
Ganoderma lucidum immunoregulatory protein on HL60 cells.
Zhongguo Mian Yi Xue Za Zhi. 26:520–522. 2010.(In Chinese).
|
|
6
|
Liang CY, Zhang SQ and Sun F: The dynamic
observation: cellular localization of fluorescein isothiocyanate
labeled recombinant Ganoderma lucidum immunoregulatory
protein (rLZ-8) in NB4 APL cell. Chemical Journal of Chinese
Universities: CJCU. 30:479–483. 2009.
|
|
7
|
Kino K, Mizumoto K, Sone T, Yamaji T,
Watanabe J, Yamashita A, Yamaoka K, Shimizu K, Ko K and Tsunoo H:
An immunomodulating protein, Ling Zhi-8 (LZ-8) prevents insulitis
in non-obese diabetic mice. Diabetologia. 33:713–718. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haak-Frendscho M, Kino K, Sone T and
Jardieu P: Ling Zhi-8: a novel T cell mitogen induces cytokine
production and upregulation of ICAM-1 expression. Cell Immunol.
150:101–113. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh KY, Hsu CI, Lin JY, Tsai CC and Lin
RH: Oral administration of an edible-mushroom-derived protein
inhibits the development of food-allergic reactions in mice. Clin
Exp Allergy. 33:1595–1602. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YH, Kao MC, Lai YL and Tsai JJ:
Efficacy of local nasal immunotherapy for 10. Dp2-induced airway
inflammation in mice: using Dp2 peptide and fungal immunomodulatory
peptide. J Allergy Clin Immunol. 112:301–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho JC, Sze SC, Shen WZ and Liu WK:
Mitogenic antivity of edible mushroom lectins. Biochim Biophys
Acta. 1671:9–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wasser SP and Weis AL: Therapeutic effects
of substances occurring in higher Basidiomycetes mushrooms: a
modern perspective. Crit Rev Immunol. 19:65–96. 1999.PubMed/NCBI
|
|
13
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
14
|
Liang CY, Li H, Zhou H, Zhang SQ, Liu ZY,
Zhou Q and Sun F: Recombinant Lz-8 from Ganoderma lucidum
induces endoplasmic reticulum stress-mediated autophagic cell death
in SGC-7901 human gastric cancer cells. Oncol Rep. 27:1079–1089.
2012.
|
|
15
|
van der Hem LG, van der Vliet JA, Bocken
CF, Kino K, Hoitsma AJ and Tax WJ: Ling Zhi-8: studies of a new
immunomodulating agent. Transplantation. 60:438–443.
1995.PubMed/NCBI
|
|
16
|
Zhu JQ, Liang CY, Feng K, Gai XD, Sun X
and Sun F: Purification and properties of recombinant Ganoderma
lucidum immunoregulatory protein. Chem Res Chin Univ. 29:1–4.
2008.
|
|
17
|
Huang L, Sun F, Liang CY, He YX, Bao R,
Liu L and Zhou CZ: Crystal structure of LZ-8 from the medicinal
fungus Ganoderma lucidium. Proteins. 75:524–527. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin WH, Hung CH, Hsu CI and Lin JY:
Dimerisation of the N-terminal amphipathic α-helix domain of the
fungal immunomodulatory protein from Ganoderma tsugae
(Fip-gts) defined by a yeast two-hybrid system and site-directed
mutagenesis. J Biol Chem. 272:20044–20048. 1997.PubMed/NCBI
|
|
19
|
Tanaka S, Ko K, Kino K, Tsuchiya K,
Yamashita A, Murasugi A, Sakuma S and Tsunoo H: Complete amino acid
sequence of an immunomodulatory protein, ling zhi-8 (LZ-8). An
immunomodulator from a fungus, Ganoderma lucidium, having
similarity to immunoglobulin variable regions. J Biol Chem.
264:16372–16377. 1989.PubMed/NCBI
|
|
20
|
Roberts MJ, Bentley MD and Harris JM:
Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev.
54:459–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fee CJ and Van Alstine JM: PEG-proteins:
Reaction engineering and separation issues. Chem Eng Sci.
61:924–939. 2005.
|
|
22
|
Kinstler O, Molineux G, Treuheit M, Ladd D
and Gegg C: Mono-N-terminal poly(ethylene glycol)-protein
conjugates. Adv Drug Deliv Rev. 54:477–485. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greenwald RB: PEG drugs: an overview. J
Control Release. 74:159–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seyfried BK, Siekmann J, Belgacem O,
Wenzel RJ, Turecek PL and Allmaier G: MALDI linear TOF mass
spectrometry of PEGylated (glyco)proteins. J Mass Spectrom.
45:612–617. 2010.PubMed/NCBI
|
|
25
|
Ronda L, Pioselli B, Bruno S, Faggiano S
and Mozzarelli A: Electrophoretic analysis of PEGylated
hemoglobin-based blood substitutes. Anal Biochem. 408:118–123.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhai Y, Zhao Y, Lei J, Su Z and Ma G:
Enhanced circulation half-life of site-specific PEGylated rhG-CSF:
optimization of PEG molecular weight. J Biotechnol. 142:259–266.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moosmann A, Christel J, Boettinger H and
Mueller E: Analytical and preparative separation of PEGylated
lysozyme for the characterization of chromatography media. J
Chromatogr A. 1217:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|