Introduction
Sepsis is an complex clinical condition
characterized by a dysregulated inflammatory response to infection
resulting in multiorgan failure with a fatal outcome (1–4).
Currently, sepsis accounts for 12% patients admitted to the ICU
despite the use of antibiotics (5). Gram-negative sepsis is also one of
the most common and serious post-operative complications in
abdominal surgery (6,7). Despite major improvements in
antimicrobial therapies, up to now, even highly effective
treatments for sepsis syndrome have not been able to eliminate
mortality. In addition, the excessive utilization of antibiotics
increases the risk of development of sepsis caused by
drug-resistant bacteria, particularly Gram-negative bacteria
(8). Therefore, novel approaches
for the prevention and early treatment of sepsis are likely to
reduce the incidence and mortality of the syndrome. To date, the
pathogenesis of sepsis remains unknown. The current understanding
of Gram-negative sepsis is that the syndrome is evoked by the
endotoxin produced by microbes and an overwhelming innate
inflammatory response to a microbial infection, which correlates
with the excessive release of immune factors, including interleukin
(IL)-1, IL-6 and tumor necrosis factor (TNF)-α (4,9).
Thus, an effective therapy may be directed at inhibiting the
release of immune factors and interrupting the cytokine
cascade.
As potent inducers of immune tolerance,
CD4+CD25+Foxp3+ regulatory T cells
(Tregs) are important for the control of the immune response and
prevention of the development of excessive immune-induced tissue
damage (10–12). Tregs exert a pronounced
anti-inflammatory effect largely by the contact-mediated direct
inhibition of effector T cells and secretion of immunosuppressive
cytokines, including IL-10 and transforming growth factor (TGF)-β,
subsequently suppressing inflammatory response-induced damage
(10,11). Various studies have documented that
the proportions of certain Tregs are increased and the suppressive
functions of Tregs are amplified following sepsis or acute insult
(13–15), but the role of these changes
remains unclear as the depletion of Tregs in a septic mouse model
was found to produce variable conclusions between models,
improving, enhancing or bearing no effect on mortality (16–18).
However, the vital role of Tregs in regulating proinflammatory
cytokine formation secondary to severe insults is well
established.
Pseudomonas aeruginosa is an extracellular,
Gram-negative bacteria that increases the proliferative response of
normal adult peripheral blood lymphocytes (19). Immunization with a vaccine derived
from the outer membrane proteins of P. aeruginosa has been
demonstrated to induce high titers of serum IgG antibody in rabbits
and humans (20). A P.
aeruginosa vaccine has been found to have anti-infective and
anti-inflammatory functions as an immune modulator. P.
aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) is a form
of peritrichous MSHA fimbria P. aeruginosa, which was
established by Mu (21).
Heat-inactivated PA-MSHA is currently available as a vaccine and
functions as an effective immune modulator via activation of the
proliferation and differentiation of dendritic cells to increase
antigen presentation (22).
However, its anti-inflammatory effect is not well understood and
other mechanisms may be involved in this role.
In the present study, PA-MSHA was identified to
induce an increased proportion of
CD4+CD25+Foxp3+ T cells in
peripheral blood mononuclear cells (PBMCs). In addition, the
stimulation of healthy PBMCs with serum isolated from Gram-negative
sepsis patients was found to initiate the release of inflammatory
mediators, whereas the exposure of PBMCs to PA-MSHA promoted the
release of the immunosuppressive factor, IL-10 and reduced the
production of the pro-inflammatory cytokine, TNF-α. The potential
mechanisms of PA-MSHA in inhibition of the inflammatory response
are also discussed.
Materials and methods
Subjects
The samples analyzed in this study were derived from
healthy donors and sepsis patients, who were enrolled in an
open-label randomized trial. Patients with Gram-negative sepsis
(n=6) were consecutively hospitalized at the ICU of the Second
Xiangya Hospital of Central South University (CSU) within 1 week,
and met the severe sepsis criteria following abdominal surgery. The
study was approved by the human ethics committee of the Institute
of CSU. Informed consent was obtained from all participants in the
study.
Serum
To prevent platelet activation and phospholipase
activity, blood samples were collected in EDTA-containing tubes.
Serum was obtained from peripheral blood samples by centrifuging
and was kept at −80°C until experimental assays were performed.
Cell preparation and cell treatment
PBMCs were derived from fresh heparinized blood
samples from healthy adult donors. PBMCs were isolated by density
gradient centrifugation and cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum. PA-MSHA
(donated by Professor XY Mu in 1 ml aliquots, containing
inactivated PA-MSHA strain, 1.8×109) used in this study
was scale-cultured at 37°C for 24 h, inactivated using a chemical
method and purified by centrifuging. The septic serum was enriched
by ultracentrifuging. The healthy PBMCs were treated with PA-MSHA
(5×108/ml) and then stimulated with septic serum (1 ml)
for various times, as indicated. Total RNA and cell culture
supernatant were collected for further analysis.
RNA isolation and real-time quantitative
RT-PCR
PBMCs were stimulated with septic or normal serum,
following exposure to PA-MSHA. Total RNA was isolated from the
cells with TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA). Real-time quantitative RT-PCR was performed using iQ5
(Bio-Rad, Hercules, CA, USA). Analyses were performed with 20 μl
reaction volumes containing 10 μl 2X SYBR® Premix Ex Taq
(Takara Bio, Inc., Shiga, Japan), 0.4 μM each primer, 1 μl cDNA
template and 8.2 μl deionized water. PCR amplifications were
performed using the following parameters: 95°C for 10 sec and 40
cycles of 95°C for 5 sec and 60°C for 30 sec. Melting curve
analysis was also performed to exclude non-specific PCR products.
All PCR products were confirmed by melting curve analysis to
exclude the possibility of multiple products or incorrect product
size. PCR analyses were conducted in triplicate for each sample.
Primers used were as follows: GAPDH, forward AGAAGGCTGGGGCTCATTTG
and reverse AGGGGCCATCCACAGTCTTC; TNF-α, forward CCTGTGAGGAGGACGAAC
and reverse CCTGTGAGGAGGACGAAC; IL-10, forward
TGAGAACAGCTGCACCCACTT and reverse TCGGAGATTCGAAGCATGTTA.
Flow cytometry
PBMCs were treated with septic or healthy serum,
following treatment with PA-MSHA. Following this, the treated cells
were stained with antibodies against FITC-conjugated CD4, APC-CD25
and PE-Foxp3 (BD Biosciences, San Jose, CA, USA). The percentage of
CD4+CD25+Foxp3+ cells was measured
by flow cytometry (FACSCalibur; BD Biosciences) and blank and
isotype controls were used to eliminate autofluorescence and
non-specific fluorescence.
ELISA measurement of cytokines
Secretion of TNF-α and IL-10 was determined by
ELISA. PBMCs were plated in 24-well plates and treated with PA-MSHA
prior to stimulation with septic serum. Next, media were harvested
for measurement of cytokines. ELISA was performed according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
Analysis of data was performed using the unpaired
t-test. Data are presented as the mean ± SD. Analysis was performed
using SPSS 16.0 and P<0.05 was considered to indicate a
statistically significant difference.
Results
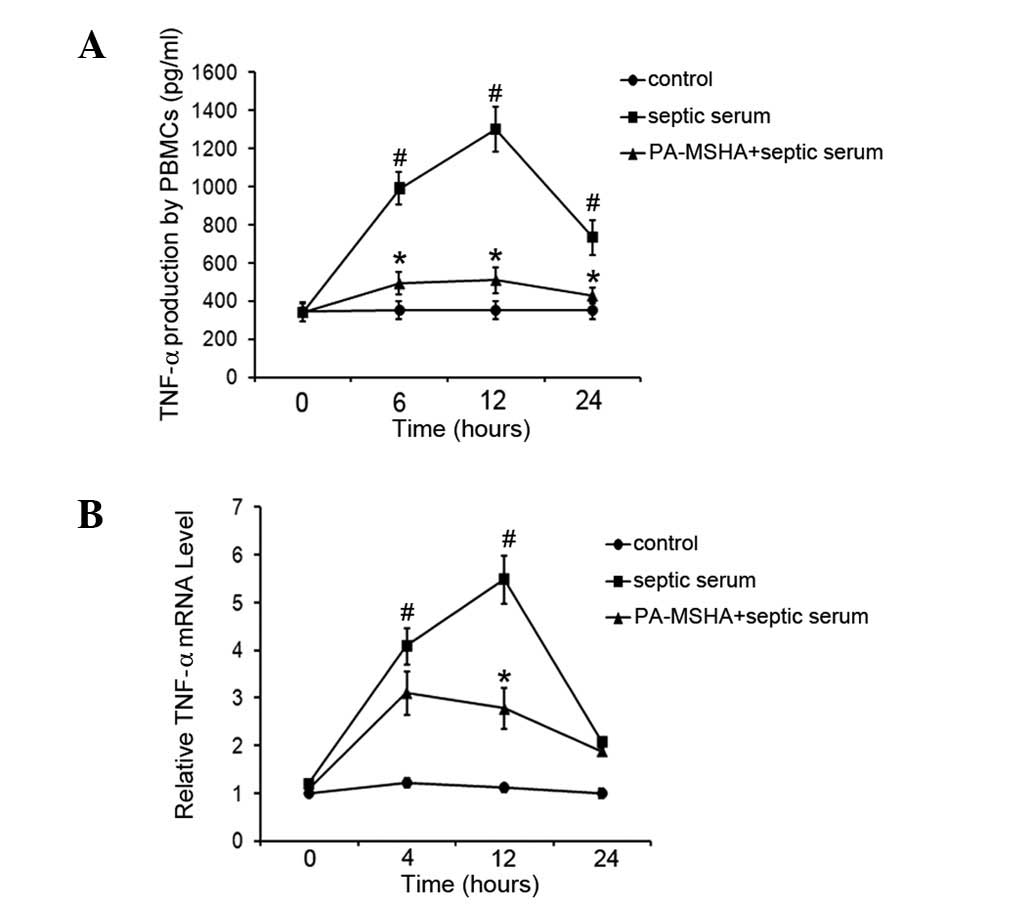
PA-MSHA vaccine reduces TNF-α levels
The P. aeruginosa vaccine is known to exhibit
anti-inflammatory effects. In the present study, the effect of the
PA-MSHA vaccine on septic serum-induced TNF-α expression was
examined in PBMCs. Healthy PBMCs were exposed to PA-MSHA, followed
by stimulation using septic serum. Following this, total RNA and
cell culture supernatant were collected at various times and TNF-α
expression levels were determined by real-time RT-PCR and ELISA. As
demonstrated in Fig. 1A, septic
serum stimulation significantly increased the production of TNF-α
during all the incubation periods tested compared with the
untreated cells. The TNF-α levels increased and reached peak levels
after 12 h of septic serum stimulation and decreased subsequently.
However, the PA-MSHA-pretreated cells were found to release
significantly lower levels of TNF-α compared with the cells
stimulated with septic serum only at each time point. Consistent
with ELISA results, the real-time RT-PCR results in Fig. 1B also revealed that stimulation of
PBMCs with septic serum led to marked increases in TNF-α mRNA
levels compared with those in the untreated cells, while PA-MSHA
treatment markedly attenuated the TNF-α expression (Fig. 1B). These results indicate that
PA-MSHA suppresses the production of septic serum-induced
TNF-α.
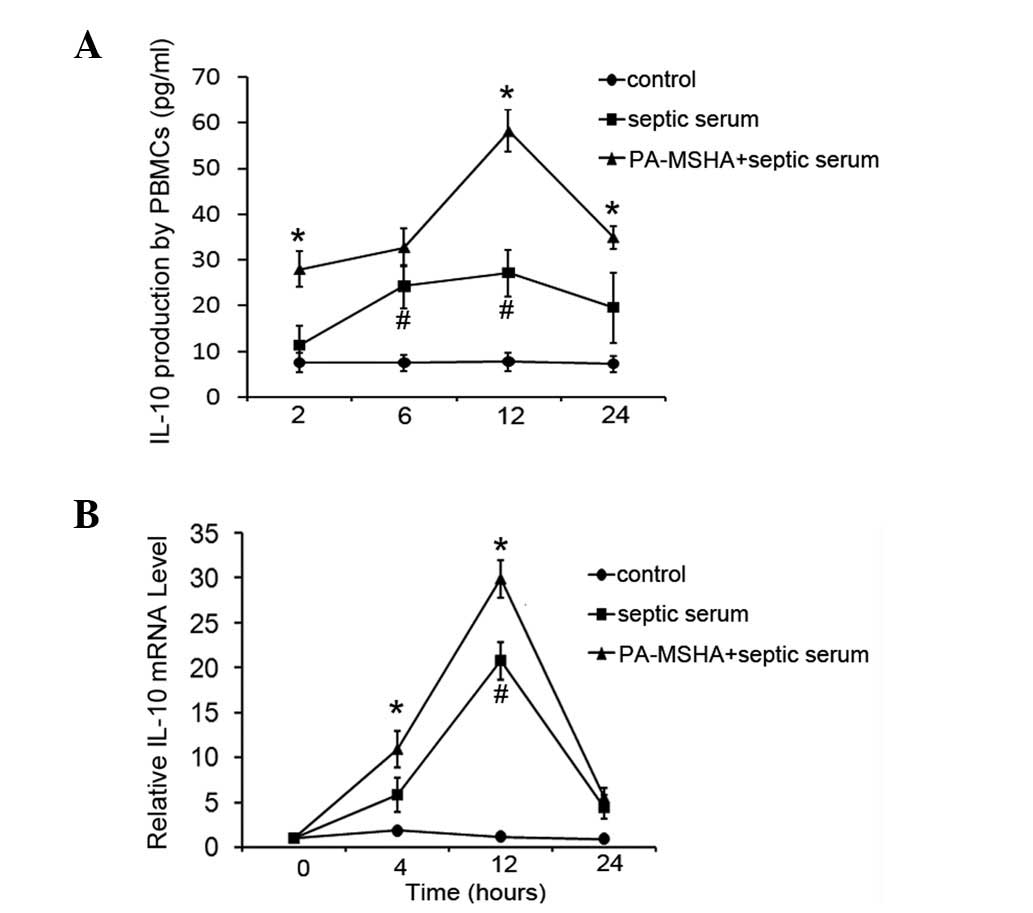
PA-MSHA vaccine increases IL-10
levels
Since PA-MSHA represses the induction of TNF-α by
septic serum stimulation, the effect of the PA-MSHA vaccine on the
levels of the anti-inflammatory factor, IL-10, was investigated.
Using the septic serum-stimulated culture system, pretreatment of
healthy PBMCs with PA-MSHA was observed to increase the induction
of IL-10 levels by septic serum stimulation. As revealed in
Fig. 2A, IL-10 levels in the
supernatants of cells cultured in septic serum increased 2- or
3-fold compared with those in untreated cells, while PA-MSHA
pretreatment was identified to result in a significant enhancement
of the induction of IL-10 levels. IL-10 mRNA expression, as
determined by RT-PCR, was consistent with the effects on protein
concentration (Fig. 2B).
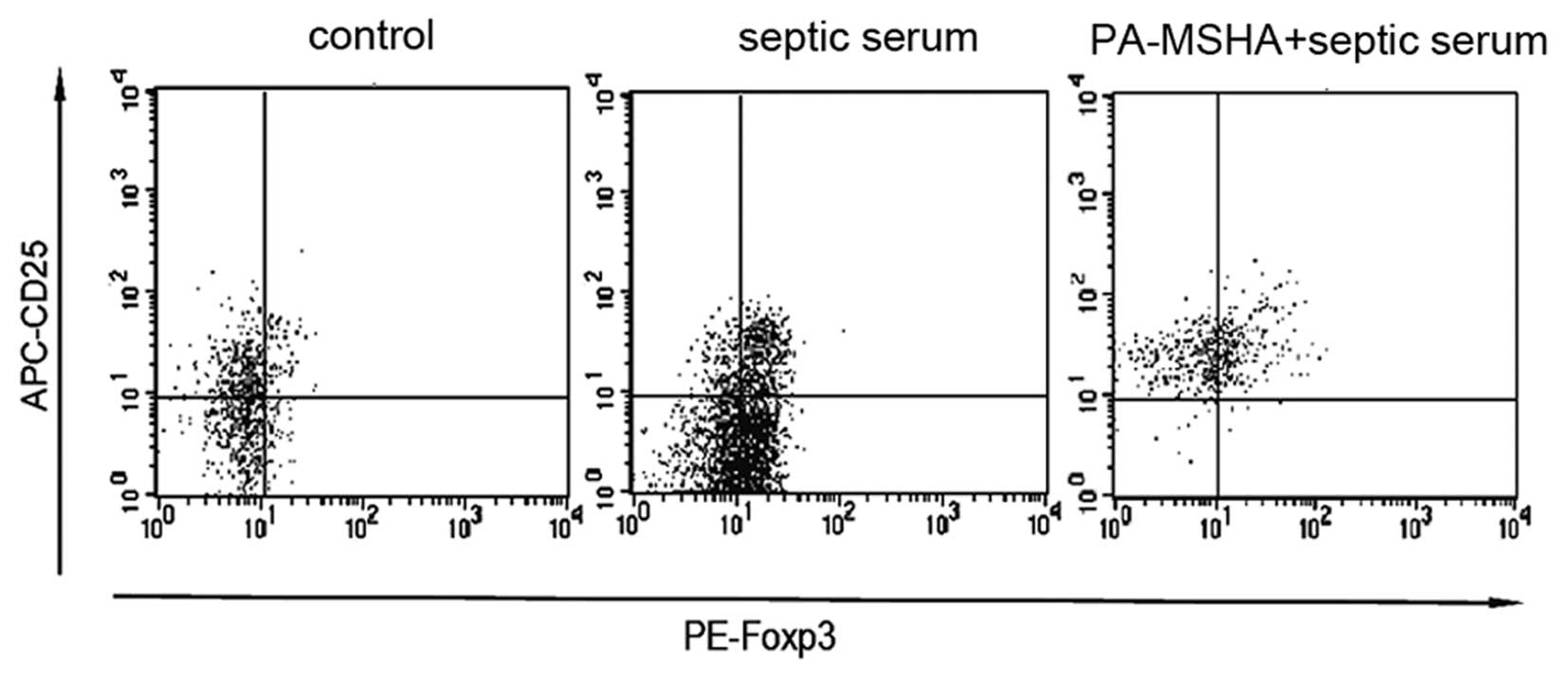
Effect of the PA-MSHA vaccine on the
generation of CD25+Foxp3+Tregs
IL-10 is produced by cells of the immune lineage,
mainly by macrophages and Tregs. Considering the significance of
Tregs in sepsis, the effect of PA-MSHA on the generation of Tregs
was determined. PBMCs were treated with PA-MSHA and then stimulated
with septic serum. Post-stimulation (4 h), cells were collected and
stained with antibodies against FITC-conjugated CD4, APC-CD25 and
PE-Foxp3. Firstly, CD4+ T cells were sorted and then the
proportion of CD25+Foxp3+ T cells in the
CD4+ T cells was determined. A significant increase in
the percentage of Tregs was identified in the cells stimulated by
septic serum [17.74% (16.04–18.89%)] compared with the control
[9.04% (7.75–10.23%)]. When the cells were pretreated with PA-MSHA,
a more marked increase in the percentage of
CD25+Foxp3+ T cells [34.12% (29.01–43.80%)]
was observed (Fig. 3). These
results indicate that the PA-MSHA vaccine promotes the septic
serum-stimulated induction of Tregs.
Discussion
The P. aeruginosa vaccine is currently widely
used for anti-infective and -inflammatory purposes as an immune
modulator. In a clinical study, we observed that the exposure of
surgical wounds to a P. aeruginosa vaccine increased the
rate of healing (unpublished data). To explore the potential
mechanisms by which the P. aeruginosa vaccine represses the
inflammatory response, changes in the cell immune and inflammatory
factors produced by healthy PBMCs were analyzed following exposure
to a P. aeruginosa vaccine, PA-MSHA, and stimulation by
septic serum. The results indicated that exposure to PA-MSHA
induced a more marked suppressive immune response, thereby
inhibiting the release of pro-inflammatory cytokines.
Previous studies have demonstrated that PA-MSHA
increases the antigen-presenting function by activating the
proliferation and differentiation of dendritic cells, thus breaking
down the complete and incomplete immunological tolerance,
completely activating polyclonal T and B cells, increasing the
number and proportion of T cells and T cell subgroups and
stimulating the differentiation of intrinsic immunological active
factors (22). The
anti-inflammatory action of PA-MSHA indicates that the vaccine may
also trigger a suppressive immune response to balance excessive
inflammation. As a subset of T cells, Tregs may inhibit the
activation of effector T-cell subsets in a contact-dependent manner
or by producing inhibitory cytokines, including IL-10 and TGF-β
(10,11). As a transcription factor of Tregs,
FoxP3 is a relatively specific marker of Tregs and also considered
to be the key regulator (23). In
the present study, stimulation of PBMCs with septic serum was
demonstrated to induce an increased proportion of
CD4+CD25+Foxp3+ cells, while
pretreatment of the cells with PA-MSHA elicited a more marked
increase, indicating that Tregs may be involved in the
anti-inflammatory role of PA-MSHA.
In vitro and in vivo studies in
animals have indicated a protective role of IL-10 in
atherosclerotic lesion formation and stability (24,25).
IL-10 is an important anti-inflammatory cytokine, which
downregulates innate and adaptive immune responses and suppresses
tissue inflammation and damage (26). IL-10 potently represses the
inflammatory response largely through blocking the maturation of
antigen-presenting cells, inhibiting the production of
pro-inflammatory cytokines and directly suppressing the
differentiation of T cells into effector subsets (27). IL-10 is known to be produced by
various inflammatory cells, among them macrophages and Tregs. In
the present study, the exposure of PBMCs to PA-MSHA was revealed to
induce the suppressive inflammatory factor, IL-10, which may reduce
the magnitude of the immune response against septic serum, thus
minimizing consequent damage due to sepsis. In addition, the
observation of enhanced IL-10 release by PBMCs pretreated with
PA-MSHA is supported by the increased Treg numbers and may be
explained by this increase. Thus, these observations indicate that
PA-MSHA may induce the differentiation and development of Tregs and
promote the increased production of IL-10, resulting in an
alleviated inflammatory response.
Previous studies have revealed that in sepsis, the
serum concentration of IL-10 is also increased. This suppressive
factor functions to inhibit the release of TNF-α, IL-1β and IL-6,
and reduces the circulating concentrations of these cytokines. A
number of the classical features of inflammation in sepsis are
attributed to the actions of TNF-α (9). Serum concentrations of TNF-α
correlate with mortality in specific types of human sepsis
(28,29). In the current study, PBMCs exposed
to PA-MSHA were found to produce lower levels of TNF-α than cells
which had not been pretreated, following stimulation with septic
serum. This result indicates that PA-MSHA may reduce the release of
the key cytokine TNF-α in sepsis. Considering the role of IL-10 in
regulating the release of TNF-α in sepsis, we hypothesize that
PA-MSHA may exert its anti-inflammatory role through promoting the
development of Tregs, consequently suppressing the release of
TNF-α.
In summary, results of the present study indicate
that PA-MSHA suppresses the inflammatory response during sepsis.
PA-MSHA represses the key inflammatory cytokine, TNF-α. This
process may be mediated by the induction of Treg development and
promotion of IL-10 production. Since the vaccine reveals inhibitory
potential against the inflammatory response, PA-MSHA may be
suitable for the prevention of early sepsis.
Acknowledgements
This study was supported by a grant from the Nature
Scientific Foundation of Hunan Province (no. 2009FJ3186 and
2012Rs4021).
References
|
1
|
Bone RC, Balk RA, Cerra FB, et al:
Definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee American College of Chest Physicians/Society
of Critical Care Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar
|
|
2
|
Warren HS: Strategies for the treatment of
sepsis. N Engl J Med. 336:952–953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stone R: Search for sepsis drugs goes on
despite past failures. Science. 264:365–367. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curns AT, Steiner CA, Sejvar JJ and
Schonberger LB: Hospital charges attributable to a primary
diagnosis of infectious diseases in older adults in the United
States, 1998 to 2004. J Am Geriatr Soc. 56:969–975. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukazawa A, Yokoi Y, Kurachi K, et al:
Implication of B lymphocytes in endotoxin-induced hepatic injury
after partial hepatectomy in rats. J Surg Res. 137:21–29. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcea G and Maddern GJ: Liver failure
after major hepatic resection. J Hepatobiliary Pancreat Surg.
16:145–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wright AJ, Unger S, Coleman BL, Lam PP and
McGeer AJ: Maternal antibiotic exposure and risk of antibiotic
resistance in neonatal early-onset sepsis: a case-cohort study.
Pediatr Infect Dis J. 31:1206–1208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis DH, Chan DL, Pinheiro D,
Armitage-Chan E and Garden OA: The immunopathology of sepsis:
pathogen recognition, systemic inflammation, the compensatory
anti-inflammatory response and regulatory T cells. J Vet Intern
Med. 26:457–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaguchi S, Wing K, Onishi Y,
Prieto-Martin P and Yamaguchi T: Regulatory T cells: how do they
suppress immune responses? Int Immunol. 21:1105–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Workman CJ, Szymczak-Workman AL, Collison
LW, Pillai MR and Vignali DA: The development and function of
regulatory T cells. Cell Mol Life Sci. 66:2603–2622. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shalev I, Schmelzle M, Robson SC and Levy
G: Making sense of regulatory T cell suppressive function. Semin
Immunol. 23:282–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leng FY, Liu JL, Liu ZJ, Yin JY and Qu HP:
Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells
during the early-stage sepsis in ICU patients. J Microbiol Immunol
Infect. Aug 23–2012.(Epub ahead of print).
|
|
14
|
Monneret G, Debard AL, Venet F, et al:
Marked elevation of human circulating
CD4+CD25+ regulatory T cells in
sepsis-induced immunoparalysis. Crit Care Med. 31:2068–2071. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito K, Wagatsuma T, Toyama H, et al:
Sepsis is characterized by the increases in percentages of
circulating CD4+CD25+ regulatory T cells and
plasma levels of soluble CD25. Tohoku J Exp Med. 216:61–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heuer JG, Zhang T, Zhao J, et al: Adoptive
transfer of in vitro-stimulated CD4+CD25+
regulatory T cells increases bacterial clearance and improves
survival in polymicrobial sepsis. J Immunol. 174:7141–7146.
2005.PubMed/NCBI
|
|
17
|
Scumpia PO, Delano MJ, Kelly KM, et al:
Increased natural CD4+CD25+ regulatory T
cells and their suppressor activity do not contribute to mortality
in murine polymicrobial sepsis. J Immunol. 177:7943–7949.
2006.PubMed/NCBI
|
|
18
|
Wisnoski N, Chung CS, Chen Y, Huang X and
Ayala A: The contribution of CD4+CD25+
T-regulatory-cells to immune suppression in sepsis. Shock.
27:251–257. 2007.PubMed/NCBI
|
|
19
|
Porwoll JM, Gebel HM, Rodey GE and Markham
RB: In vitro response of human T cells to Pseudomonas
aeruginosa. Infect Immun. 40:670–674. 1983.PubMed/NCBI
|
|
20
|
Lee N, Ahn B, Jung SB, Kim YG, Kim H and
Park WJ: Conformation-dependent antibody response to Pseudomonas
aeruginosa outer membrane proteins induced by immunization in
humans. FEMS Immunol Med Microbiol. 27:79–85. 2000.
|
|
21
|
Mu XY: Success in establishing the
MSHA-positive Pseudomonas aeruginosa fimbrial strain. Wei
Sheng Wu Xue Bao. 26:176–179. 1986.(In Chinese).
|
|
22
|
Jia L, Wang C, Kong H, et al: Effect of
PA-MSHA vaccine on plasma phospholipids metabolic profiling and the
ratio of Th2/Th1 cells within immune organ of mouse IgA
nephropathy. J Pharm Biomed Anal. 43:646–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Borde M, Heissmeyer V, et al: FOXP3
controls regulatory T cell function through cooperation with NFAT.
Cell. 126:375–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mallat Z, Besnard S, Duriez M, et al:
Protective role of interleukin-10 in atherosclerosis. Circ Res.
85:e17–e24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pinderski Oslund LJ, Hedrick CC, Olvera T,
et al: Interleukin-10 blocks atherosclerotic events in vitro and in
vivo. Arterioscler Thromb Vasc Biol. 19:2847–2853. 1999.PubMed/NCBI
|
|
26
|
Ait-Oufella H, Taleb S, Mallat Z and
Tedgui A: Cytokine network and T cell immunity in atherosclerosis.
Semin Immunopathol. 31:23–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davidson NJ, Leach MW, Fort MM, et al: T
helper cell 1-type CD4+ T cells, but not B cells,
mediate colitis in interleukin 10-deficient mice. J Exp Med.
184:241–251. 1996.PubMed/NCBI
|
|
28
|
Damas P, Reuter A, Gysen P, Demonty J,
Lamy M and Franchimont P: Tumor necrosis factor and interleukin-1
serum levels during severe sepsis in humans. Crit Care Med.
17:975–978. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Girardin E, Grau GE, Dayer JM,
Roux-Lombard P and Lambert PH: Tumor necrosis factor and
interleukin-1 in the serum of children with severe infectious
purpura. N Engl J Med. 319:397–400. 1988. View Article : Google Scholar : PubMed/NCBI
|