Introduction
Hypoxic ischemic encephalopathy (HIE) is a serious
condition due to the inadequate oxygen supply to the brain, and is
associated with oxygen deprivation in the neonate. For HIE
neonates, regeneration of neural cells is a critical process for
repairing the damaged brain (1).
Several inhibitors are capable of reducing the ability of central
nervous system (CNS) repair, among which Nogo A is important
(2). Nogo has been identified as
an inhibitor of neurite outgrowth that is specific to the CNS. It
belongs to the family of reticulon-encoding genes, associated with
the endoplasmic reticulum (3).
Nogo is a potent neurite outgrowth inhibitor that may also block
the regeneration of the CNS in higher vertebrates (4). Nogo-66 receptor (NgR), together with
oligodendrocyte myelin glycoprotein and myelin-associated
glycoprotein, mediate axonal growth inhibition and may play a role
in regulating axonal regeneration and plasticity in the adult CNS
(5). However, NgR may inhibit the
regeneration of neurons and related nervous cells in HIE neonates
and thus hinder the repair of injured CNS. NgR mediates axonal
growth inhibition and may play a role in regulating axonal
regeneration and plasticity in the adult CNS (6).
The Wnt signaling pathway controls cell-cell
communication in the embryo and in adults, including cell
proliferation and differentiation during development and healing
(7). A previous study indicated
that the Wnt signaling pathway is involved in regeneration of
neural cells mediated by Nogo; however, the mechanism involved
remains unknown (8). Inhibition of
NgR is considered as one potentially useful method for treatment of
HIE neonates. To study the effects of inhibition of NgR on the
regeneration of injured CNS and the related transcription factors
(TFs) involved, Nogo-A receptor antagonist NEP1-40 was used in the
present study. The investigation focused on the TFs in the Wnt
signaling pathway that are regulated by inhibition of NgR during
CNS regeneration, together with the proliferation of neural
cells.
Materials and methods
Generation of animal model and drug
treatments
ewborn male Wistar rats (7 days old, weighing
16.0±3.0 g) were provided by the Animal Research Center, University
of Yangzhou, China. The newborn hypoxic ischemic encephalopathy rat
animal model was generated as described previously and rats were
named hypoxic ischemic brain damage (HIBD) rats (9). The 40 HIBD rats were divided into a
HIBD group and a HIBD + NEP1-40 group (n=20 in each group). The
HIBD + NEP1-40 group rats were treated with NEP1-40 for 7 days as
previously described (9). Rats
were sacrificed by inhalation of CO2 for 3 min. This
study was approved by the Medical Ethics Committee of the Clinical
Medical College of Yangzhou, University of Yangzhou (Yangzhou,
China).
Quantitative PCR (qPCR)
Total RNA from rat brains was isolated using TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The Rat WNT Signaling Pathway PCR
array (SABiosciences, Qiagen, Inc., Frederick, CA, USA), which
contained Apc, Apc2, Ccnd1, Ccnd2, Ccnd3, Ctnnb1, Ep300, Fgf4,
Fzd3, c-Jun, Lrp5, c-Myc, Ppp2ca, Ppp2r1a, Wisp1 and Wnt3a was used
to detect the expression of genes related to the Wnt signaling
pathway. After reverse transcription using a cDNA Synthesis kit
(Invitrogen Life Technologies), all the products were used as the
templates for the qPCR using the ABI Prism SDS 7000 (Applied
Biosystems, Inc., Foster City, CA, USA). qPCR conditions were as
follows: i) 50°C 2 min, 1 cycle; ii) 95°C 10 min, 1 cycle; iii)
95°C 15 sec, followed by 60°C 30 sec and 72°C 30 sec, 40 cycles;
iv) 72°C 10 min, 1 cycle.
Western blot analysis
Total protein extracted from rat brains (12 μg) was
boiled at 100°C with 4X loading buffer for 5 min, and then
subjected to 10% SDS-PAGE (Invitrogen Life Technologies). After
electrophoresis, the gel was transferred onto a nitrocellulose (NE)
membrane at 70 V for 2 h at 4°C. After blocking in 5% nonfat milk
for 1 h, the membrane was incubated with Apc (1:800, rabbit
polyclonal IgG; Millipore, Billerica, MA, USA), Ep300 (1:1,000,
rabbit polyclonal IgG; Sigma-Aldrich, St. Louis, MO, USA), c-Jun
(1:1,000, rabbit polyclonal IgG; Millipore), c- Myc (1:1,000,
rabbit polyclonal IgG; Millipore) and Wnt3a (1:1,000, rabbit
polyclonal IgG; Millipore) primary antibodies overnight at 4°C in
3% BSA according to the results from qPCR. After washing with 1X
tris phosphate-buffered saline (TPBS; pH 7.4), the membrane was
incubated with secondary antibody (goat anti-rabbit IgG, Cell
Signaling Technology, Inc., Boston, MA, USA) for 1 h at room
temperature, washed again with 1X TPBS (pH 7.4), and images were
captured with film exposure (Kodak, Rochester, NY, USA) for
analysis. β-actin was used as a negative control. The value of each
protein was first compared with β-actin, then the relative value
was compared between the HIBD and HIBD + NEP1-40 groups.
Immunofluorescence (IF) for cell
proliferation
Brain extracts were detected by IF for the
expression of Ki67. Cryostat rat brain coronal sections (12 μm)
were prepared (Leica, Solms, Germany) and stained with Ki67
antibody (Abcam, Cambridge, MA, USA; 1:1,000) to assess the
proliferation of neural cells in the subventricular zone (SVZ).
Images were captured using a confocal microscope, shown in the dark
(A1R MP+ Multiphoton Confocal; Nikon, Tokyo, Japan; ×100).
8-Isoprostane detection
The brains were homogenized in TBS buffer with
protease inhibitors (1:1,000, Invitrogen Life Technologies) and
centrifuged at 12,000 g/min for 40 min at 4°C. Supernatant was
transferred into another tube. The levels of 8-isoprostane, a
special marker for reactive oxygen species (ROS), were determined
using an 8-Isoprostane EIA kit (Cayman Chemical Company, Ann Arbor,
MI, USA) according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as the means ± SD. The
results were evaluated by Student’s t-tests. Statistically
significant differences between groups were defined as P<0.05
and P<0.01. Calculations were performed using SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA).
Results
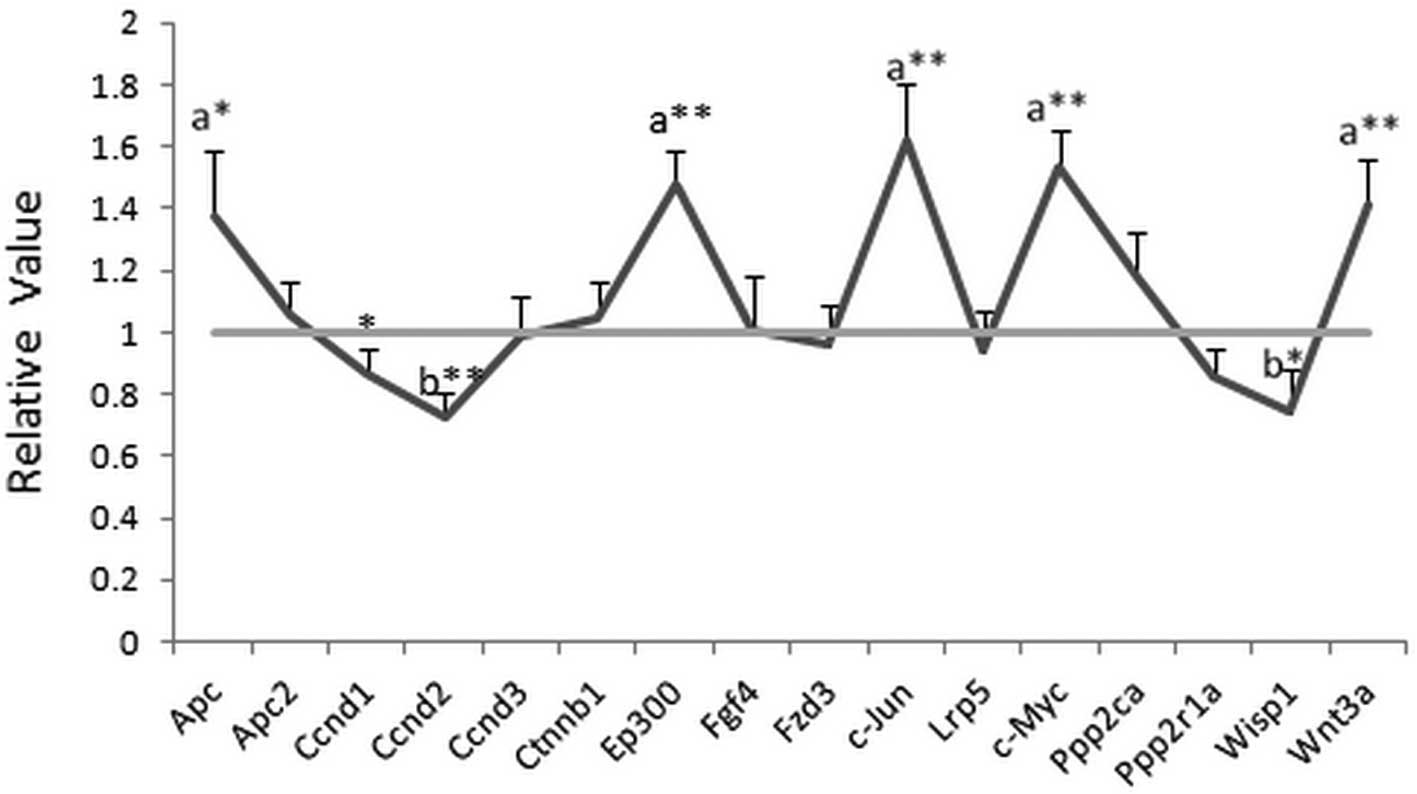
Effects of NEP1-40 on gene
expression
The gene expression of Apc, Ep300, c-Jun, c-Myc and
Wnt3a was significantly increased (>1.35-fold) while Ccnd2 and
Wisp1 (<0.75-fold) were decreased in the HIBD + NEP1-40 group
after treatment with NEP1-40 for 7 days. For other genes, no
significant changes (>1.5- or <0.75-fold) were detected. The
value in the HIBD group was set as 1, while the relative value was
calculated by comparing the HIBD + NEP1-40 group with the HIBD
group. All data are shown in Fig.
1.
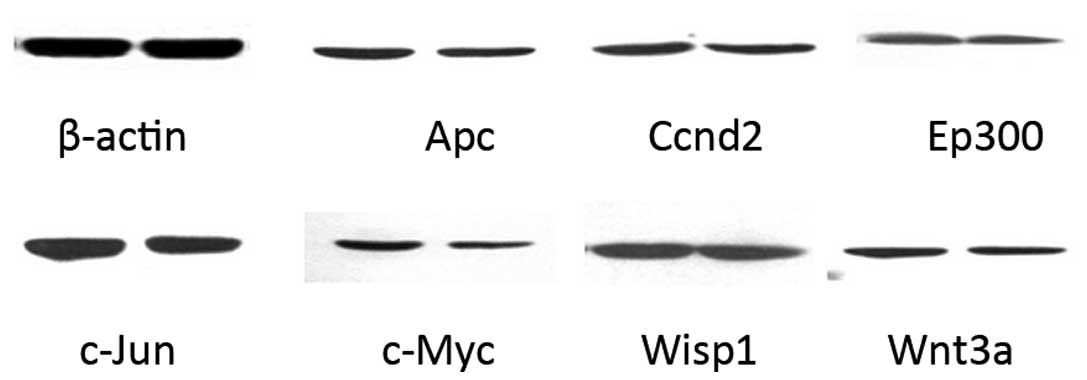
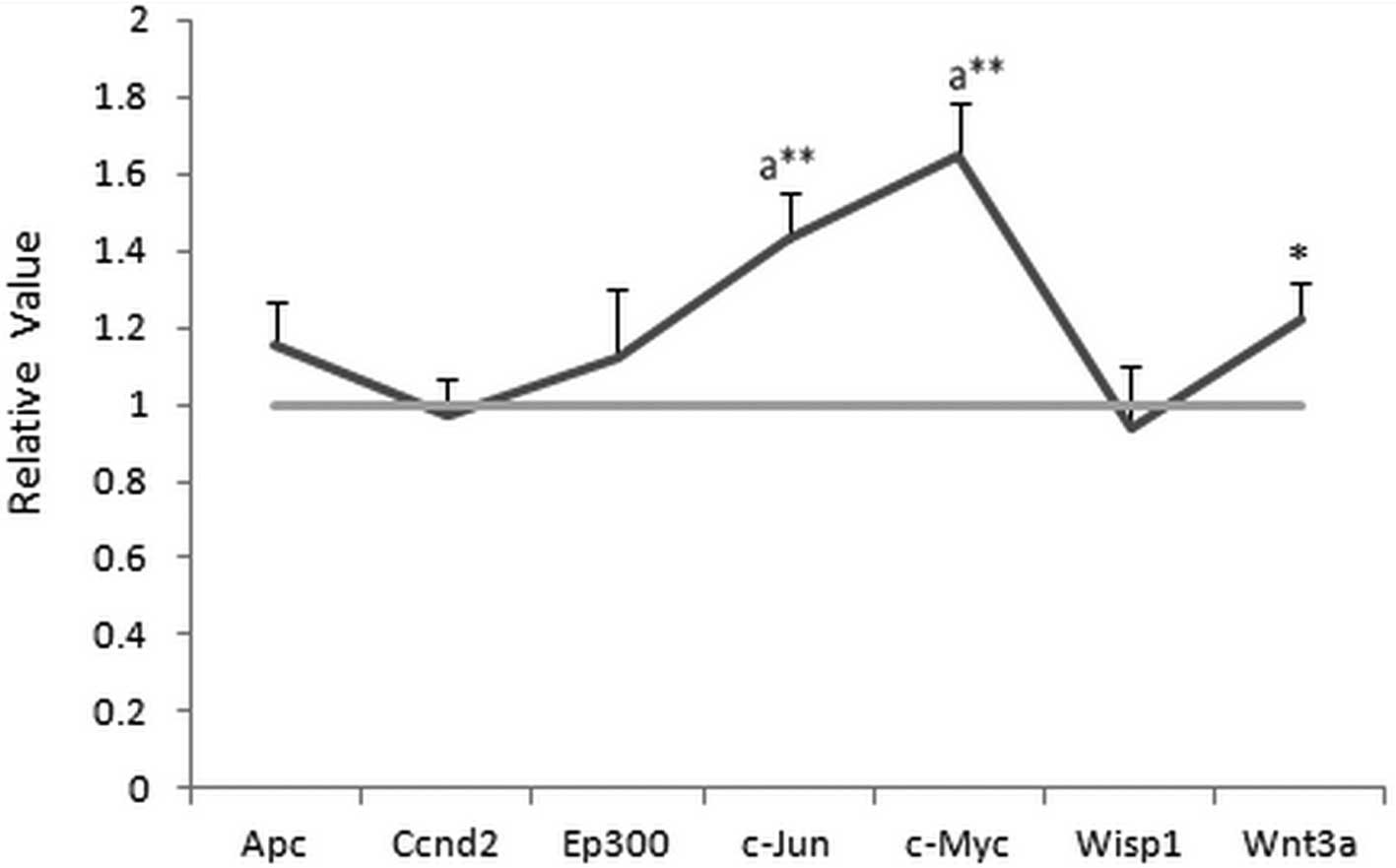
Effects of NEP1-40 on protein
expression
As shown in Fig. 2,
the expression of c-Jun and c-Myc at the protein level were
upregulated (>1.5-fold) after treatment with the Nogo-A receptor
antagonist NEP1-40 for 7 days, which correlated with the changes
observed for gene expression. However, no marked changes in Apc,
EP300, Wnt3a, Ccnd2 and Wisp1 expression (>1.35- or
<0.75-fold) were detected, in contrast with the gene expression
results. The value in the HIBD group was set as 1, while the
relative value was calculated by comparing the HIBD + NEP1-40 group
with the HIBD group. All data were analyzed in Fig. 3.
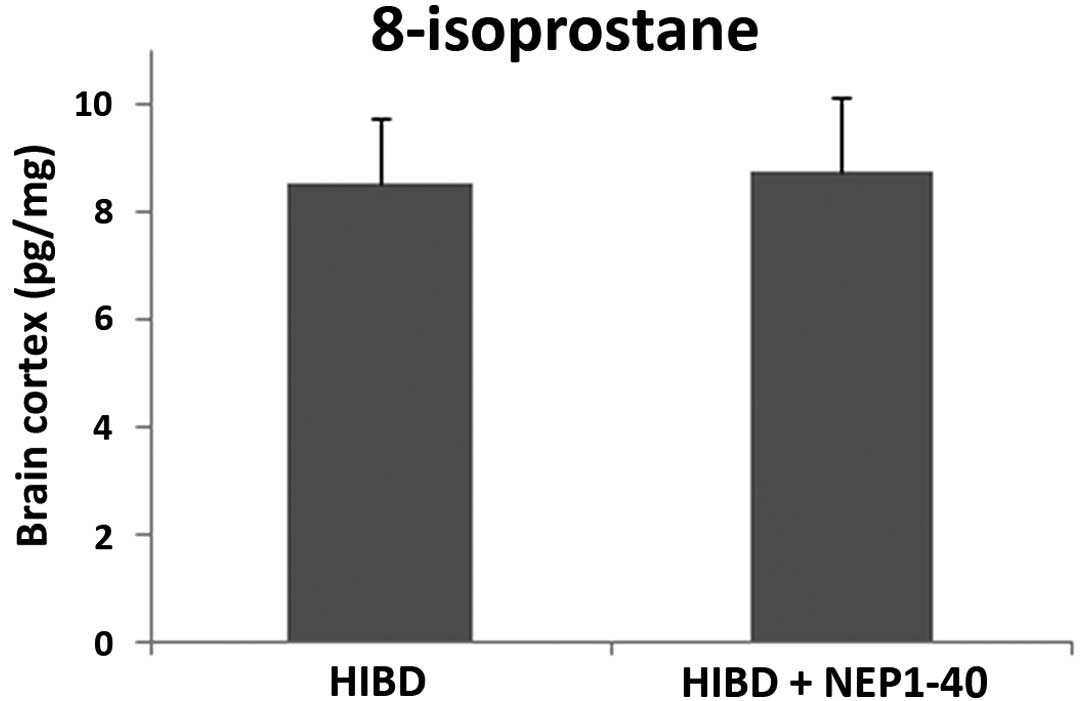
Analysis of 8-isoprostane detection
8-Isoprostane is an ideal biomarker for detecting
oxidative stress in animal tissues and organs. No significant
changes in 8-isoprostane were detected between the HIBD + NEP1-40
group and the HIBD group (Fig. 4).
This study indicated that the Nogo-A receptor antagonist NEP1-40
did not affect oxidative stress in the CNS during HIE.
Analysis of the regeneration of neural
cells
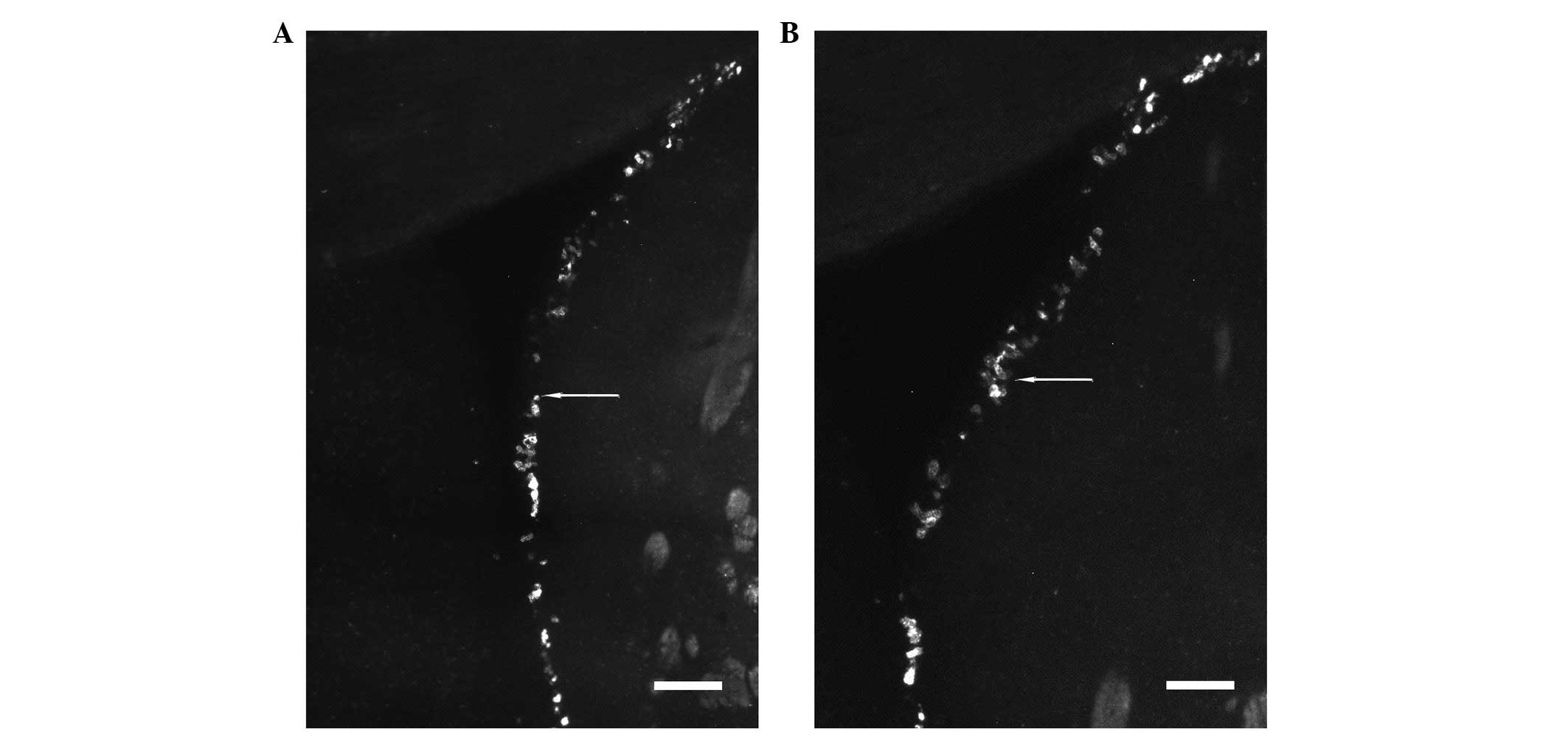
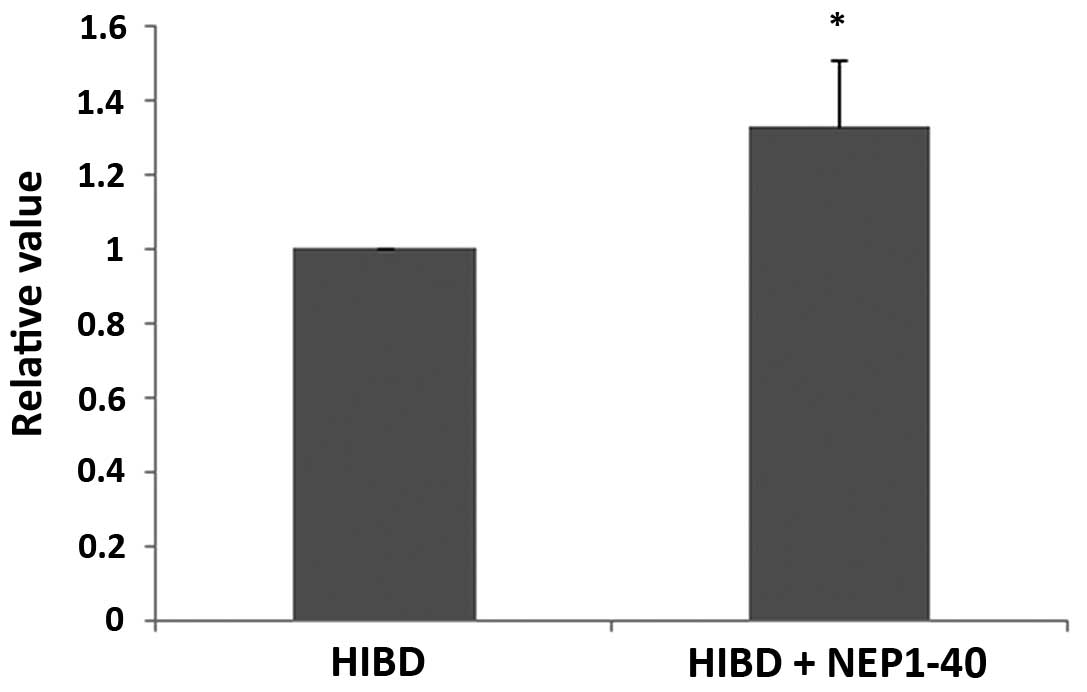
As indicated by arrows showing Ki67 in Fig. 5 (bar, 200 μm), increased
regeneration of neural cells was detected in the HIBD + NEP1-40
group (Fig. 5A) compared with the
HIBD group (Fig. 5B) in the SVZ,
the site of neural cellular proliferation in the adult brain, which
may be useful for repair of damage to the CNS. Promotion of neural
cellular proliferation is a potential method for HIE treatment. The
value in the HIBD group was set as 1, while the relative value was
calculated by comparing the HIBD + NEP1-40 group with the HIBD
group. All data were analyzed in Fig.
6.
Discussion
Numerous proteins are involved in HIE, including
Nogo, which is involved in neuroendocrine secretion or in membrane
trafficking in neuroendocrine cells (10). Nogo-A has two known inhibitory
domains including amino-Nogo, at the N-terminus, and Nogo-66
(4). Blocking Nogo-A during
neuronal damage may help to protect or restore the damaged neurons.
NgR is a high-affinity binding receptor for a region of Nogo, a
myelin-associated protein that inhibits axon outgrowth, which
requires membrane-spanning co-receptors to transduce growth
inhibitory signals (11). NgR is
implicated in neuronal plasticity and regeneration (12). However, the mechanism involved
remains unknown, among which the Wnt signaling pathway is valuable
to study. Wnt signaling pathways play a variety of roles in
embryonic development, cell differentiation and cell polarity
generation (13), particularly for
shifting ventral genes into dorsal regions of the neural tube
(14). In this study, c-Jun and
c-Myc were found to be upregulated. c-Jun plays an important role
in cellular proliferation and apoptosis of the endometrium
throughout the menstrual cycle (15). Cyclical changes of the c-Jun
protein levels are significant in the proliferation and apoptosis
of glandular epithelial cells (16). c-Myc activates the expression of a
number of genes through binding to enhancer box sequences and
recruiting histone acetyltransferases (HATs) (17), activated upon various mitogenic
signals, including the Wnt signaling pathway (18).
8-Isoprostane is an ideal biomarker of oxidative
stress and increased concentrations are detected during this
progress, indicating that an imbalance between the systemic
manifestation and clearance of reactive oxygen species results in
body or organ damage. (19). Our
research indicated that no significant change in 8-isoprostane
levels was detected between the HIBD + NEP1-40 group and the HIBD
group. This result suggested that oxidative stress was not related
to or involved in the inhibition of Nogo-A during neuronal damage
and neuronal repair. Ki67 is a cellular marker for proliferation.
Ki67 is strictly associated with cell proliferation and is present
during all active phases of the cell cycle (G1, S,
G2 and mitosis), but is absent from resting cells
(G0) (20). Increased
Ki67 expression means that increased regeneration of neural cells
was detected in the HIBD + NEP1-40 group in the SVZ, the area of
neural cellular proliferation in the adult brain. Promotion of
neural cellular proliferation is a potential method for HIE
treatment, which requires further study. Given that this antagonist
may be a good potential drug target for the treatment of HIE, the
importance of this in vivo study is currently under intense
investigation.
This study focused on the effects of the Nogo-A
receptor antagonist, NEP1-40, on regulation of the Wnt signaling
pathway and neural cell proliferation in newborn HIE rats. It was
indicated by inhibition of NgR that c-Jun and c-Myc were the main
TFs in the Wnt signaling pathway, while neural cell proliferation
in the SVZ was increased during this process.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81071466) and Research
Project Foundation on Social Development of Science and Technology
of Yangzhou (YZ2011082).
References
|
1
|
Pietrini D, Piastra M, Luca E, Mancino A,
Conti G, Cavaliere F and De Luca D: Neuroprotection and hypothermia
in infants and children. Curr Drug Targets. 13:925–935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pernet V and Schwab ME: The role of Nogo-A
in axonal plasticity, regrowth and repair. Cell Tissue Res.
49:97–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skaper SD: Neuronal growth-promoting and
inhibitory cues in neuroprotection and neuroregeneration. Methods
Mol Biol. 846:13–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borrie SC, Baeumer BE and Bandtlow CE: The
Nogo-66 receptor family in the intact and diseased CNS. Cell Tissue
Res. 349:105–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDonald CL, Bandtlow C and Reindl M:
Targeting the Nogo receptor complex in diseases of the central
nervous system. Curr Med Chem. 18:234–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Z, Gao Y, Deng K, Williams G, Doherty
P and Walsh FS: Receptors for myelin inhibitors: structures and
therapeutic opportunities. Mol Cell Neurosci. 43:1–14. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Budnik V and Salinas PC: Wnt signaling
during synaptic development and plasticity. Curr Opin Neurobiol.
21:151–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cerpa W, Toledo EM, Varela-Nallar L and
Inestrosa NC: The role of Wnt signaling in neuroprotection. Drug
News Perspect. 22:579–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Y, Shumsky JS, Sabol MA, Kushner RA,
Strittmatter S, Hamers FP, Lee DH, Rabacchi SA and Murray M:
Nogo-66 receptor antagonist peptide (NEP1–40) administration
promotes functional recovery and axonal growth after lateral
funiculus injury in the adult rat. Neurorehabil Neural Repair.
22:262–278. 2008.
|
|
10
|
Llorens F, Gil V and del Río JA: Emerging
functions of myelin-associated proteins during development,
neuronal plasticity, and neurodegeneration. FASEB J. 25:463–475.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng FY and Tang BL: Nogo-A and Nogo-66
receptor in amyotrophic lateral sclerosis. J Cell Mol Med.
12:1199–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Zhang Q, Zhang JH and Qin X: NgR
acts as an inhibitor to axonal regeneration in adults. Front
Biosci. 13:2030–2040. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cadigan KM: TCFs and Wnt/β-catenin
signaling: more than one way to throw the switch. Curr Top Dev
Biol. 98:1–34. 2012.
|
|
14
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
15
|
Otsuki Y: Apoptosis in human endometrium:
apoptotic detection methods and signaling. Med Electron Microsc.
34:166–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doucas H, Garcea G, Neal CP, Manson MM and
Berry DP: Changes in the Wnt signalling pathway in gastrointestinal
cancers and their prognostic significance. Eur J Cancer.
41:365–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honeycutt KA and Roop DR: c-Myc and
epidermal stem cell fate determination. J Dermatol. 31:368–375.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS, Lee K, Kang KA, Lee NH, Hyun JW
and Kim HS: Phloroglucinol exerts protective effects against
oxidative stress-induced cell damage in SH-SY5Y cells. J Pharmacol
Sci. 119:186–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin T, Liu Y, Shi M, Liu X, Li L, Liu Y
and Zhao G: Promotive effect of ginsenoside Rd on proliferation of
neural stem cells in vivo and in vitro. J Ethnopharmacol.
142:754–761. 2012. View Article : Google Scholar : PubMed/NCBI
|