Introduction
A number of clinical settings, including acute
mesenteric ischemia, emergency abdominal aortic aneurysm surgery,
strangulated hernias and neonatal necrotizing enterocolitis,
contribute to small intestinal ischemia/reperfusion injury (IIRI)
(1,2). The disruption of intestinal mucosal
integrity is highly associated with the development of multiple
organ dysfunction. Despite intensive research, the morbidity and
mortality caused by IIR remains high (3). Therefore, it is imperative to find
novel remedies to attenuate IIRI and IIRI-mediated mortality.
Mast cells (MCs) are immune effector cells known for
releasing their cytoplasmic granules and play a key role in the
inflammatory process (4). When
activated, MCs can release three classes of pro-inflammatory
mediators, including tryptase, histamine, TNF-α and IL-6 cytokines
(5). Among these released
mediators, histamine and tryptase exacerbate inflammation by
enhancing blood vessel permeability or directly causing damage to
the tissue (6,7). Stabilizing MCs using cromolyn sodium
(CS, 50 mg·kg−1 i.v. at 5 min prior to reperfusion and
once daily for three days following reperfusion), as demonstrated
by our previous study, exhibited promising therapeutic benefits
against IIRI as evidenced by marked increases in long-term survival
and attenuations in IIRI-mediated inflammation in rats (8).
By contrast, intestinal mucosal MCs (IMMCs) are
present mostly in close proximity to intestinal epithelial surfaces
and connect to vessels and nerves, where they are strategically
located for optimal interaction with the environment and for their
putative functions for host defense (9). Groschwitz et al reported that
MC activation exhibits a protective role against gut inflammation
and is important in maintaining homeostasis of the gastrointestinal
tract (10). Endothelin-1 (ET-1),
mostly secreted by endothelial cells, is upregulated in the
post-ischemic period of IIRI (11), and suppressing or reducing ET-1
production significantly attenuates the damage to the intestine
caused by ischemia/reperfusion (12). MCs releasing carboxypeptidase A3
and other MC peptidases can directly degrade ET-1 and exhibit
protective roles against sepsis (13). Boros et al have noted that
MC degranulation prior to ischemia significantly limits IIRI in a
canine model (14). Those
paradoxical results prompted us to hypothesize that stabilizing MCs
at various time points may result in different outcomes induced by
IIRI.
In certain predictable clinical conditions of IIRI,
such as bowel transplantation, the treatment to attenuate IIRI may
be initiated either prior to ischemia or after reperfusion.
However, the optimum therapeutic method for blocking IIRI by
stabilizing MCs remains poorly understood. The present study was
designed to explore improved methods of treatment using CS, which
was administered intravenously either 15 min prior to ischemia or
after reperfusion in order to attenuate IIRI.
Materials and methods
Experimental model of IIR and animal
groups
Four sets of healthy male Kunming mice weighing
20–22 g (provided by the Animal Center of Guangdong Province,
Guangdong, China) were anesthetized by intraperitoneal injection of
10% chloral hydrate (3.0 ml/kg) after they were fasted for 16 h
prior to surgery. Animals had free access to water prior to
surgery. After ensuring an adequate depth of anesthesia, the mice
were fixed in the supine position. In the IIR group (M group), the
abdomen was opened and the superior mesenteric artery (SMA) was
identified and clamped for 30 min. The clamp was then released and
reperfusion of the splanchnic region was maintained for 3 days to
observe survival rates or maintained for 3 h to define early small
intestinal injury. In the sham-operated group (SH group), the
abdomen was opened and the SMA was isolated but not clamped. In the
other two treatment groups, mice subjected to IIR in the PreCr
group and PostCr group were injected intravenously with CS (ICN,
USA; 25 mg/kg in 0.1 ml) through the caudal vein at 15 min prior to
ischemia and 15 min after releasing of the clamp, respectively.
Mice in the SH and M groups received the same volumes of normal
saline at 15 min prior to ischemia. In addition, pre-warmed normal
saline (0.033 ml/g body weight) was administered subcutaneously to
avoid fluid loss following surgery. The dose of CS was selected in
accordance with previous publications (8,15).
All the protocols in the present study were approved by the
Institutional Animal Care and Use Committee of Sun Yat-Sen
University (Guangzhou, Guangdong, China) and followed the national
guidelines for the treatment of animals.
Preparation of specimens and
measurements
The surviving mice were sacrificed by anesthetic
overdose at 3 h after clamp release and 7 mice in each group were
collected. A segment of 1.0 cm intestine was cut from 10 cm to the
terminal ileum and fixed in 10% formaldehyde, then embedded in
paraffin for sectioning. The whole small intestine was removed
carefully, and washed thoroughly with 0°C normal saline and then
opened longitudinally to expose the intestinal epithelium, rinsed
completely with 0°C normal saline and dried with suction paper, and
then stored at −70°C for further measurements.
Pathohistological changes in the
intestine
Sections of 5 μm thickness were prepared from
paraffin-embedded intestinal tissues, and the segment was then
stained with hematoxylin-eosin. Damage to the intestinal mucosa was
evaluated by two different histopathologists who were initially
blind to the experiment, according to the criteria of Chiu’s method
(16).
Immunohistochemical detection of MC
counts in the intestine
Immunohistochemical staining was determined as
previously described (8). Briefly,
5-μm sections were prepared from paraffin-embedded intestinal
tissues. After deparaffinization and blocking of nonspecific
binding sites, the sections were incubated with polyclonal human
anti-mast cell tryptase (dilution 1:50) for 30 min at 37°C,
followed by incubation with biotinylated goat-anti-rat IgG at room
temperature for 10–15 min. The antibody binding sites were
visualized by incubating with
diaminobenzidine-H2O2 solution. MC counts
were assessed by brownish granules in the cytoplasm using Image-Pro
Plus 5.0 software (Media Cybernetics, Warrendale, PA, USA) in 5
randomly selected areas per slide at ×200 magnification.
Western blotting
Intestinal tissue samples were homogenized for 30
sec in a mortar and pestle with liquid nitrogen. Homogenates were
centrifuged at 9,447 × g for 10 min at 4°C and the supernatant was
collected as the source of sample protein. The protein expression
of mast cell protease 7 (MCP7) was determined as described
previously (7). Densitometry was
quantitated using Quantity One software (Bio-Rad Laboratories,
Hercules, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Small intestinal tissues were homogenized with cold
normal saline, and then spun at 894 × g for 15 min. Supernatants
were transferred into fresh tubes for detection of histamine,
TNF-α, IL-6 and ET-1. Tissue total protein was measured by a BCA
Protein Assay kit provided by KenGen Biotech Company (Nanjing,
Jiangsu, China). The levels of histamine, TNF-α, IL-6 and ET-1 were
measured by ELISA kits (R&D Systems Inc., Minneapolis, MN, USA)
according to the manufacturer’s instructions. The intestine tissue
levels of histamine, TNF-α and IL-6 were normalized by tissue
weight.
Detection of myeloperoxidase (MPO)
activity in small intestine tissue
The MPO activities were determined by the
O-dianisidine method (17)
according to the manufacturer’s instructions (Jiancheng
Bioengineering Ltd, Nanjing, Jiangsu, China). MPO activity was
defined as the quantity of enzyme degrading 1 μmol of peroxide per
min at 37°C and was expressed in units per g weight of wet
tissue.
Statistical analysis
The data (with the exception of the survival curves)
were expressed as the means ± SEM. Analysis of variance was
performed using Graphpad Prism software (GraphPad Software, La
Jolla, CA, USA). One-way analysis of variance was used for multiple
comparisons, followed by Bonferroni’s Student’s t-test for unpaired
values. The survival rate was expressed as the percentage of live
animals, and the Mantel-Cox log rank test was used to determine
differences between survival curves. P<0.05 was considered to
indicate a statistically significant result.
Results
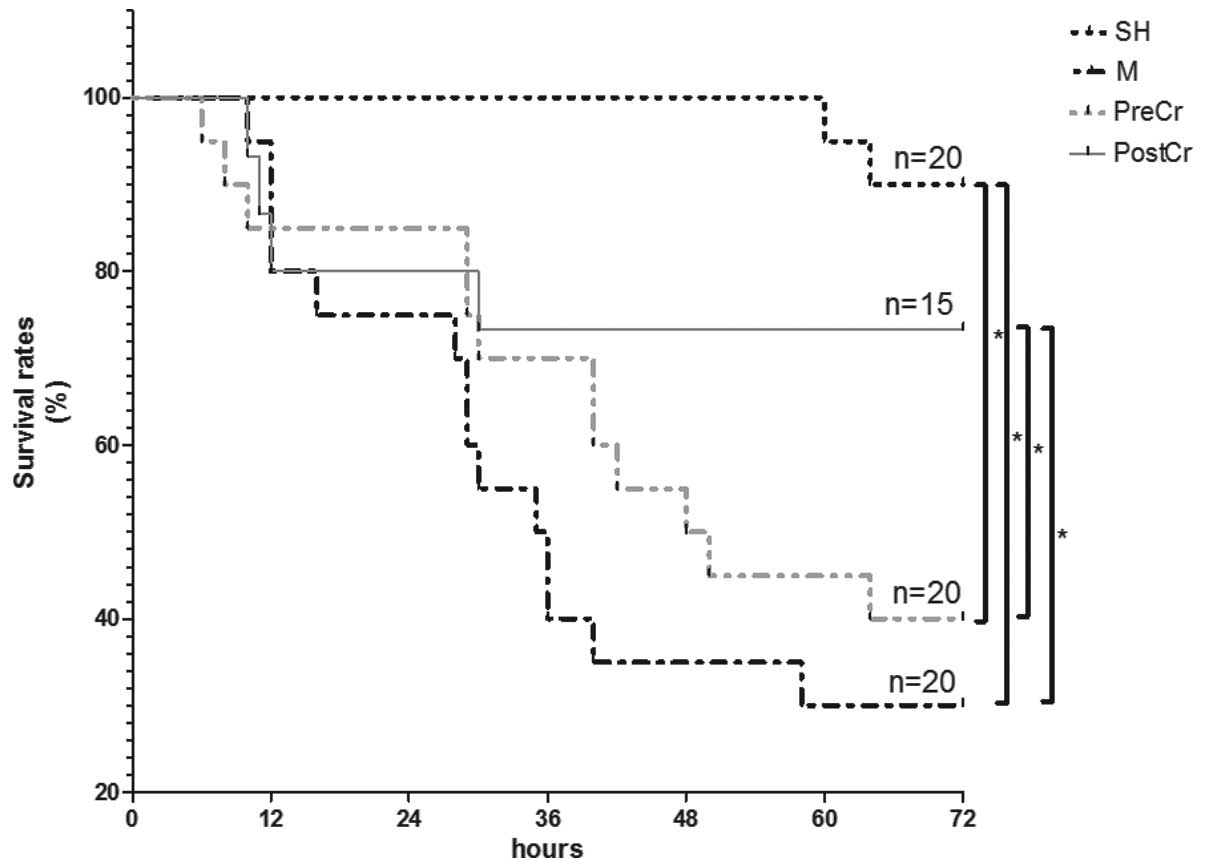
Survival
Our previous study revealed that the majority of
experimental animals no longer died 3 days after reperfusion
(8); therefore, the 3-day survival
rate after reperfusion was assessed in the present study. As shown
in Fig. 1, 30 min mesenteric
artery occlusion induced severe damage in mice, and the survival at
3 days decreased to ~30% in the M group from 90% in the SH group.
Treatment with CS 15 min after reperfusion had a protective role,
as demonstrated by increases in the survival rates to ~74% in the
PostCr group. Survival in the PostCr group was comparable with that
of the SH group. Administration with CS at 15 min prior to ischemia
slightly reduced IIR-mediated mortality. Furthermore, the treatment
of IIR mice with CS after reperfusion showed improved therapeutic
benefits compared with treatment prior to ischemia. The findings
suggest that stabilizing MCs after reperfusion showed promising
therapeutic benefits (Fig. 1).
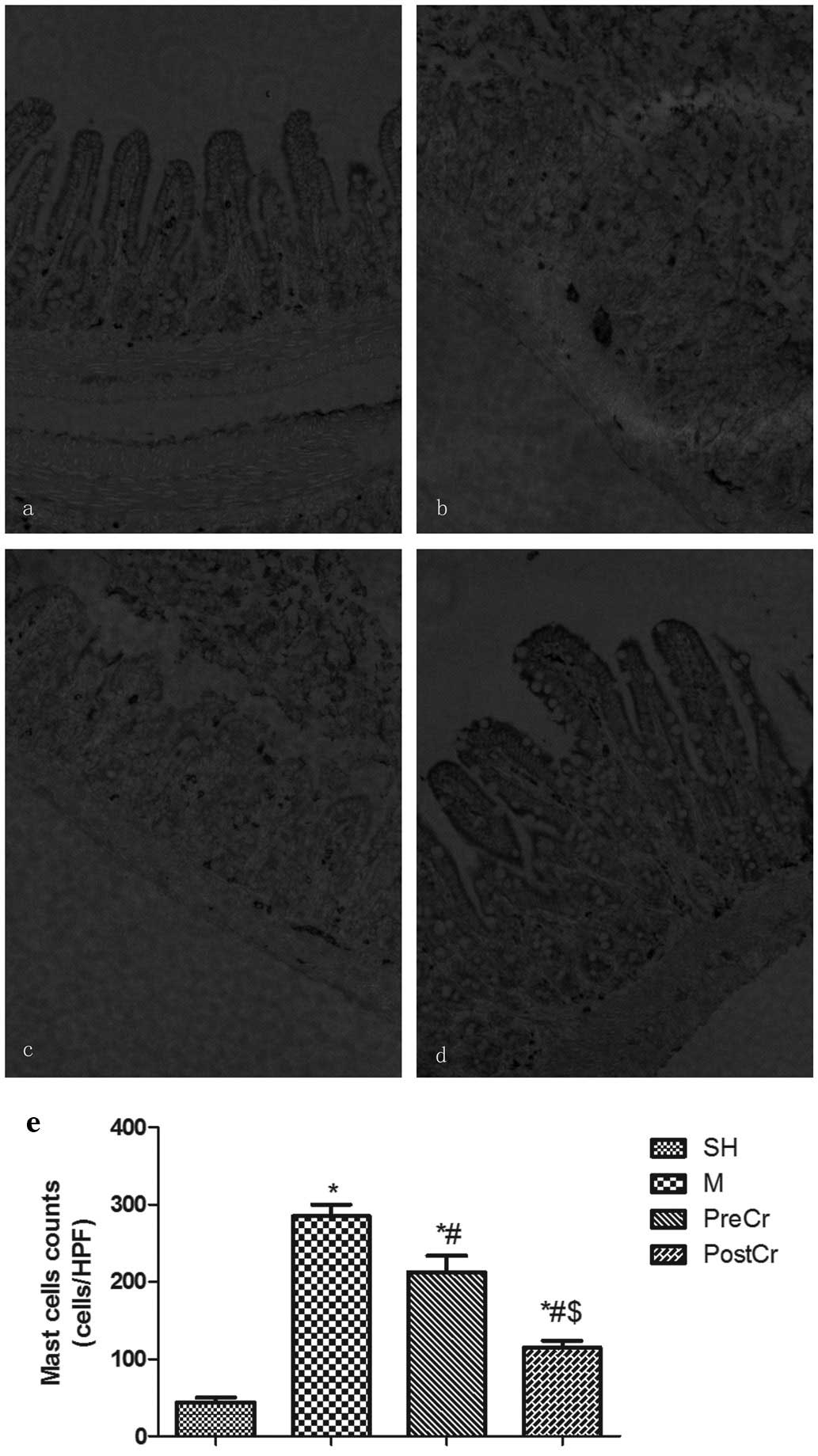
Pathology of small intestinal mucosa
We next evaluated the small intestine injury at 3 h
after releasing the clamp, as this is the optimal time when the
most severe mucosal injuries could be observed without unduly high
mortality. The villus and glands were normal, and no inflammatory
cell infiltration was observed in the mucosal epithelial layer in
the SH group (Fig. 2a). However,
large numbers of inflammatory cells infiltrating the mucosal
epithelial layer, as well as bleeding, were observed in the M
group, in which mice were subjected to 30 min ischemia followed by
3 h reperfusion (Fig. 2b). Slight
edema of mucosa villus and infiltration of a small number of
necrotic epithelial inflammatory cells were observed in the mucosa
epithelial layer in the PostCr group, in which mice received CS at
15 min after reperfusion (Fig.
2d). However, there was more inflammatory cell sequestration
and bleeding in the small intestine mucosa of the PreCr group
(Fig. 2c). In line with the
histological data, the Chiu’s scores in the M group were higher
than in the SH group. As illustrated in Fig. 2e, treatment of IIR mice with CS 15
min after reperfusion, but not before ischemia, markedly reduced
the scores compared with the M group.
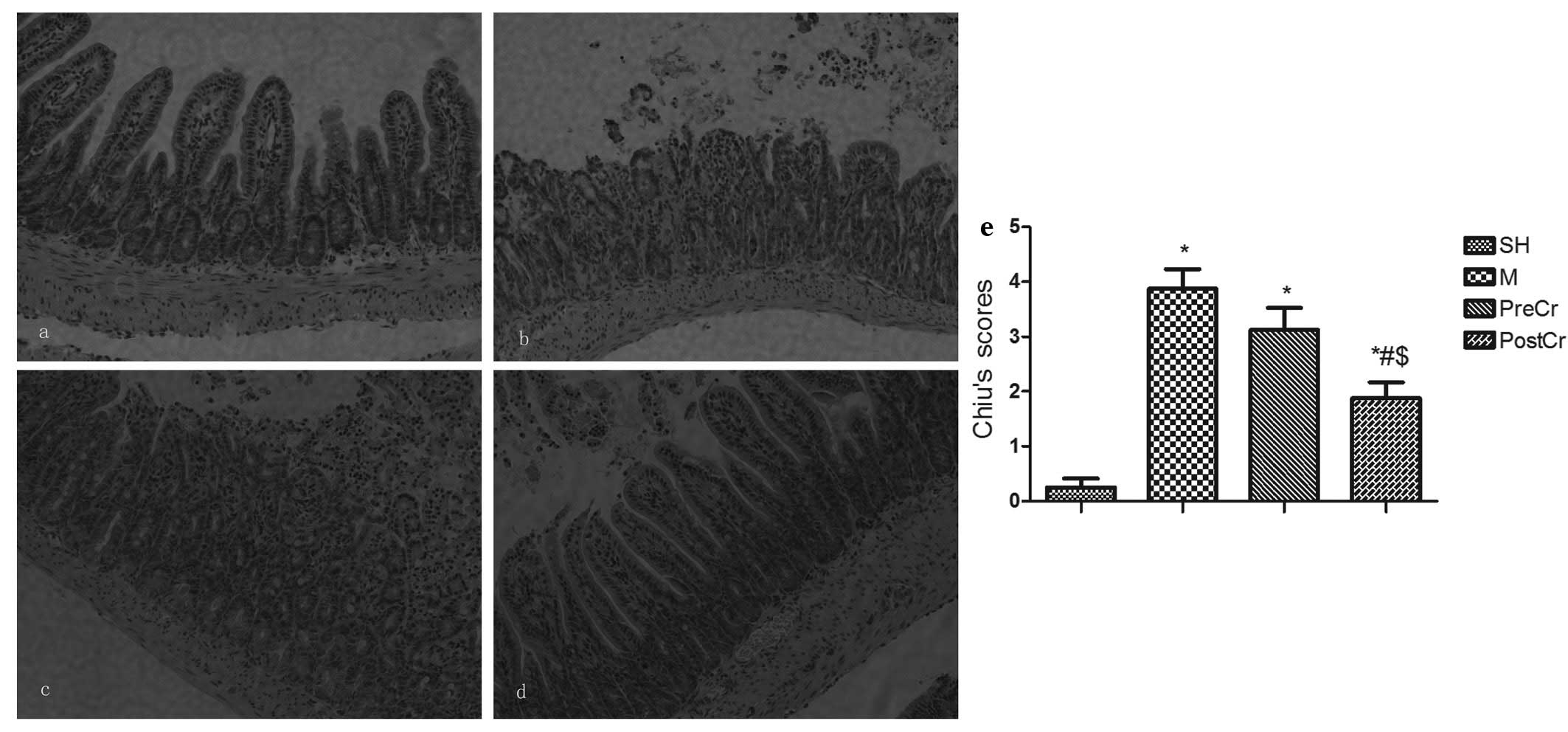 | Figure 2Morphological changes of intestine and
intestinal histological score under a light microscope after IIR
injury. (a–d) Representative images of the SH group (control,
sham-operated group), the M group (30 min intestinal ischemia and
3-h reperfusion), the PreCr group (25 mg/kg CS-treated 15 min prior
to ischemia) and the PostCr group (25 mg/kg CS-treated 15 min after
reperfusion). HE staining, ×200. (e) Quantification of intestine
histological scores. Results are expressed as the means ± SEM; n=7
per group. *P<0.05, compared with the SH group;
#P<0.05, compared with the IIR group;
$P<0.05, compared with the PreCr group. CS, cromolyn
sodium; IIR, intestinal ischemia/reperfusion; HE, hematoxylin and
eosin. |
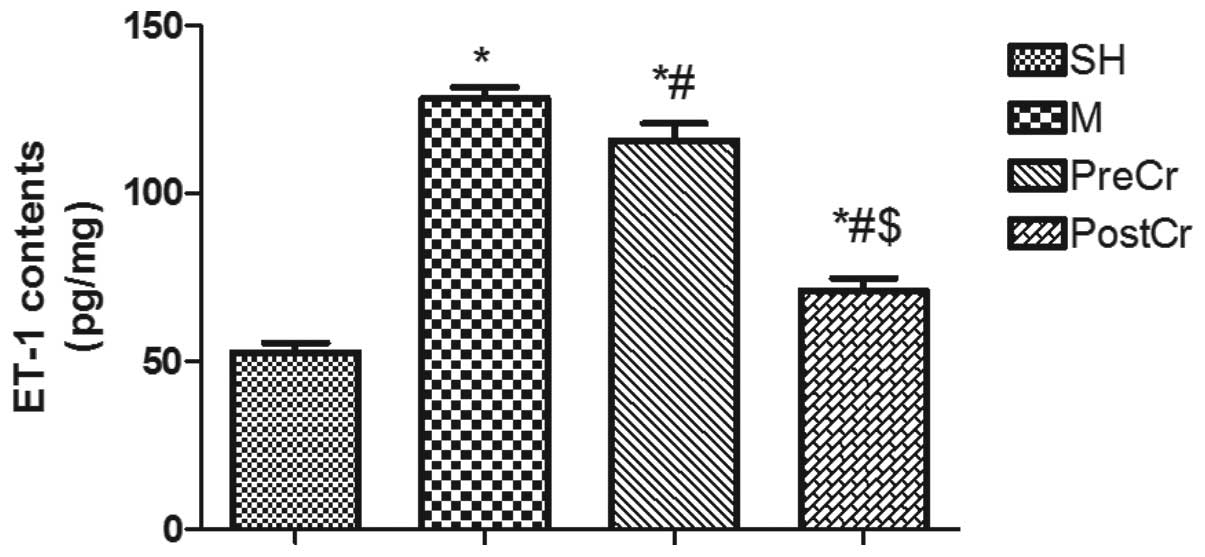
ET-1 contents in the small intestine
Elevated ET-1 contributes to the deleterious injury
in the small intestine challenged by SMA occlusion, and MC
activation can detoxify ET-1 for host defense against bacterial
invasion. Therefore, we hypothesized that treatment of IIR mice at
various time points may result in different alterations of ET-1,
and as expected, we found that ET-1 in the small intestine was
greatly upregulated in mice subjected to 30 min ischemia followed
by 3 h reperfusion compared with the SH group, as shown in Fig. 3. Treatment of IIR mice with CS
after reperfusion, but not prior to ischemia, significantly reduced
the upregulation of ET-1. These notable results indicate that
stabilizing MCs prior to ischemia shows no protection against IIRI,
possibly by inhibiting the degradation of ET-1 by mast cell
peptides.
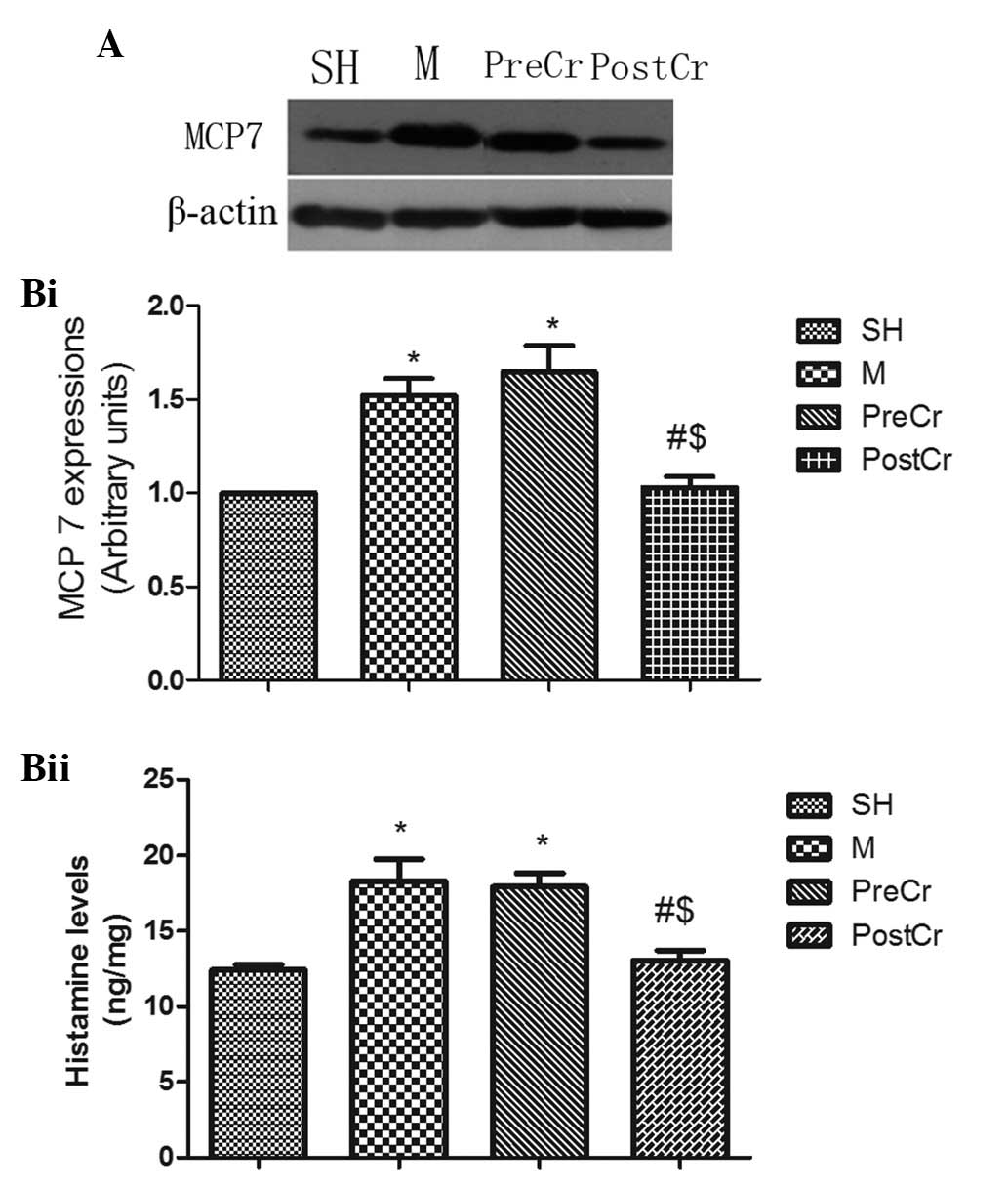
MCP7 protein expression and histamine
levels in the small intestine
MCs can release three types of proteases after
degranulation, including chymases, carboxypeptidases and tryptase.
Previous studies suggested that MCs releasing MCP7, one subtype of
tryptase, and histamine play a pivotal role in the exacerbation of
inflammatory responses (18,19).
In the current study, we demonstrated that IIR led to significant
elevations in MCP7 protein expression and histamine levels in the M
group compared with the SH group. Of note, treatment of IIR mice
with CS after reperfusion, but not prior to ischemia, markedly
attenuated MCP7 protein expression and histamine levels in the
intestine. The findings from the present study suggest that
treatment of IIR mice with CS after reperfusion confers protection,
possibly through suppressing sustained MC activation (Fig. 4A and B).
MPO activity in the small intestine
IIRI is also characterized by neutrophil
infiltration into ischemic areas, and ET-1 is an important mediator
for neutrophil recruitment (20).
In line with the alterations of ET-1 contents, the MPO activities
in the small intestine of mice subjected to IIR in the M group were
significantly higher than in the SH group. Treatment of IIR mice
with CS 15 min after reperfusion, but not prior to ischemia,
significantly reduced the tissue MPO activities as compared with
the M group (Fig. 5).
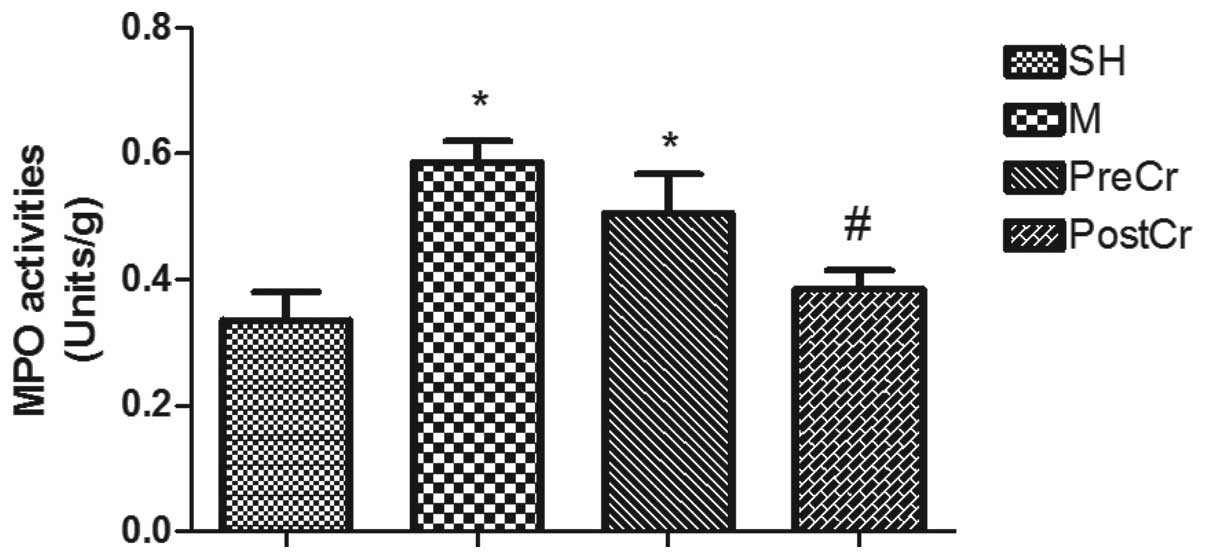 | Figure 5MPO activities in the intestine after
IIR injury. SH group (control, sham-operated group), M group (30
min intestinal ischemia and 3-h reperfusion), PreCr group (25 mg/kg
CS-treated 15 min prior to ischemia), PostCr group (25 mg/kg
CS-treated 15 min after reperfusion). Bar graphs quantified MPO
activities. Results are expressed as the means ± SEM; n=7 per
group. *P<0.05, compared with SH group;
#P<0.05, compared with IIR group;
$P<0.05, compared with PreCr group. MPO,
myeloperoxidase; CS, cromolyn sodium; IIR, intestinal
ischemia/reperfusion. |
IMMC counts in the small intestine
Chemokines released from neutrophils play a central
role in mast cell accumulation. In agreement with the results of
MPO activities, IMMC counts in the small intestine of the mice
subjected to IIR injury were markedly increased compared with the
SH group; however, treatment of IIR mice with CS after reperfusion,
but not prior to ischemia, revealed inhibition of IIR-induced mast
cell accumulation (Fig. 6).
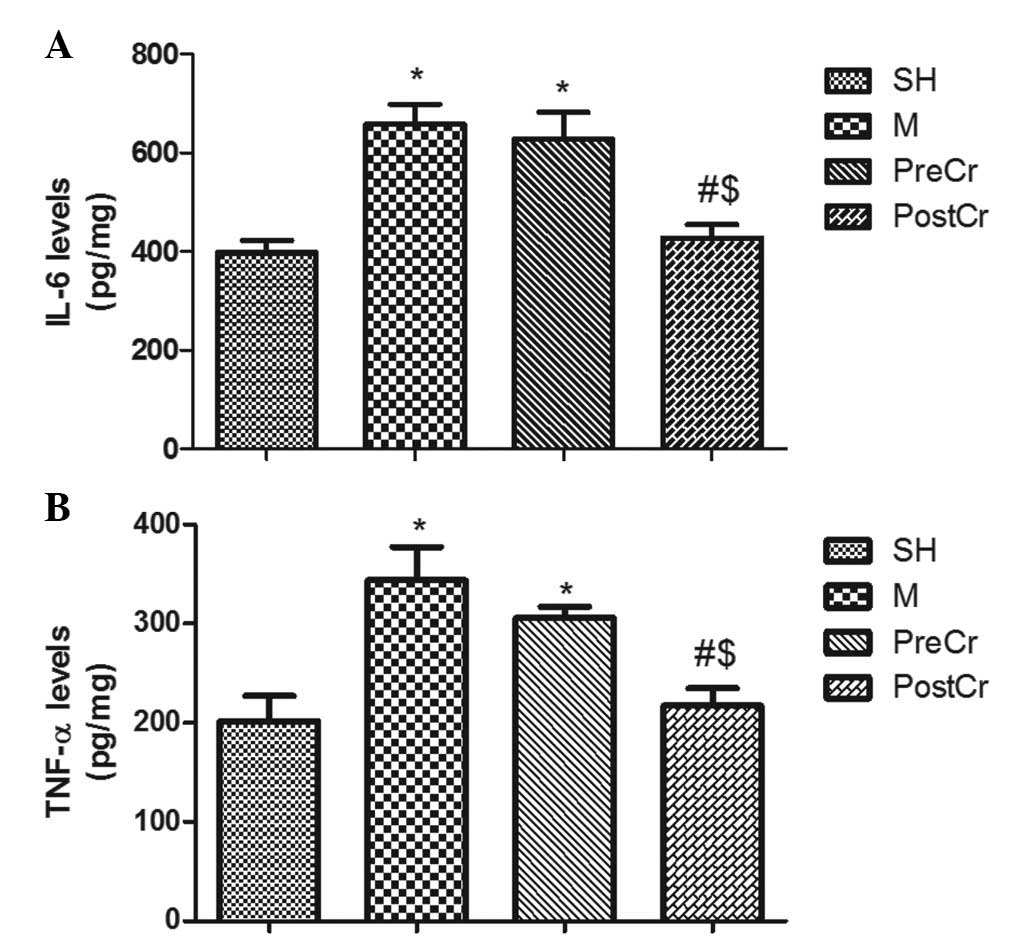
IL-6 and TNF-α levels in the small
intestine
As shown in Fig. 7A and
B, IIR led to significant increases in the levels of IL-6 and
TNF-α in the M group compared with the SH group. Treatment with CS
15 min after reperfusion significantly reduced tissue levels of
TNF-α and IL-6 in the PostCr group as compared with the M group;
however, treatment with CS at 15 min prior to ischemia did not show
any reduction of IL-6 and TNF-α levels in the PreCr group compared
with the M group.
Discussion
In the present study, we observed that stabilizing
MCs after reperfusion, but not prior to ischemia, showed promising
therapeutic benefits against IIRI, as evidenced by significant
elevations in survival, as well as marked reductions in
inflammation, the underlying mechanism by which stabilizing MCs
after reperfusion, but not prior to ischemia, contribute to
attenuate IIRI in part through appropriate MC activation to degrade
ET-1.
IMMCs are widely present throughout the
gastrointestinal tract, and play a significant role in host defense
against pathogens (21),
particularly bacterial infections, and in tissue repair, by
releasing a large number of pro-inflammatory mediators, proteases
and cytokines (22). Furthermore,
MC activation can detoxify elevated neurotensin, which contributes
to high mortality during septic shock, and stabilizing MCs can
increase sepsis-mediated mortality (13). Proteases, largely stored in MC
granules and specifically released by MCs, include chymase,
tryptase and carboxypeptidase A (MC-CPA) (23). MC-CPA released by MC activation can
greatly alleviate sepsis by antagonizing ET-1 (24). ET-1 is important in small
intestinal ischemia/reperfusion-induced injury, and endothelin
receptor antagonist significantly alleviates IIRI (12). In the present study, it was shown
that treatment of IIR mice with MC stabilizer prior to ischemia
caused unchanged high levels of ET-1 in the intestine as well as
high mortality. The data indicated that ET-1 plays a central role
in the pathogenesis of IIRI (25)
and stabilizing MCs prior to ischemia demonstrated no protective
role against IIRI. Our results are different from Kalia et
al’s findings, which showed that pretreatment of rats with
ketotifen for three days prior to ischemia significantly limited
IIRI (26). We speculated that
different treatments and animal models may contribute to the
discrepancy, and it should be noted that ketotifen mainly functions
as a histamine antagonist. These data indicate that appropriate MC
activation may suppress inflammation through the degradation of
ET-1.
By contrast, continued activation of MCs can
exacerbate IIRI by releasing tryptase and MCP7 (7), and sustained activation of MCs has
been shown to be highly associated with inflammatory bowel disease
(27). Abonia et al
discovered that mouse mast cell protease 5 is responsible for
irreversible damage in skeletal muscle ischemia-reperfusion injury
in mice (28). Histamine, another
pivotal mediator mostly released from MCs, induces vasodilation and
elevation in capillary permeability, and augments the injury
induced by ischemia/reperfusion (29). In the current study, we also showed
that treatment of IIR mice with CS after reperfusion, but not prior
to ischemia, significantly reduced the elevations in MCP7 protein
expression and histamine level induced by IIR. We hypothesized that
the administration of a single dose of CS prior to ischemia could
not stabilize the sustained activation of IMMCs after reperfusion,
as the elimination half-life of CS is ~1 h (15). The paradoxical results further
confirmed a dual role of MC stabilization in inflammation.
In addition to the key roles in vasoconstriction and
delayed transit of the small intestine induced by IIRI (12), the increased ET-1 production in
intestinal mucosa triggered by endotoxin stimulation results in
leukocytes infiltrating into the ischemic area. Zarpelon et
al recently reported that ET-1 induced neutrophil recruitment
in adaptive inflammation through a TNF-α and CXCL1/CXCR2-dependent
mechanism (20). We found in the
present study that pretreatment of IIR mice with CS did not lead to
downregulation of ET-1, and the high level of neutrophil
recruitment caused by IIR remained, as demonstrated by significant
elevations in intestinal MPO activities. By contrast, downregulated
ET-1 in the PostCr group led to less neutrophil recruitment when CS
was administered after reperfusion. It should also be noted that
tryptase released by MC activation can also induce neutrophil
infiltration into ischemic areas (30). The findings indicated that MC
inhibition at early reperfusion may be a promising therapeutic
method to attenuate IIRI.
TNF-α and IL-6 are the main pro-inflammatory
cytokines that induce the waterfall-like inflammation response
(31) and have been implicated in
the pathogenesis of IIRI, as using antibodies to inhibit TNF-α and
IL-6 can block IIRI (32,33). The production of TNF-α and IL-6 in
the intestine mainly originate from IMMC degranulation (34). Our study clearly showed that TNF-α
and IL-6 levels were markedly increased after IIR associated with
MC activation, and stabilizing MCs with CS after reperfusion
significantly reduced the elevation of TNF-α and IL-6 levels in the
intestine. However, pretreatment of IIR mice with CS prior to
ischemia showed slight reductions of TNF-α and IL-6 levels. These
findings suggest that the sustained MC activation during the period
of reperfusion can aggravate IIRI.
In conclusion, treatment of mice with CS at early
reperfusion, but not prior to ischemia, displays promising
therapeutic benefits against IIRI. Appropriate MC activation can
suppress inflammation by degrading ET-1; however, sustained MC
activation may exacerbate inflammation by releasing tryptase,
histamine and pro-inflammatory cytokines.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30901408).
References
|
1
|
Acosta S and Nilsson T: Current status on
plasma biomarkers for acute mesenteric ischemia. J Thromb
Thrombolysis. 33:355–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Debus ES, Muller-Hulsbeck S, Kolbel T and
Larena-Avellaneda A: Intestinal ischemia. Int J Colorectal Dis.
26:1087–1097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta PK, Natarajan B, Gupta H, Fang X and
Fitzgibbons RJ Jr: Morbidity and mortality after bowel resection
for acute mesenteric ischemia. Surgery. 150:779–787. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiemann F, Brandt E, Gross R, et al: The
cathelicidin LL-37 activates human mast cells and is degraded by
mast cell tryptase: counter-regulation by CXCL4. J Immunol.
183:2223–2231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fifadara NH, Aye CC, Raghuwanshi SK,
Richardson RM and Ono SJ: CCR1 expression and signal transduction
by murine BMMC results in secretion of TNF-alpha, TGFbeta-1 and
IL-6. Int Immunol. 21:991–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei JF, Wei XL, Mo YZ and He SH: Induction
of mast cell accumulation, histamine release and skin edema by N49
phospholipase A2. BMC Immunol. 10:212009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gan X, Liu D, Huang P, Gao W, Chen X and
Hei Z: Mast-cell-releasing tryptase triggers acute lung injury
induced by small intestinal ischemia-reperfusion by activating
PAR-2 in rats. Inflammation. 35:1144–1153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hei ZQ, Gan XL, Huang PJ, Wei J, Shen N
and Gao WL: Influence of ketotifen, cromolyn sodium, and compound
48/80 on the survival rates after intestinal ischemia reperfusion
injury in rats. BMC Gastroenterol. 8:422008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bischoff SC: Physiological and
pathophysiological functions of intestinal mast cells. Semin
Immunopathol. 31:185–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groschwitz KR, Ahrens R, Osterfeld H, et
al: Mast cells regulate homeostatic intestinal epithelial migration
and barrier function by a chymase/Mcpt4-dependent mechanism. Proc
Natl Acad Sci USA. 106:22381–22386. 2009. View Article : Google Scholar
|
|
11
|
Wang JY, Cheng KI, Yu FJ, Tsai HL, Huang
TJ and Hsieh JS: Analysis of the correlation of plasma NO and ET-1
levels in rats with acute mesenteric ischemia. J Invest Surg.
19:155–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sunose Y, Ohwada S, Takeyoshi I, et al:
Effects of endothelin receptor antagonist TAK-044 on small bowel
autograft from a controlled non-heart-beating donor model. Surgery.
130:819–825. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caughey GH: Mast cell proteases as
protective and inflammatory mediators. Adv Exp Med Biol.
716:212–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boros M, Kaszaki J, Ordogh B and Nagy S:
Mast cell degranulation prior to ischemia decreases
ischemia-reperfusion injury in the canine small intestine. Inflamm
Res. 48:193–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vural KM, Liao H, Oz MC and Pinsky DJ:
Effects of mast cell membrane stabilizing agents in a rat lung
ischemia-reperfusion model. Ann Thorac Surg. 69:228–232. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu CJ, Scott HJ and Gurd FN: Intestinal
mucosal lesion in low-flow states. II The protective effect of
intraluminal glucose as energy substrate. Arch Surg. 101:484–488.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krawisz JE, Sharon P and Stenson WF:
Quantitative assay for acute intestinal inflammation based on
myeloperoxidase activity. Assessment of inflammation in rat and
hamster models. Gastroenterology. 87:1344–1350. 1984.PubMed/NCBI
|
|
18
|
McNeil HP, Shin K, Campbell IK, et al: The
mouse mast cell-restricted tetramer-forming tryptases mouse mast
cell protease 6 and mouse mast cell protease 7 are critical
mediators in inflammatory arthritis. Arthritis Rheum. 58:2338–2346.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Funaba M, Ikeda T, Murakami M, et al:
Transcriptional activation of mouse mast cell Protease-7 by activin
and transforming growth factor-beta is inhibited by
microphthalmia-associated transcription factor. J Biol Chem.
278:52032–52041. 2003. View Article : Google Scholar
|
|
20
|
Zarpelon AC, Pinto LG, Cunha TM, et al:
Endothelin-1 induces neutrophil recruitment in adaptive
inflammation via TNFalpha and CXCL1/CXCR2 in mice. Can J Physiol
Pharmacol. 90:187–199. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
McNeil HP, Adachi R and Stevens RL: Mast
cell-restricted tryptases: structure and function in inflammation
and pathogen defense. J Biol Chem. 282:20785–20789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malaviya R, Ikeda T, Ross E and Abraham
SN: Mast cell modulation of neutrophil influx and bacterial
clearance at sites of infection through TNF-alpha. Nature.
381:77–80. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pejler G, Abrink M, Ringvall M and
Wernersson S: Mast cell proteases. Adv Immunol. 95:167–255. 2007.
View Article : Google Scholar
|
|
24
|
Pejler G, Knight SD, Henningsson F and
Wernersson S: Novel insights into the biological function of mast
cell carboxypeptidase A. Trends Immunol. 30:401–408. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ozel SK, Yuksel M, Haklar G, Durakbasa CU,
Dagli TE and Aktan AO: Nitric oxide and endothelin relationship in
intestinal ischemia/reperfusion injury (II). Prostaglandins Leukot
Essent Fatty Acids. 64:253–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalia N, Brown NJ, Wood RF and Pockley AG:
Ketotifen abrogates local and systemic consequences of rat
intestinal ischemia-reperfusion injury. J Gastroenterol Hepatol.
20:1032–1038. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Philpott H, Gibson P and Thien F:
Irritable bowel syndrome - An inflammatory disease involving mast
cells. Asia Pac Allergy. 1:36–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abonia JP, Friend DS, Austen WG Jr, et al:
Mast cell protease 5 mediates ischemia-reperfusion injury of mouse
skeletal muscle. J Immunol. 174:7285–7291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akerstrom G and Lisander B:
Antihistaminergic pretreatment prevents tissue extravasation of
albumin from intra-abdominal trauma in rats. Acta Anaesthesiol
Scand. 38:569–574. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dharancy S, Body-Malapel M, Louvet A, et
al: Neutrophil migration during liver injury is under
nucleotide-binding oligomerization domain 1 control.
Gastroenterology. 138:1546–1556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spanos CP, Papaconstantinou P, Spanos P,
Karamouzis M, Lekkas G and Papaconstantinou C: The effect of
L-arginine and aprotinin on intestinal ischemia-reperfusion injury.
J Gastrointest Surg. 11:247–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An S, Hishikawa Y, Liu J and Koji T: Lung
injury after ischemia-reperfusion of small intestine in rats
involves apoptosis of type II alveolar epithelial cells mediated by
TNF-alpha and activation of Bid pathway. Apoptosis. 12:1989–2001.
2007. View Article : Google Scholar
|
|
33
|
Cuzzocrea S, De Sarro G, Costantino G, et
al: IL-6 knock-out mice exhibit resistance to splanchnic artery
occlusion shock. J Leukoc Biol. 66:471–480. 1999.PubMed/NCBI
|
|
34
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|