Introduction
The structure of the arteries is important as serve
as a target for a number of substances that regulate smooth muscle
tension. The endothelium is a cell layer that lines the inside of
blood vessels, and also produces and releases mediators that
modulate the contraction of arteries (1,2).
Endothelial damage occurs in the course of various
pathological processes, particularly atherosclerosis, and leads to
vascular disorders with regard to diameter regulation, which
potentially result in occlusion of the vessels. Although platelet
aggregation is a natural recovery process following the injury of
blood vessel walls, activation of the coagulation system following
plaque rupture may result in a reduction or complete elimination of
blood flow. Nitric oxide (NO) is a vasodilator agent that is
produced by the endothelial cells by constitutive NO synthase
(3). In smooth muscle cells, NO
activates soluble guanylyl cyclase (GC) and the resulting increase
in cyclic guanosine monophosphate (cGMP) levels leads to blood
vessel dilatation (4,5). This effect may be as a result of the
reduction in the concentration of Ca2+ in the cytoplasm
(due to the influx of calcium into the cell or the inhibition of
the release of calcium from intracellular stores),
dephosphorylation of myosin light chains or interaction with the
contractile system (6–10). The release of NO occurs under
physiological conditions; however, it may be further stimulated by
various factors, such as acetylcholine (Ach), bradykinin and
histamine (11).
The aim of this study was to determine the
significance of the signaling pathway associated with Ach in the
contraction induced via the extracellular pool of Ca2+
by Bay K8644 (an agonist of calcium channels located in the cell
membrane) and KCl (at depolarizing concentrations), and also to
determine the importance of the vascular endothelium in the
activity of Bay K8644.
Materials and methods
Reagents
The study was performed on perfused male Wistar rat
tail arteries. Rats, weight 250–350 g, were narcotized by
intraperitoneal injection of 120 mg/kg of body mass. After being
dissected and cleared from the surrounding tissue, 2.5–3-cm long
segments of rat tail arteries were cannulated and connected to
perfusion apparatus. Perfusion pressure was measured continuously.
The distal part was weighted with a 500 mg weight and placed in a
20 ml container filled with oxygenated Krebs solution at 37°C. The
perfusion solution flow was gradually increased using a peristaltic
pump until 1 ml/min was reached.
Two types of Krebs fluid were used in this study to
determine the importance of the intracellular and extracellular
pools of Ca2+ in the reactions induced by Bay K8644 (30
μM/l) and KCl (110 mM/l) under control conditions, following the
addition of nitro-L-arginine (L-NNA; nitric oxide synthase, 10
μM/l) or 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ; an
inhibitor of soluble guanylyl cyclase; 10 μM/l), and in the
presence of increasing Ach concentrations. The two Krebs solutions
were as follows: i) fluid without Ca2+-EGTA [Krebs (no
calcium); calcium-free physiological salt solution, FPSS]; and ii)
fluid with Ca2+-EGTA [Krebs (normal); physiological salt
solution, PSS]. The composition of the fluid without
Ca2+-EGTA was as follows: NaCl (71.8 mM/l), KCl (4.7
mM/l), NaHCO3 (28.4 mM/l), MgSO4 (2.4 mM/l),
KH2PO4 (1.2 mM/l), glucose (11.1 mM/l) with
the addition of EGTA (30 μM/l). The composition of the fluid with
Ca2+-EGTA was as follows: NaCl (71.8 mM/l), KCl (4.7
mM/l), CaCl2 (1.7 mM/l), NaHCO3 (28.4 mM/l),
MgSO4 (2.4 mM/l), KH2PO4 (1.2
mM/l), glucose (11.1 mM/l) with addition of EGTA (30 μM/l),
following the emptying of the intracellular pool of
Ca2+. All reagents were purchased from Sigma-Aldrich
(Poznań, Poland).
Removal of the endothelium
In a number of cases, the endothelium of the
arteries was removed using compressed air to determine the
importance of the endothelium in the responses induced by Bay K8644
and following the addition of increasing concentrations of 8Br-cGMP
(12).
Concentration-response curves (CRCs)
CRCs were determined using the van Rossum method of
increasing concentrations (12).
Dose-dependency was determined for arteries with and without
endothelium under control conditions and in the presence of
8Br-cGMP (10, 30 and 100 μM/l). The concentration of 8Br-cGMP that
resulted in the half maximal effective concentration
(EC50) was determined using the method of linear
regression for 20–80% maximal effect. Vessel contraction was
measured as increase in perfusion pressure.
Ethical compliance
The Guiding Principles for the Care and Use of
Animals in the Field of Physiological Sciences as well as specific
national law were followed. The study was approved by the Ethics
Committee for the Affairs of Experiments on Animals in Bydgoszcz
(no. 1/2008-4).
Statistical analysis
Results are presented as the mean ± standard
deviation. Statistical differences were analyzed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
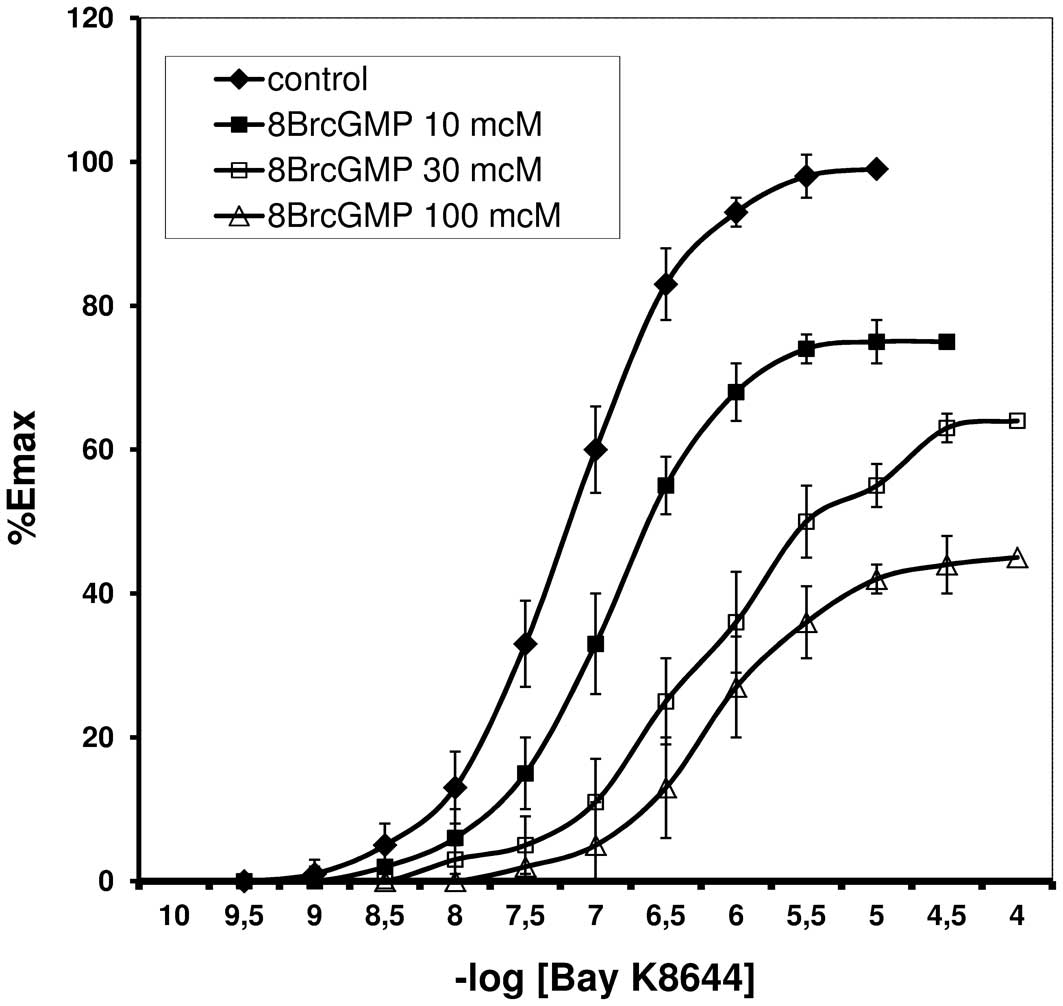
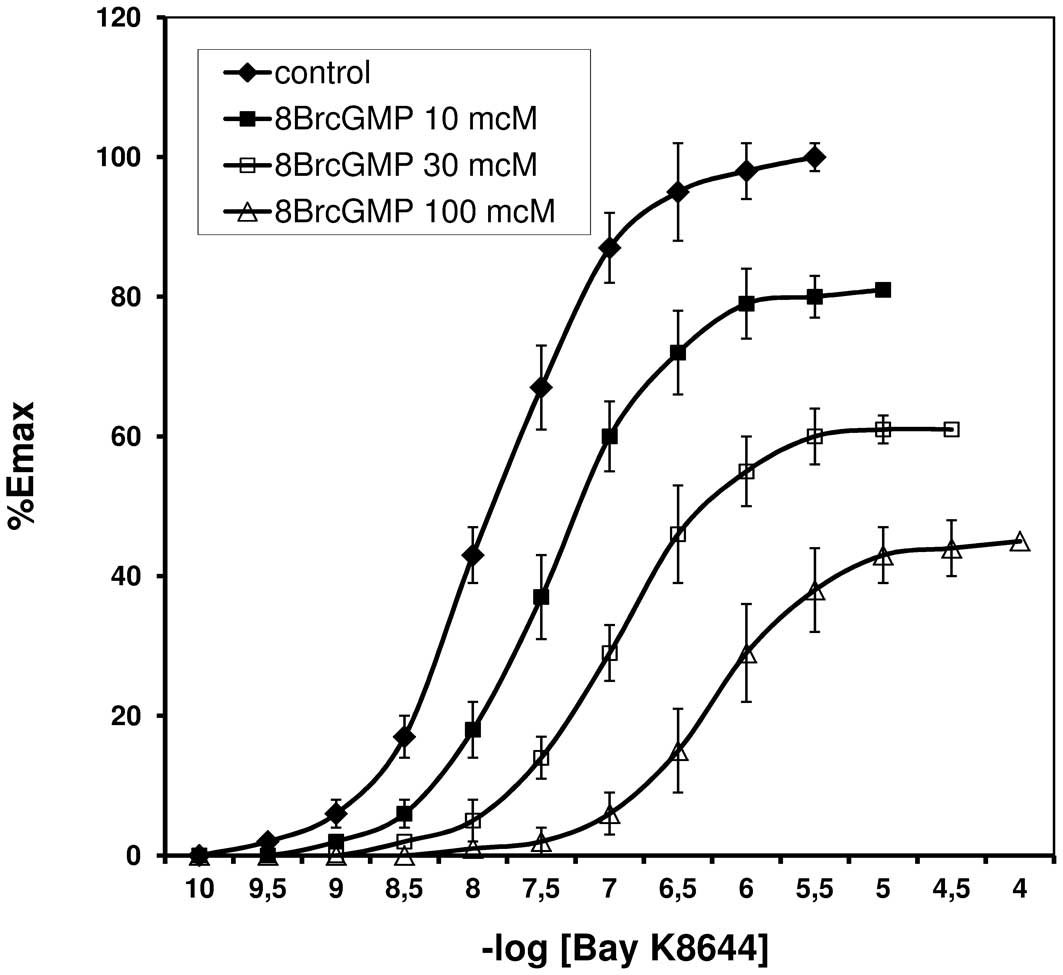
Bay K8644 induced contraction of the rat tail
arteries with and without endothelium. CRCs for Bay K8644, in the
presence of 8Br-cGMP are shown in Figs. 1 and 2. The resulting EC50 values
are listed in Table I.
 | Table IEC50 values for Bay K8644
in the presence of 8Br-cGMP (experiments performed on arteries
with/without endothelium). |
Table I
EC50 values for Bay K8644
in the presence of 8Br-cGMP (experiments performed on arteries
with/without endothelium).
| Group | EC50
arteries with endothelium | EC50
arteries without endothelium |
|---|
| Bay K8644
(control) |
5.97(±0.21)×10−8 |
1.37(±0.24)×10−8 |
| 8Br-cGMP (10
μM/l) |
4.07(±0.29)×10−7 |
3.47(±0.21)×10−8 |
| 8Br-cGMP (30
μM/l) |
5.67(±0.26)×10−7 |
2.17(±0.26)×10−7 |
| 8Br-cGMP (100
μM/l) |
7.12(±0.32)×10−7 |
6.12(±0.22)×10−7 |
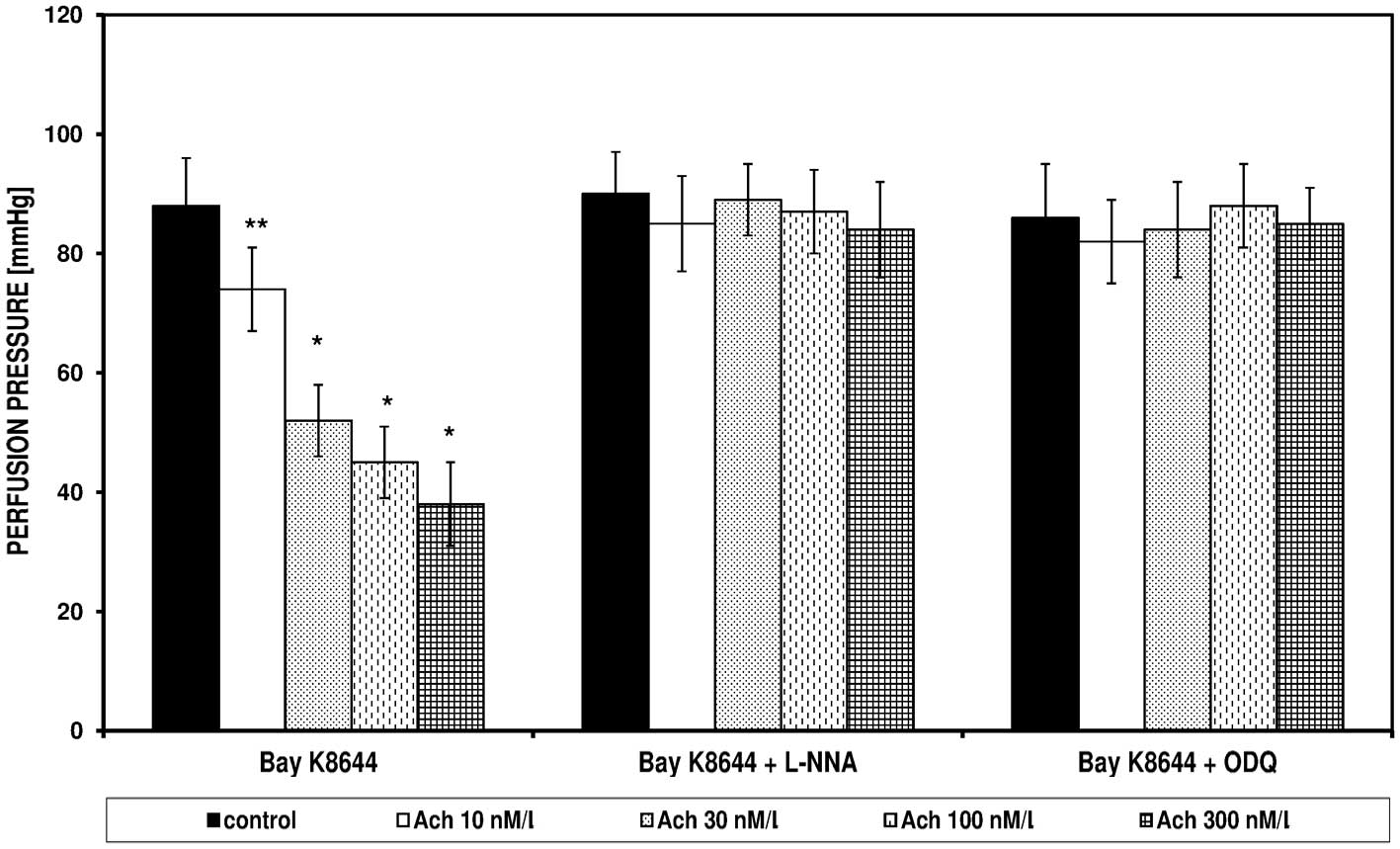
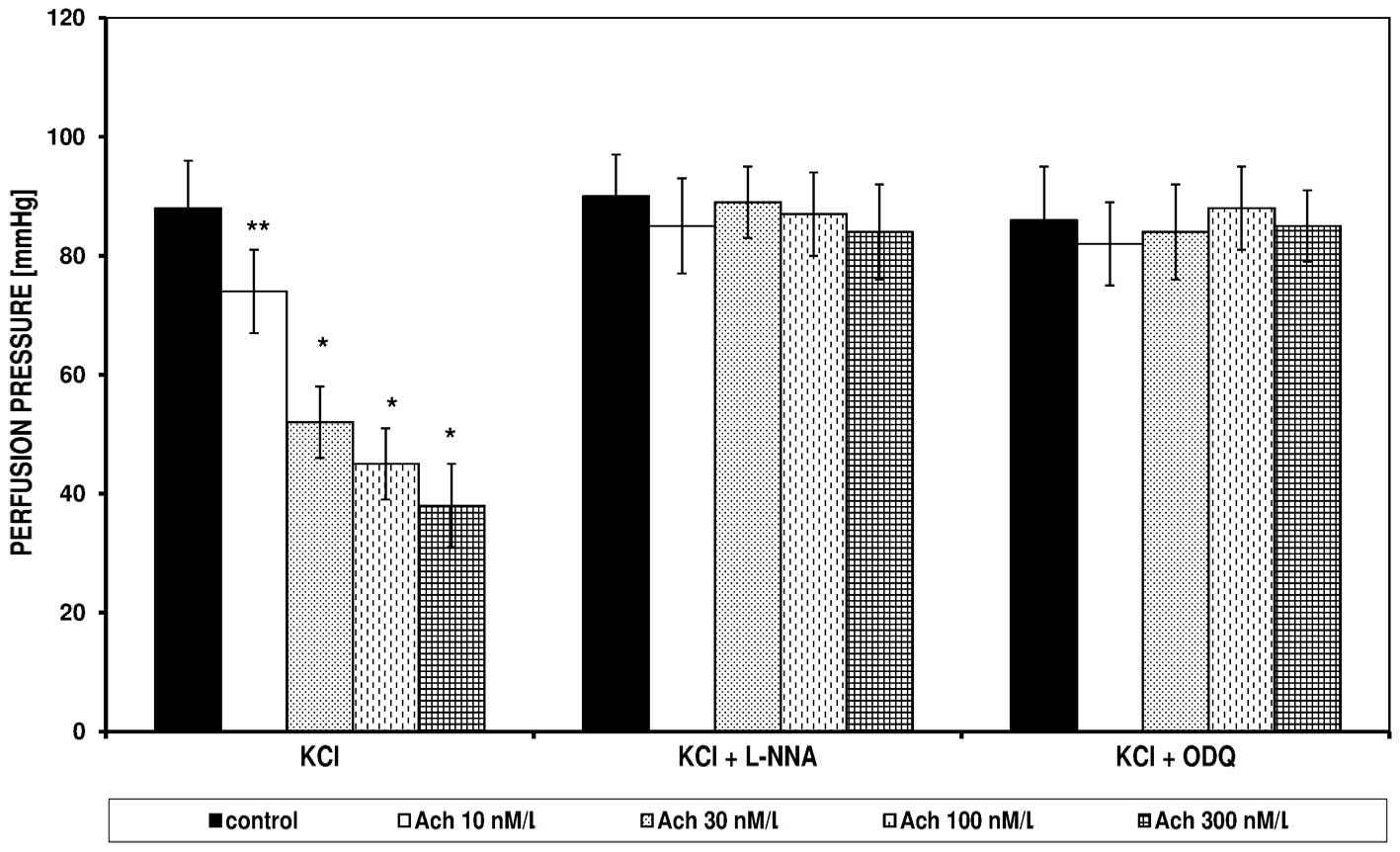
In the FPSS group, perfusion pressure under the
influence of Bay K8644 and KCl did not change; the pressures were
14±6 and 15±5 mmHg, respectively (data not shown). Bay K8644 and
KCl in the PSS induced an increase of perfusion pressure, which was
significantly reduced in the presence of Ach (Figs. 3 and 4). The spasmolytic Ach action did not
occur in the presence of L-NNA and ODQ. Figs. 3 and 4 present the effect of increasing
concentrations of Ach on the perfusion pressure induced in the PSS
by Bay K8644 and KCl, respectively, in the presence of L-NNA and
ODQ.
Discussion
Arterial tension is dependent upon the structure and
function of each layer of the arterial wall, the availability of
Ca2+ and the presence of certain vasodilator and
vasoconstrictor factors. Endothelial NO is important in the
induction of vasodilation (1), and
acts through a signaling system involving cGMP (4,5). Bay
K8644 and KCl are agents that stimulate blood vessel contraction by
inducing an influx of Ca2+ into the cells through
channels in the cell membrane. In the present study, the role of
the endothelium in vascular contraction induced by Bay K8644 and
the involvement of the Ach/NO/cGMP cascade in Bay K8644 and KCl
activity were investigated.
Arterial spasm induced by Bay K8644 was demonstrated
to be reduced by 8Br-cGMP, in a dose-dependent manner. The CRCs
were shifted to the right with increasing 8Br-cGMP concentrations.
Similar changes in the shape and position of the CRCs were
demonstrated in the experiments performed in arteries without
endothelium, thus the reaction was determined to be independent of
this layer of the vessel wall. Similar findings were observed in
previous studies, which utilized angiotensin II (ANG II) as a
substance that stimulated vessel contraction (13,14).
These studies also demonstrated that in arteries without
endothelium, in contrast to vessels with an intact inner layer, no
inhibition of ANG II-stimulated contraction following ischemia was
observed. Numerous studies have confirmed that ischemia/reperfusion
results in an impaired endothelial function, which reduces the
synthesis of endothelial vasodilatators, such as prostaglandins and
NO (15–18).
Under these conditions, vasodilatation stimulated by
Ach, bradykinin, adenosine-5′-diphosphate (ADP), serotonin and
thrombin, among others, is disturbed; however, the effect of NO
donors remains to be observed (17,19).
In the present study, experiments in FPSS and PSS demonstrated that
Bay K8644 and KCl induce an increase in the perfusion pressure, due
to an influx of Ca2+ from outside of the cell, as
observed in previous studies (20–23).
Other studies have indicated that KCl (at depolarizing
concentrations) and Bay K8644 contract smooth muscle by opening the
Ca2+ L-type channels located in the cell membrane, and
by increasing concentrations of free Ca2+ in the
cytoplasm. This effect may be eliminated in the presence of calcium
channel Ca2+ antagonists, nifedipine and diltiazem
(24–27). Subsequent experiments demonstrated
that the contraction induced by Bay K8644 and KCl in the PSS was
reduced at increasing Ach concentrations, and this effect was
eliminated in the presence of L-NNA (nitric oxide synthase
inhibitor) or ODQ (an inhibitor of soluble GC). In addition,
similar observations have been demonstrated in studies of human
mesenteric arteries (22). Ji
et al demonstrated that Ach blocked phenylephrine-triggered
contraction of endothelium-intact rat aorta, but did not affect the
responses of arteries without endothelium (28).
The studies have also indicated that L-NNA and
methylene blue (an inhibitor of soluble GC) may abolish the
spasmolytic action of Ach. It was demonstrated that KCl stimulates
the influx and increase of the calcium ion concentration in the
cytoplasm, and also increases the myosin light chain kinase
activity (29–31). However, the cellular mechanism of
contraction induced by KCl remains to be elucidated.
This mechanism of action was the basis for the use
of KCl in the present study investigating cell signaling as a
factor in cell membrane depolarizing (electromechanical coupling),
compared with agents that stimulate muscle contraction when
combined with the corresponding receptors (pharmacomechanical
coupling) (31,32). Other studies have demonstrated that
KCl may lead to the release of Ca2+ from intracellular
stores (33,34). It was also shown that membrane
depolarization alone led to the sensitivity to
Ca2+(35). Studies have
indicated that KCl also blocked the myosin light chain phosphatase
through RhoA kinase activation, which led to the sensitivity of
Ca2+(36–38).
Signal pathways that control smooth muscle tension
remain an important focus of research, as the mechanisms regulating
vascular contraction may contribute to the understanding of
physiological and pathological processes in the circulatory system,
and may also be beneficial in determining novel treatment methods
for cardiovascular diseases.
In conclusion, it was demonstrated that the increase
in vascular tone induced by Bay K8644 and KCl was independent of
the intracellular pool of Ca2+. The relaxant effect of
Ach on the responses stimulated by Bay K8644 and KCl indicated that
NO was involved in modulating the reactivity of the arteries to the
examined factors, which contributes to the influx of
Ca2+ into the cell.
References
|
1
|
Furchgott RF and Zawadzki JW: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lüscher TF and Barton M: Biology of the
endothelium. Clin Cardiol. 20(Suppl 2): II-3–II-10. 1997.
|
|
3
|
Palmer RM, Ashton DS and Moncada S:
Vascular endothelial cells synthesize nitric oxide from L-arginine.
Nature. 333:664–666. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furchgott RF and Vanhoutte PM:
Endothelium-derived relaxing and contracting factors. FASEB J.
3:2007–2018. 1989.PubMed/NCBI
|
|
5
|
Murad F: The nitric oxide-cyclic GMP
signal transduction system for intracellular and intercellular
communication. Recent Prog Horm Res. 49:239–248. 1994.PubMed/NCBI
|
|
6
|
Blatter LA and Wier WG: Nitric oxide
decreases [Ca2+]i in vascular smooth muscle by
inhibition of the calcium current. Cell Calcium. 15:122–131.
1994.
|
|
7
|
Collins P, Griffith TM, Henderson AH and
Lewis MJ: Endothelium-derived relaxing factor alters calcium fluxes
in rabbit aorta: a cyclic guanosine monophosphate-mediated effect.
J Physiol. 381:427–437. 1986. View Article : Google Scholar
|
|
8
|
Karaki H, Sato K, Ozaki H and Murakami K:
Effects of sodium nitroprusside on cytosolic calcium level in
vascular smooth muscle. Eur J Pharmacol. 156:259–266. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McDaniel NL, Chen XL, Singer HA, Murphy RA
and Rembold CM: Nitrovasodilators relax arterial smooth muscle by
decreasing [Ca2+]i and uncoupling stress from myosin
phosphorylation. Am J Physiol. 263:C461–C467. 1992.
|
|
10
|
Murad F: Cyclic guanosine monophosphate as
a mediator of vasodilation. J Clin Invest. 78:1–5. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.
|
|
12
|
Van Rossum JM: Cumulative dose-response
curves. II Technique for the making of dose-response curves in
isolated organs and the evaluation of drug parameters. Arch Int
Pharmacodyn Ther. 143:299–330. 1963.PubMed/NCBI
|
|
13
|
Koller A, Sun D, Huang A and Kaley G:
Corelease of nitric oxide and prostaglandins mediates
flow-dependent dilation of rat gracilis muscle arterioles. Am J
Physiol. 267:H326–H332. 1994.PubMed/NCBI
|
|
14
|
Szadujkis-Szadurska K, Slupski M,
Szadujkis-Szadurski R, Szadujkis-Szadurski L, Jasiñski M and
Kolodziejska R: The role of the endothelium in the regulation of
vascular smooth muscle cell contractions induced by angiotensin II
after ischemia and reperfusion. Arch Pharm Res. 33:1019–1024. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dignan RJ, Dyke CM, Abd-Elfattah AS, Lutz
HA, Yeh T Jr, Lee KF, Parmar J and Wechsler AS: Coronary artery
endothelial cell and smooth muscle dysfunction after global
myocardial ischemia. Ann Thorac Surg. 53:311–317. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fullerton DA, Hahn AR, Koike K, Banerjee A
and Harken AH: Intracellular mechanisms of pulmonary vasomotor
dysfunction in acute lung injury caused by mesenteric
ischemia-reperfusion. Surgery. 114:360–367. 1993.PubMed/NCBI
|
|
17
|
Fullerton DA, Mitchell MB, McIntyre RC Jr,
Banerjee A, Campbell DN, Harken AH and Grover FL: Cold ischemia and
reperfusion each produce pulmonary vasomotor dysfunction in the
transplanted lung. J Thorac Cardiovasc Surg. 106:1213–1217.
1993.PubMed/NCBI
|
|
18
|
Hashimoto K, Pearson PJ, Schaff HV and
Cartier R: Endothelial cell dysfunction after ischemic arrest and
reperfusion: a possible mechanism of myocardial injury during
reflow. J Thorac Cardiovasc Surg. 102:688–694. 1991.PubMed/NCBI
|
|
19
|
Dauber IM, VanBenthuysen KM, McMurtry IF,
Wheeler GS, Lesnefsky EJ, Horwitz LD and Weil JV: Functional
coronary microvascular injury evident as increased permeability due
to brief ischemia and reperfusion. Circ Res. 66:986–998. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pesic A, Madden JA, Pesic M and Rusch NJ:
High blood pressure upregulates arterial L-type Ca2+
channels: is membrane depolarization the signal? Circ Res.
94:e97–e104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pratt PF, Bonnet S, Ludwig LM, Bonnet P
and Rusch NJ: Upregulation of L-type Ca2+ channels in
mesenteric and skeletal arteries of SHR. Hypertension. 40:214–219.
2002.
|
|
22
|
Szadujkis-Szadurski R, Tafil-Klawe M,
Szadujkis-Szadurska K, Szadujkis-Szadurski L, Slupski M, Grzesk G,
Matusiak G, Gajdus M and Glaza I: Modulation of the contractile
effect of Bay K8644 on human vascular smooth muscle cells by
acetylocholine and calcium ions. Med Biol Sci. 24:59–64. 2010.
|
|
23
|
Szadujkis-Szadurska K, Grzesk G,
Szadujkis-Szadurski L, Gajdus M and Matusiak G: Role of nitric
oxide and cGMP in modulation of vascular contraction induced by
angiotensin II and Bay K8644 during ischemia/reperfusion. Exp Ther
Med. 5:616–620. 2013.PubMed/NCBI
|
|
24
|
Iesaki T and Wolin MS: Thiol oxidation
activates a novel redox-regulated coronary vasodilator mechanism
involving inhibition of Ca2+ influx. Arterioscler Thromb
Vasc Biol. 20:2359–2365. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matchkov VV, Aalkjaer C and Nilsson H: A
cyclic GMP- dependent calcium-activated chloride current in
smooth-muscle cells from rat mesenteric resistance arteries. J Gen
Physiol. 123:121–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piper AS and Large WA: Direct effect of
Ca2+-calmodulin on cGMP-activated
Ca2+-dependent Cl-channels in rat mesenteric artery
myocytes. J Physiol. 559:449–457. 2004.PubMed/NCBI
|
|
27
|
Szadujkis-Szadurski L, Talar J, Wisniewski
K, Tomaszewski W, Lukowicz M and Szadujkis-Szadurski R: Modulatory
effects of laser radiation on extracellular and intracellular
calcium pool and vascular resistance of rat’s tail artery.
Physiother Pol. 2:11–20. 2002.
|
|
28
|
Ji J, Benishin CG and Pang PK: Nitric
oxide selectively inhibits intracellular Ca2+ release
elicited by inositol trisphosphate but not caffeine in rat vascular
smooth muscle. J Pharmacol Exp Ther. 285:16–21. 1998.PubMed/NCBI
|
|
29
|
Brozovich FV: Rho signaling: agonist
stimulation and depolarization come together. Circ Res. 93:481–483.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu C, Zuo J, Pertens E, Helli PB and
Janssen LJ: Regulation of Rho/ROCK signaling in airway smooth
muscle by membrane potential and [Ca2+]i. Am J Physiol
Lung Cell Mol Physiol. 289:L574–L582. 2005.PubMed/NCBI
|
|
31
|
Somlyo AV and Somlyo AP: Electromechanical
and pharmacomechanical coupling in vascular smooth muscle. J
Pharmacol Exp Ther. 159:129–145. 1968.PubMed/NCBI
|
|
32
|
Karaki H, Ozaki H, Hori M, Mitsui-Saito M,
Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ and Sato K:
Calcium movements, distribution, and functions in smooth muscle.
Pharmacol Rev. 49:157–230. 1997.PubMed/NCBI
|
|
33
|
Kobayashi S, Kanaide H and Nakamura M:
Complete overlap of caffeine- and K+
depolarization-sensitive intracellular calcium storage site in
cultured rat arterial smooth muscle cells. J Biol Chem.
261:15709–15713. 1986.PubMed/NCBI
|
|
34
|
Ureña J, del Valle-Rodríguez A and
López-Barneo J: Metabotropic Ca2+ channel-induced
calcium release in vascular smooth muscle. Cell Calcium.
42:513–520. 2007.
|
|
35
|
Yanagisawa T and Okada Y: KCl
depolarization increases Ca2+ sensitivity of contractile
elements in coronary arterial smooth muscle. Am J Physiol.
267:H614–H621. 1994.PubMed/NCBI
|
|
36
|
Ratz PH, Berg KM, Urban NH and Miner AS:
Regulation of smooth muscle calcium sensitivity: KCl as a
calcium-sensitizing stimulus. Am J Physiol Cell Physiol.
288:C769–C783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ratz PH and Miner AS: Role of protein
kinase Czeta and calcium entry in KCl-induced vascular smooth
muscle calcium sensitization and feedback control of cellular
calcium levels. J Pharmacol Exp Ther. 328:399–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakurada S, Takuwa N, Sugimoto N, Wang Y,
Seto M, Sasaki Y and Takuwa Y: Ca2+-dependent activation
of Rho and Rho kinase in membrane depolarization-induced and
receptor stimulation-induced vascular smooth muscle contraction.
Circ Res. 93:548–556. 2003.
|