Introduction
Cyclin D1 (CCND1) is a key regulatory
protein that plays a vital role in cell cycle control, particularly
in the transition from G1 to S phase, which is regulated by
cyclin-dependent kinases (1). It
results in the release of E2F transcription factors and allows
cells to enter the S phase (2).
Located on chromosome 11q13 (3), the cyclin D1 gene activation
(due to amplification or chromosomal rearrangement), as well as its
protein overexpression have been described in a wide variety of
tumour types, including colon (4–6),
breast (7,8), head and neck (9,10)
and lung (11,12) cancer, as its abnormal expression
disrupts normal cell cycle control, hence possibly promoting the
development and progression of cancer (13).
Single nucleotide polymorphism (SNP) of cyclin
D1 at G870A has been studied in various cancers (14–20),
demonstrating its role in modulating the risk of cancers in
different populations.
This common G to A polymorphism in the splice donor
region of exon 4 in the cyclin D1 gene located at codon 242
(nucleotide 870) is implicated on the splicing of the cyclin
D1 transcript (14,21). The dominant allele A preferentially
transcribes the truncated transcript (transcript b), encoding a
cyclin D1 protein with a longer half-life. The transcript b
results in deregulated cell proliferation since it lacks a PEST
sequence postulated to target for rapid degradation (22). The higher levels of this protein
may be associated with proliferation and a great risk of developing
adenomas and cancer. The allele A, particularly in the homozygous
state (AA genotype), has been associated with an increased risk of
colorectal cancer (CRC) and adenomas, mostly in younger patients
and in patients with family history of the illness (23,24).
However, no association was found between the AG or GG genotypes
(25,26) and others (27,28).
The role of cyclin D1 SNP in CRC risk remains
controversial.
In the present case-control study we evaluated the
potential impact of cyclin D1 (G870A) gene polymorphism on
the risk of CRC in the Kashmiri population. We also investigated
whether or not there was a link between the clinicopathological
variables of the cyclin D1 variant genotype (AA), as well as
its role in modulating the risk of CRC.
Materials and methods
Population study
This study comprised 130 CRC cases. All the
participants were patients of the Department of General Surgery of
the Sher-I-Kashmir Institute of Medical Sciences in Kashmir. Blood
samples were collected from 160 age- and gender-matched
individuals, with no signs of any malignancy, serving as external
controls. The mean age of both the patients and the control group
was 53 years (Table I).
 | Table IDemographic and clinical
characteristics of study subjects. |
Table I
Demographic and clinical
characteristics of study subjects.
| Variable | CRC cases
(n=130) | Healthy controls
(n=160) | P-value |
|---|
| Age group |
| ≤50 | 48 (36.9%) | 56 (35.0%) | 0.80 |
| >50 | 82 (63.1%) | 104 (65.0%) | |
| Gender |
| Female | 54 (41.54%) | 72 (45.0%) | 0.63 |
| Male | 76 (58.46%) | 88 (55.0%) | |
| Dwelling |
| Rural | 91 (70.0%) | 104 (65.0%) | 0.38 |
| Urban | 39 (30.0%) | 56 (35.0%) | |
| Smoking status |
| Ever | 81 (62.3%) | 90 (56.3%) | 0.33 |
| Never | 49 (37.7%) | 70 (43.7%) | |
| Tumour
location |
| Colon | 52 (40.0%) | | |
| Rectum | 78 (60.0%) | | |
| Tumour grade |
|
Well-differentiated | 98 (75.4%) | | |
| Moderately/poorly
differentiated | 32 (24.6%) | | |
Data on all CRC patients were obtained from personal
interviews with patients and/or their guardian, as well as from
their medical records. All patients and/or guardians were informed
of the study and they provided written consent in the form of a
pre-designed questionnaire (available on request). The collection
and use of blood samples (from patients and controls) for this
study was approved by the appropriate institutional ethics
committee.
DNA extraction and polymerase chain
reaction (PCR)
DNA extraction was performed using the ammonium
precipitation method. Genotyping for the cyclin D1 A870G
polymorphism was determined by the method described previously by
Satinder et al(3). The
oligonucleotide primers used for the amplification of the target
region were: forward 5′-AGT TCATTTCCAATCCGCCC-3′ and reverse
5′-TTTCCGTGGCACTAGGTGTC-3′. PCR was carried out in a final volume
of 20 μl, containing 50 ng genomic DNA template, 1X PCR buffer
(Fermentas, MD, USA), with 2 mM MgCl2, 0.5 μM of each
primer (Sigma-Aldrich, Bangalore, India), 50 μM deoxynucleotide
triphosphates (dNTPs) (Cinnagen, Tehran, Iran) and 0.25 units of
Taq DNA polymerase (Invitrogen, Bangalore, India). For PCR
amplification, the standard program was used as follows: one
initial denaturation step at 94°C for 7 min, followed by 40
denaturation cycles of 30 sec at 94°C, 30 sec of annealing at 60°C
and 30 sec of extension at 72°C for 40 cycles, followed by a final
elongation cycle at 72°C for 7 min.
The PCR product of cyclin D1 was 212 bp in
length and was then digested with 2 units of MspI in a
reaction mixture of 20 μl for 3 h at 37°C. The digestion resulted
in the generation of 3 bands of 141, 37 and 34 bp for the wild
genotype (GG), whereas for the homozygous variant genotype (AA) 2
bands of 175 and 37 bp were produced (Fig. 1).
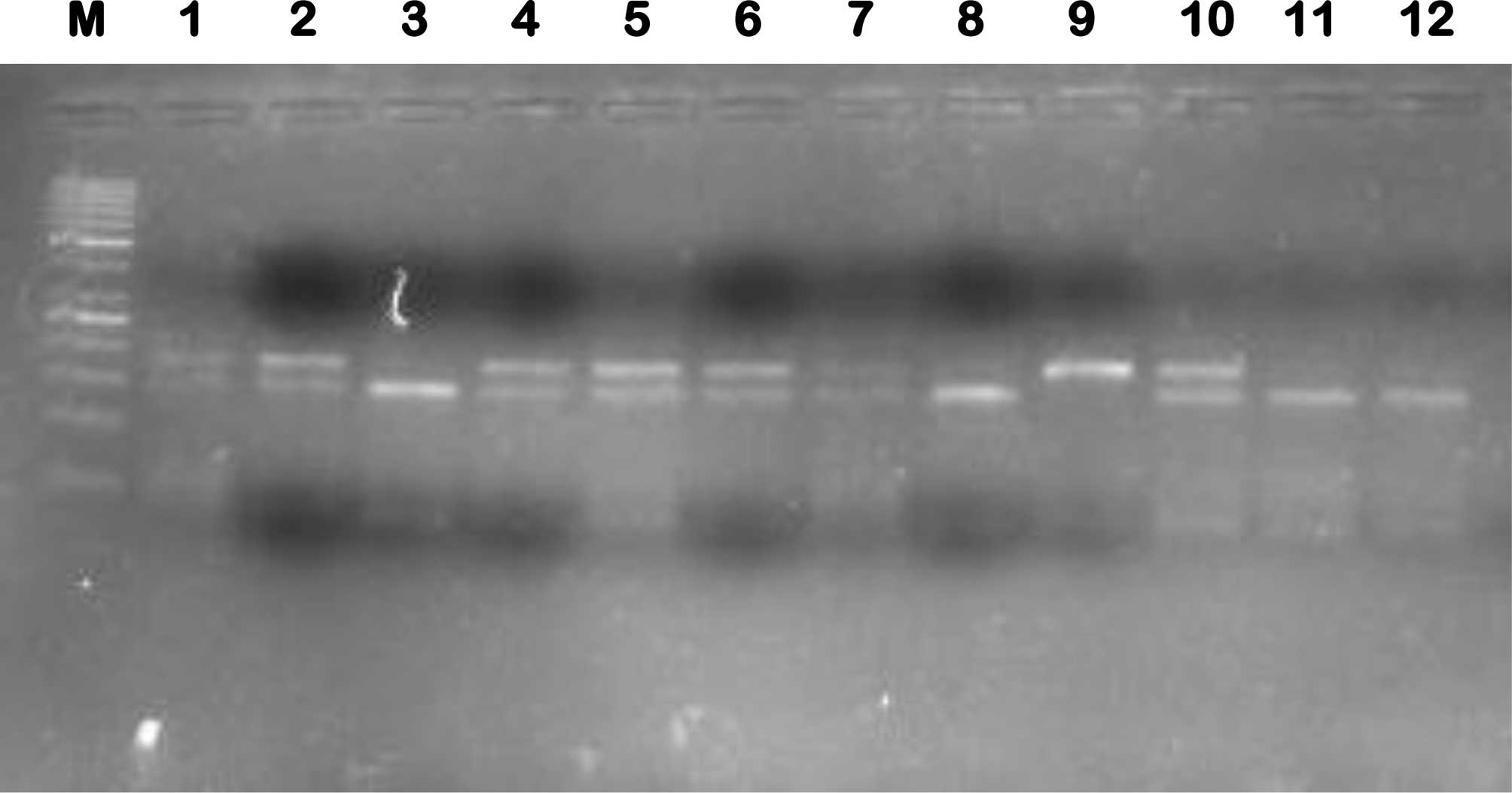 | Figure 1Representative gel of cyclin
D1 A870G polymorphism, representing amplicon digest with MspI
(C|CGG), where variant (AA) is cleaved to generate a visible 175-bp
band and wild-type (GG) is cleaved to generate a visible 141-bp
band. Lanes: M, 50-bp ladder; 3, 8, 11 and 12, homozygous wild-type
(GG) form; 1, 2, 4, 5, 6, 7 and 10, heterozygous (AG) form; 9,
homozygous (AA) variant form. |
DNA amplicons, as well as the digestion products,
were electrophoresed through a 2–3% agarose gel (Genie, Bangalore,
India) for resolution. The genotypes of >20% of the samples were
reassessed in a double-blind manner by 2 independent researchers to
confirm the results. A positive control for each polymorphism was
used for 50% of the samples.
Statistical analysis
The observed frequencies of genotypes in CRC
patients were compared with controls using Chi-square or Fisher’s
exact (FET) tests, when the expected frequencies were small. The
Chi-square test was used to verify whether or not the genotype
distributions were in Hardy-Weinberg equilibrium. P≤0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using PASW version 18
software.
Results
The present study comprised 130 CRC cases
and 160 control subjects
The patients comprised 76 males and 54 females (M/F
ratio=1.41), while the control subjects were 88 males and 72
females (M/F ratio=1.2). The mean age in the patient and control
groups was 52 years. No significant gender- or age-related
differences were observed between the groups (p>0.05).
Furthermore, out of 130 confirmed cases of CRC, 125 cases were
sporadic, 4 were familial adenomatous polyposis and one case was
hereditary non-polyposis (Lynch Syndrome) CRC. All but one case had
adenocarcinoma, one had squamous cell carcinoma of the basal cell
type, and 52 had carcinoma in the colon, while 78 had carcinoma in
the rectum. A total of 78 resided in rural areas, while 39 resided
in urban areas; 81 were smokers and 49 non-smokers (Table I).
Among the CRC cases, we found the frequency of the
cyclin D1 genotype to be 14.61% (19/130) for GG, 53.85%
(70/130) for AG and 31.54% (41/130) for AA, while the frequency in
the general control population was 25.62% (41/160) for GG, 47.50%
(76/160) for AG and 26.88% (43/160) for AA. The overall association
between the cyclin D1 polymorphism and the CRC cases was
found to be non-significant (p>0.05; Table II). However, a separate analysis
for the AG and AA genotypes revealed a marked association with the
risk of CRC (p<0.05). The overall hazard ratio of the cyclin
D1 A allele in CRC was 2.01 (95% CI=1.10–3.68).
 | Table IIGenotype frequencies of cyclin
D1 gene polymorphism in CRC cases and controls. |
Table II
Genotype frequencies of cyclin
D1 gene polymorphism in CRC cases and controls.
| Cyclin D1
genotype | CRC cases (n=
130) | Controls
(n=160) | OR (95% CI)
Pa,Fb | P-value
(overall) |
|---|
| Co-dominant
inheritance |
| GG (wild) | 19 (14.6%) | 41 (25.6%) | 1.0 (Ref) | 5.31;0.07 |
| AG
(heterozygous) | 70 (53.9%) | 76 (47.5%) | 1.99 (1.05–3.74);
0.03; 0.04 | |
| AA (variant) | 41 (31.5%) | 43 (26.9%) | 2.05 (1.03–4.10);
0.03; 0.05 | |
| Dominant
inheritance |
| GG | 19 (14.6%) | 41 (25.6%) | 1.0 (Ref) | |
| AG + AA | 111 (85.4%) | 119 (52.5%) | 2.01 (1.10–3.68);
0.02; 0.03 | |
| Recessive
inheritance |
| GG + AG | 89 (68.5%) | 117 (73.1%) | 1.0 (Ref) | |
| AA | 41 (31.5%) | 43 (26.9%) | 1.25 (0.75–2.08);
0.38; 0.44 | |
The correlation between the cyclin D1
polymorphic status and the clinicopathological characteristics was
also carefully analysed. A marked association (p<0.05) was
observed among the A allele, age and dwelling (p<0.05; Table III), while the remaining
parameters were not found to be markedly associated with the
variant A allele of the cyclin D1 gene.
 | Table IIIAssociation between cyclin D1
polymorphism and clinicopathological characteristics. |
Table III
Association between cyclin D1
polymorphism and clinicopathological characteristics.
| Casesa | |
|---|
|
| |
|---|
| Variables | All cases
n=130 | GG
19 (14.6%) | AG
70 (53.9%) | AA
41 (31.5%) | P-value |
|---|
| Age group |
| ≤50 | 48 (36.9%) | 6 | 33 | 9 | 7.32; 0.035 |
| >50 | 82 (63.1%) | 13 | 37 | 32 | |
| Gender |
| Female | 54 (41.54%) | 8 | 27 | 19 | 0.65; 0.720 |
| Male | 76 (58.46%) | 11 | 43 | 22 | |
| Dwelling |
| Rural | 91 (70.0%) | 11 | 57 | 23 | 9.45;
0.008 |
| Urban | 39 (30.0%) | 8 | 13 | 18 | |
| Smoking status |
| Ever | 81 (62.3%) | 10 | 48 | 23 | 2.6; 0.272 |
| Never | 49 (37.7%) | 9 | 22 | 18 | |
| Tumour
location |
| Colon | 52 (40.0%) | 7 | 31 | 14 | 1.2; 0.548 |
| Rectum | 78 (60.0%) | 12 | 39 | 27 | |
| Nodal status |
| Involved | 88 (67.7%) | 14 | 49 | 25 | 1.33; 0.514 |
| Not involved | 42 (32.3%) | 5 | 21 | 16 | |
| Tumour grade |
|
Well-differentiated | 98 (75.4%) | 15 | 54 | 29 | 0.72; 0.697 |
| Moderately/poorly
differentiated | 32 (24.6%) | 4 | 16 | 12 | |
Discussion
The Kashmiri population is exposed to a special set
of environmental and dietary risks, such as exposure to nitroso
compounds or amines and nitrates reported to be present in local
foodstuffs, most of which have been shown to contain important
irritants and carcinogens (29–32).
CRC is the third most common cancer in males and the
second in females worldwide (33).
In the Kashmir valley, it represents the third most common
gastrointestinal tract (GIT) cancer, following oesophageal and
gastric cancer (30,31,34).
The cyclin D1 A870G polymorphism has 3
distinct genotypes: GG (wild-type), AG and AA (variants). All 3
forms synthesise similar proteins due to their identical biological
functionality. However, the difference among the genotypes is the
capability of A allele to cause the truncation of the transcript,
which in turn increases the half-life of the resulting protein
(1,28).
In the present study, we investigated the
association between the cyclin D1 A870G polymorphism and CRC
in the Kashmiri population. Although no association was found
between this polymorphism and the risk of CRC, our results
demonstrated a statistically significant (p<0.05) 1.99-fold
increase in the OR for the AG genotype and a 2.05-fold increase in
the OR for the AA genotype (Table
II), when compared to the GG genotype. Furthermore, there was a
statistically significant (p<0.05) 2.01-fold increase in the OR
for the A allele in a dominant model of inheritance, although only
a 1.25-fold increase in the OR for the A allele in a recessive
model of inheritance. These results are quite different from those
of Jian et al(25), who
reported a recessive model of inheritance for this polymorphism in
the Indian population on the basis of high fold increase in OR
(1.56). The frequency of the different genotypes of cyclin
D1 polymorphism in our Kashmiri CRC cases was 14.61% (19/130)
for the GG, 53.85% (70/130) for the AG and 31.54% (41/130) for the
AA genotype. The frequencies for the rest of Indian population
reported by Jian et al were 5.28% (46/301) for the GG,
43.19% (130/301) for the AG and 41.52% (125/301) for the AA
genotype. The differences between the Kashmiri and the Indian
populations may be due to the fact that the former belongs to the
Persian genotypic pool, having descended from Persian migrants
settled in the Kashmir valley during the 15th century. However, the
present study was consistent with a Brazilian study regarding the
frequencies of the 3 genotypes of cyclin D1 A870G
polymorphism (28). Moreover,
consistent with our findings, results supporting the dominant model
of inheritance have been reported by Tan et al(35).
Zheng et al(17) previously reported the frequency of
the AA genotype to be higher in patients with squamous cell
carcinoma of the head and neck (SCCHN) (23.6%) compared to controls
(16.5%) in a non-hispanic white population, and concluded beyond
doubt that the subjects with the AA genotype had a higher
probability of developing SCCHN at an earlier stage than those with
the GG genotype. However, in the present study we found quite the
reverse. Subsequent to statistical analysis of the data using
clinicopathological parameters (Table III), a significant association of
the AA genotype with the older age group (>50 years) was
observed (p<0.05), demonstrating that older patients were at a
higher risk of developing CRC, compared to younger ones. These
results are also contradictory to those reported by Huang et
al in the Taiwanese population (36). The AA genotype was also found to be
markedly associated with dwelling, suggesting that rural dwellers
were at increased risk of CRC.
In a recent meta-analysis by Zhang et
al(37) it was determined that
the A allele significantly elevated the risk of CRC in co-dominant
and dominant models. This supports our own observations from the
present study. Furthermore, on the basis of ethnic stratification,
significant associations were found in Caucasian populations,
although not in Asians, thereby suggesting a possible role of
ethnic differences in genetic background in addition to
environmental factors (38,39).
Pabalan et al reported in a meta-analysis on
all cancers that the cyclin D1 G870A polymorphism confers
susceptibility to cancer development, irrespective of the
population studied (40). Its
interaction with other genetic variants and environmental factors
was also observed to have resulted in an elevated risk of cancer
(OR, 1.6–7.1).
Furthermore, the interaction between polymorphism
and various environmental factors induces and increases the overall
susceptibility to CRC in any population (37–39).
Therefore, we also suggest that since the Kashmiri population is
exposed to a special set of environmental and dietary risks, which
include the consumption of sun-dried and smoked fish and meat,
dried and pickled vegetables, red chilli, hakh (a leafy vegetable
of the Brassica family), hot noon chai (salted tea) and hukka
(water pipe) smoke (30–32), this may play a significant role in
modulating the effect of polymorphism on the dominant model of
inheritance. The etiology and incidence of various GIT cancers in
the Kashmiri population has been previously reported to be
attributed to probable exposure to nitroso compounds, amines and
nitrates that are present in local foodstuffs, most of which have
been proven to contain notable irritants and carcinogens (29).
In conclusion, we found a clear association between
the cyclin D1 A870G polymorphism and the risk of CRC in the
ethnic Kashmiri population. Nevertheless, these correlations need
to be verified in a large-scale study, in order to discern racial
differences and determine the aggressiveness of CRC.
Acknowledgements
The authors gratefully acknowledge the financial
support provided by the Sher-I-Kashmir Institute of Medical
Sciences, and would also like to thank the head and technical staff
of the operating theatre at the Department of General Surgery of
the Sher-I-Kashmir Institute of Medical Sciences, for their
assistance with tissue procurement. The authors also thank the
anonymous pathologists at the Department of Pathology of the
Sher-I-Kashmir Institute of Medical Sciences for their
histopathological assessment of the tumour tissues.
References
|
1
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satinder K, Chander SR, Pushpinder K, Indu
G and Veena J: Cyclin D1 (G870A) polymorphism and risk of cervix
cancer: A case control study in north Indian population. Mol Cell
Biochem. 315:151–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartkova J, Lukas J, Strauss M and Bartek
J: The PRAD-1/cyclin D1 oncogene product accumulates aberrantly in
a subset of colorectal carcinomas. Int J Cancer. 58:568–573. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arber N, Hibshoosh H, Moss SF, Sutter T,
Zhang Y, Begg M, Wang S, Weinstein IB and Holt PR: Increased
expression of cyclin D1 is an early event in multistage colorectal
carcinogenesis. Gastroenterology. 110:669–674. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maeda K, Chung Y, Kang S, Ogawa M, Onoda
N, Nishiguchi Y, Ikehara T, Nakata B, Okuno M and Sowa M: Cyclin D1
overexpression and prognosis in colorectal adenocarcinoma.
Oncology. 55:145–151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gillett C, Smith P, Gregory W, Richards M,
Millis R, Peters G and Barnes D: Cyclin D1 and prognosis in human
breast cancer. Int J Cancer. 69:92–99. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh KL and Varley JM: Frequent
alterations of cell cycle regulators in early-stage breast lesions
as detected by immunohistochemistry. Br J Cancer. 77:1460–1468.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Callender T, el-Naggar AK, Lee MS,
Frankenthaler R, Luna MA and Batsakis JG: PRAD-1 (CCND1)/cyclin D1
oncogene amplification in primary head and neck squamous cell
carcinoma. Cancer. 74:152–158. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jares P, Fernández PL, Campo E, Nadal A,
Bosch F, Aiza G, Nayach I, Traserra J and Cardesa A: PRAD-1/cyclin
D1 gene amplification correlates with messenger RNA overexpression
and tumor progression in human laryngeal carcinomas. Cancer Res.
54:4813–4817. 1994.PubMed/NCBI
|
|
11
|
Betticher DC, Heighway J, Hasleton PS,
Altermatt HJ, Ryder WD, Cerny T and Thatcher N: Prognostic
significance of CCND1 (cyclin D1) overexpression in primary
resected non-small cell lung cancer. Br J Cancer. 73:294–300. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shapiro GI, Edwards CD, Kobzik L, Godleski
J, Richards W, Sugarbaker DJ and Rollins BJ: Reciprocal Rb
inactivation and p16INK4 expression in primary lung cancers and
cell lines. Cancer Res. 55:505–509. 1995.PubMed/NCBI
|
|
13
|
Zhou P, Jiang W, Weghorst CM and Weinstein
IB: Overexpression of cyclin D1 enhances gene amplification. Cancer
Res. 56:36–39. 1996.PubMed/NCBI
|
|
14
|
Akkiz H, Bayram S, Bekar A, Akgöllü E and
Ozdil B: Cyclin D1 G870A polymorphism is associated with an
increased risk of hepatocellular carcinoma in the Turkish
population: case-control study. Cancer Epidemiol. 34:298–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong YK, Lin SC, Chang CS, et al: Cyclin
D1 genotype in areca-associated oral squamous cell carcinoma. J
Oral Pathol Med. 32:265–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang S, Kim JW, Park NH, Song YS, Kang SB
and Lee HP: Cyclin D1 polymorphism and the risk of endometrial
cancer. Gynecol Oncol. 97:431–435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Y, Shen H, Sturgis EM, et al: Cyclin
D1 polymorphism and risk for squamous cell carcinoma of the head
and neck: A case-control study. Carcinogenesis. 22:1195–1199. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong S, Amos CI, Luthra R, Lynch PM, Levin
B and Frazier ML: Effects of cyclin D1 polymorphism on age of onset
of hereditary nonpolyposis colorectal cancer. Cancer Res.
60:249–252. 2000.PubMed/NCBI
|
|
19
|
Catarino R, Matos A, Pinto D, et al:
Increased risk of cervical cancer associated with cyclin D1 gene
A870G polymorphism. Cancer Genet Cytogenet. 160:49–54. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thakur N, Hussain S, Kohaar I, et al:
Genetic variant of CCND1: Association with HPV-mediated cervical
cancer in Indian population. Biomarkers. 14:219–225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Betticher DC, Thatcher N, Altermatt HJ,
Hoban P, Ryder WDJ and Heighway J: Alternate splicing produces a
novel cyclin D1 transcript. Oncogene. 11:1005–1011. 1995.PubMed/NCBI
|
|
22
|
Sawa H, Ohshima TA, Ukita H, Murakami H,
Chiba Y, Kamada H, et al: Alternatively spliced forms of cyclin D1
modulate entry into the cell cycle in an inverse manner. Oncogene.
16:1701–1712. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong S, Wei Q, Amos CI, Lynch PM, Levin B,
Zong J, et al: Cyclin D1 polymorphism and increased risk of
colorectal cancer at young age. J Natl Cancer Inst. 93:1106–1108.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porter TR, Richards FM, Houlston RS, Evans
DG, Jankowski JA, Macdonald F, et al: Contribution of cyclin D1
(CCND1) and E-cadherin (CDH1) polymorphisms to familial and
sporadic colorectal cancer. Oncogene. 21:1928–1933. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang J, Wang J, Suzuki S, Gajalakshmi V,
Kuriki K, Zhao Y, Nakamura S, Akasaka S, Ishikawa H and Tokudome S:
Elevated risk of colorectal cancer associated with the AA genotype
of the cyclin D1 A870G polymorphism in an Indian population. J
Cancer Res Clin Oncol. 132:193–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yaylim-Eraltan I, Arikan S, Yildiz Y,
Cacina C, Ergen HA, Tuna G, Görmüs U, Zeybek U and Isbir T: The
influence of cyclin D1 A870G polymorphism on colorectal cancer risk
and prognosis in a Turkish population. Anticancer Res.
30:2875–2880. 2010.PubMed/NCBI
|
|
27
|
McKay JA, Douglas JJ, Ross VG, Curran S,
Murray GI, Cassidy J, et al: Cyclin D1 protein expression and gene
polymorphism in colorectal cancer. Int J Cancer. 88:77–81. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forones NM, de Lima JM, de Souza LG and da
Silva ID: Cyclin D1 A870G polymorphism in Brazilian colorectal
cancer patients. J Gastrointest Cancer. 39:118–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddiqi M, Kumar R, Fazili Z,
Spiegelhalder B and Preussmann R: Increased exposure to dietary
amines and nitrate in a population at high risk of oesophageal and
gastric cancer in Kashmir (India). Carcinogenesis. 13:1331–1335.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sameer AS, Nissar S, Abdullah S, Chowdri
NA and Siddiqi MA: DNA repair gene 8-oxoguanine DNA glycosylase
Ser326Cys polymorphism and colorectal cancer risk in a Kashmiri
population. DNA Cell Biol. 31:541–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sameer AS, Shah ZA, Nissar S, Mudassar S
and Siddiqi MA: Risk of colorectal cancer associated with the
methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in
the Kashmiri population. Genet Mol Res. 10:1200–1210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rasool S, Ganai BA, Kadla SA, Ahanger AG,
Qazi F, Khan T, Rasool V and Masood A: The ECRG1 290Arg/Gln
polymorphism is related to risk of esophageal squamous cell
carcinoma in Kashmir. Asian Pac J Cancer Prev. 12:265–269.
2011.PubMed/NCBI
|
|
33
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
34
|
Javid G, Zargar SA, Rather S, Khan AR,
Khan BA, Yattoo GN, Shah A, Gulzar GM, Sodhi JS, Khan MA and
Shoukat-Deeba Bashir A: Incidence of colorectal cancer in Kashmir
valley, India. Indian J Gastroenterol. 30:7–11. 2011. View Article : Google Scholar
|
|
35
|
Tan XL, Nieters A, Kropp S, et al: The
association of cyclin D1 G870A and E-cadherin C-160A polymorphisms
with the risk of colorectal cancer in a case control study and
meta-analysis. Int J Cancer. 122:2573–2580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang WS, Tang R, Lin PY, Changchien CR,
Chen JS, Chiang JM, Yeh CY, Wang JY and Hsieh LL: Impact of the
cyclin D1 A870G polymorphism on susceptibility to sporadic
colorectalcancer in Taiwan. Dis Colon Rectum. 49:602–608. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang LQ, Huang X, Wang J, Shang JQ, Bai
J, Liu FY, Guan X and Zhou JN: The cyclin D1 G870A polymorphism and
colorectal cancer susceptibility: A meta-analysis of 20
populations. Asian Pac J Cancer Prev. 12:81–85. 2011.
|
|
38
|
Donnellan R and Chetty R: Cyclin D1 and
human neoplasia. Mol Pathol. 51:1–7. 1998. View Article : Google Scholar
|
|
39
|
Palmqvist R, Stenling R, Oberg A, et al:
Expression of cyclin D1 and retinoblastoma protein in colorectal
cancer. Eur J Cancer. 34:1575–1581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pabalan N, Bapat B, Sung L, Jarjanazi H,
Pabalan OF and Ozcelik H: Cyclin D1 Pro241Pro (CCND1-G870A)
polymorphism is associated with increased cancer risk in human
populations: A meta-analysis. Cancer Epidemiol Biomarkers Prev.
17:2773–2781. 2008. View Article : Google Scholar : PubMed/NCBI
|















