Introduction
Growth hormone (GH) and insulin-like growth factor
(IGF)-1 are important regulators of bone homeostasis and are
central to normal longitudinal bone growth and bone mass (1). GH signaling via its receptor is
mediated by cascades of protein phosphorylation resulting in
activation of nuclear proteins and transcription factors. The
growth hormone receptor (GHR) itself is not a tyrosine kinase
(2). Instead, when GH binds to the
GHR, it induces receptor homodimerization and activation of the
GHR-associated tyrosine kinase, Janus kinase 2 (Jak2) (3,4).
Jak2 is then phosphorylated and in turn, phosphorylates the GHR and
signal transducers and activators of transcription (STAT) proteins.
Upon phosphorylation, STAT proteins, homodimerize or
heterodimerize, translocate to the nucleus, bind to their
appropriate DNA response element and stimulate transcription of
GH-regulated genes, including IGF-1 (5). GH exerts its effects directly and via
IGF-1, which signals by activating the IGF-1 receptor (1R). The
IGF-1R is a cell-surface receptor that contains intrinsic tyrosine
kinase activity within its intracellular domain (6).
Bone is a living organ that undergoes remodeling
throughout life (7). Bone
remodeling involves the removal of mineralized bone by osteoclasts
followed by the formation of the bone matrix through the
osteoblasts that subsequently become mineralized (8). The major regulators of bone
remodeling include GH, parathyroid hormone (PTH), IGF-1,
transforming growth factor-β (TGF-β) and bone morphogenetic protein
(BMP) (7).
BMPs constitute the largest subgroup of the TGF-β
superfamily of cytokines (9,10).
BMP molecules appear to induce bone formation in a stepwise manner,
with individual BMP molecules functioning at various stages of
osteoblastic differentiation and osteogenesis (11,12).
Specifically, BMP7 is known to be an osteogenesis-stimulating
factor and has been widely reported to induce osteogenic
differentiation of human mesenchymal stem cells (hMSCs) (13,14).
Since BMP7 controls the development and maintenance of multiple
physiological processes in the human body, it is not surprising
that aberrant expression of BMP7 has been found to be associated
with a variety of diseases (15,16).
Sorghum is rich in phytochemical components,
including tannins, phenolic acids, anthocyanins, flavanoids and
policosanols, with a potential to benefit human health (17). Recent studies have demonstrated
that sorghum has an anti-esophageal cancer and cholesterol-lowering
effect, decreasing the risk of cardiovascular disease and in
particular, revealing a high antioxidant activity (18,19).
However, the effects of Hwanggeumchal sorghum extracts (HSE)
have not yet been reported in bone cells. Natural substances have
been investigated as candidate materials to be used in bone-related
diseases. These natural extracts have been used to develop new
drugs through a combination of effective single compounds or in
combination with existing commercial drugs, including estrogen or
GH products used to prevent bone loss (20,21).
Notably, the role of Jak/STAT signaling in
osteoblasts appears to be relatively obscure. Recently, we reported
STAT5B as a mediator of MSC proliferation in response to
methylsulfonylmethane (MSM) stimulation (22). In the present study, the role of
HSE-enhanced BMP7 and GH-signaling in Jak2/STAT5B signaling was
investigated in MC3T3-E1 cells.
Materials and methods
Antibodies and reagents
Dulbecco’s modified eagle’s medium (DMEM), MEM,
fetal bovine serum and trypsin-EDTA were purchased from Gibco-BRL
(Carlsbad, CA, USA). IGF-1R, phospho-IGF-1R, STAT5B, Jak2 and GHR
primary antibodies, and the secondary antibodies (HRP-conjugated
goat anti-mouse IgG and HRP-conjugated donkey anti-rabbit IgG) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). BMP7 antibody was purchased from Abcam (Cambridge, UK). An
antibody against phospho-STAT5B was obtained from Upstate
Biotechnology (Lake Placid, NY, USA). The anti-actin antibody and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma-Aldrich (St. Louis, MO, USA). The RNeasy
mini kit was purchased from Qiagen (Hilden, Germany). The reverse
transcription-polymerase chain reaction (RT-PCR) Premix kit and
BMP7, GHR and 18S primers for RT-PCR were purchased from Bioneer
Corporation (Daejeon, Korea). The ON-TARGETplus SMARTpool small
interference RNA (siRNA) targeting STAT5B and ON-TARGETplus
non-targeting siRNA were purchased from Dharmacon (Pittsburgh, PA,
USA). The enhanced chemiluminescence (ECL) plus detection kit was
purchased from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
The coomassie protein assay kit and Restore western blot stripping
buffer were purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA).
Extraction and characterization of
sorghum
For this study, HS was selected and extracted
according to a previously described method (23) with small modifications. The sample
(50 g) was stored at −36°C and ground well prior to use. Ground
powder was then extracted with 30% 0.1 N HCl in acetonitrile. The
solution was filtered using Whatman filter paper (Cole-Parmer,
Vernon Hills, IL, USA), concentrated and again extracted with 100%
methanol and dried overnight in a freeze dryer. The sorghum extract
was characterized for its phenolic content using high performance
liquid chromatography (data not shown).
MTT assay
Cell viability was assayed by measuring blue
formazan that was metabolized from MTT by mitochondrial
dehydrogenase, which is active only in live cells. One day prior to
drug application, MC3T3-E1 cells were seeded in 96-well
flat-bottomed microtiter plates (3,000–5,000 cells/well). MC3T3-E1
cells were incubated for 24 h with various concentrations of HSE.
MTT (20 μl; 5 mg/ml) was added to each well and incubated for 4 h
at 37°C. The formazan product was dissolved by adding 200 μl DMSO
to each well and the plates were read at 550 nm. All measurements
were performed in triplicate and each experiment was repeated at
least three times.
Western blot analysis
MC3T3-E1 cells were treated with the indicated HSE
concentrations (0, 10, 20 and 40 μg/ml) for 24 h. MC3T3-E1 cells
were untreated or pretreated with 50 μM AG490 for 4 h then treated
with 30 μg/ml HSE for 24 h. Cells were lysed in whole lysis buffer
[50 mM Tris-HCl, (pH 7.5), 5 mM EDTA, 150 mM NaCl and 1% Triton
X-100] containing protease and phosphatase inhibitors (1 mM PMSF, 2
μg/ml leupeptin, 4 μg/ml aprotinin and 1 μg/ml pepstatin) and
protein concentrations were determined using the coomassie protein
assay kit (Pierce Biotechnology, Rockford, IL, USA). An equivalent
amount of protein extracts from each sample was electrophoresed on
10% SDS-PAGE and transferred onto nitrocellulose membranes.
Membranes were blocked for 1 h with 5% non-fat milk in T-TBS buffer
[20 mM Tris-HCl (pH 7.6), 137 mM NaCl and 0.1X Tween-20] and
incubated overnight at 4°C with primary antibodies (BMP7, STAT5B,
p-STAT5B, IGF-1R, GHR, Jak2 and β-actin). The membranes were washed
three times in T-TBS and incubated with the corresponding secondary
antibody, anti-mouse or anti-rabbit IgG HRP-conjugate (1:1,000), in
T-TBS with 5% non-fat milk for 1 h under agitation at room
temperature. Following washing three times in T-TBS, the membranes
were developed using the ECL plus kit.
RT-PCR
MC3T3-E1 cells were treated with the indicated
concentrations of HSE for 24 h. MC3T3-E1 cells were untreated or
pretreated with 50 μM AG490 for 4 h then treated with 30 μg/ml HSE
for 24 h. Total RNA was prepared using the RNeasy mini kit and cDNA
was synthesized using the AccuPower RT PreMix kit (Bioneer
Corporation) according to the manufacturer’s instructions. PCR was
performed using aliquots of cDNA to detect GHR and BMP7. The PCR
primer sequences were as follows: BMP7 sense,
5′-GGCTTCTCCTACCCCTACAA-3′ and antisense,
5′-GTGGTTGCTGGTGGCTGTGA-3′; GHR sense, 5′-TTCTAAACAGCAAAGGATTAA-3′
and antisense, 5′-CACTGTGAAATTCGGGTTTA-3′; 18S primers: sense,
5′-CGGCTACCACATCCAAGGAA-3′ and antisense:
5′-CCGGCGTCCCCTCTTAATC-3′. The PCR was conducted under the
following conditions: 30 cycles at 94°C for 45 sec, 60°C for 45 sec
and 72°C for 1 min. Following amplification, the PCR products were
analyzed on a 1.2% agarose gel and visualized by ethidium bromide
staining and ultraviolet irradiation.
siRNA assay
MC3T3-E1 cells were grown to 50% confluence and
transfected with ON-TARGETplus SMARTpool siRNA targeting STAT5B or
ON-TARGETplus non-targeting siRNA using FuGene 6 (Roche, Basel,
Switzerland), according to the manufacturer’s instructions.
Following transfection (48 h), cells were cultured in serum-free
medium for 24 h and then exposed to 30 μg/ml HSE for 24 h. STAT5B,
p-STAT5B, BMP7 and IGF-1R expression levels were detected using
western blot analysis.
Statistical analysis
Data are presented as the mean ± SEM. Statistical
analysis was performed using the student’s t-test or one-way
analysis of variance (ANOVA) test of the SAS program. These were
compared by ANOVA followed by Duncan’s multiple range test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HSE cytotoxicity of osteoblast-like
cells
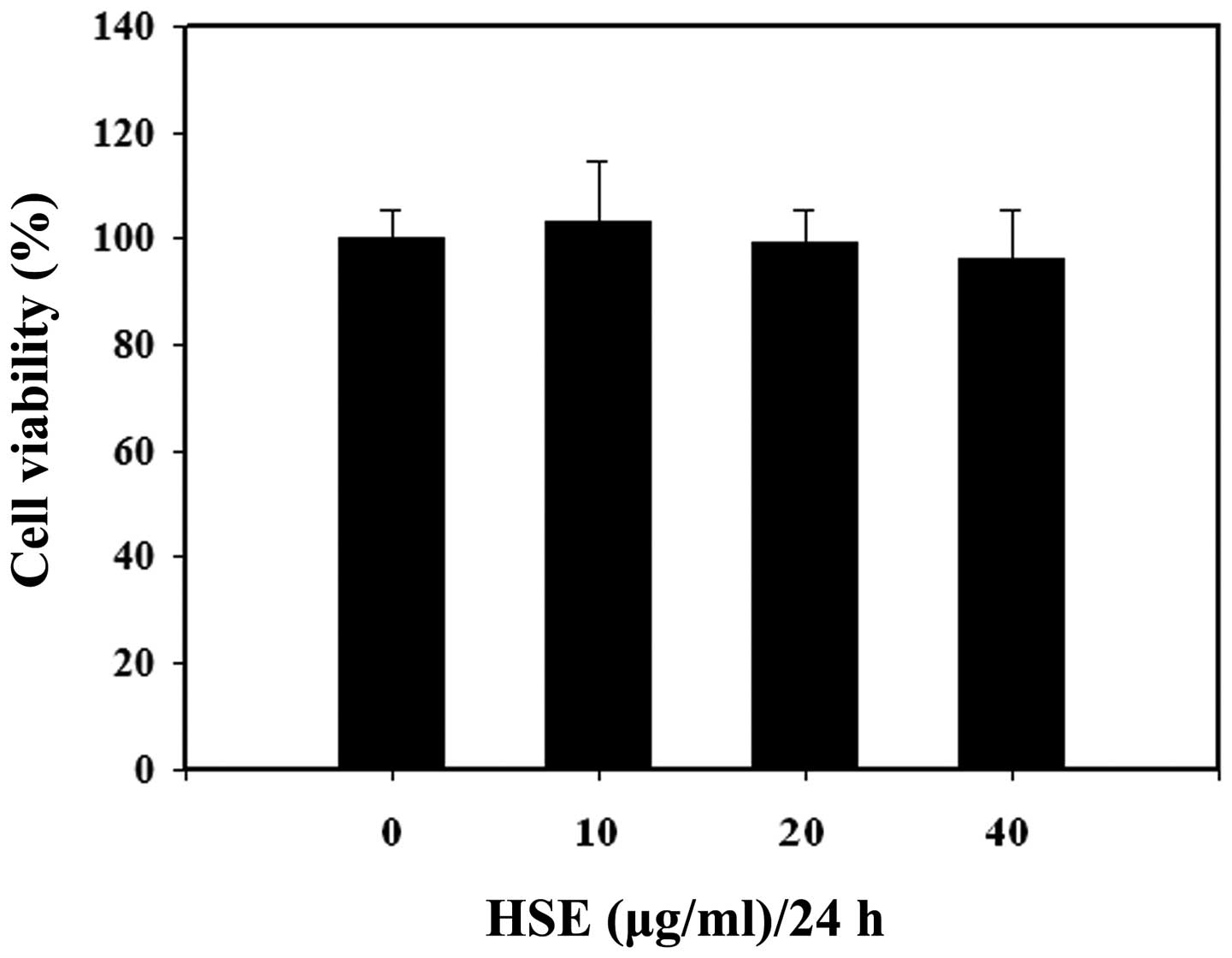
To determine the suitability of HSE as an osteoblast
growth supporting agent, cytotoxicity of HSE in osteoblast-like
cells was examined. MC3T3-E1 cells were treated with increasing
concentrations of HSE (0, 10, 20 and 40 μg/ml) for 24 h and the
impact of HSE in MC3T3-E1 cell proliferation was assayed using the
MTT assay. No notable cytotoxicity was observed when the cells were
exposed to up to 40 μg/ml for 24 h (Fig. 1). Thus, HSE was used at a
concentration of 30 μg/ml for subsequent experiments.
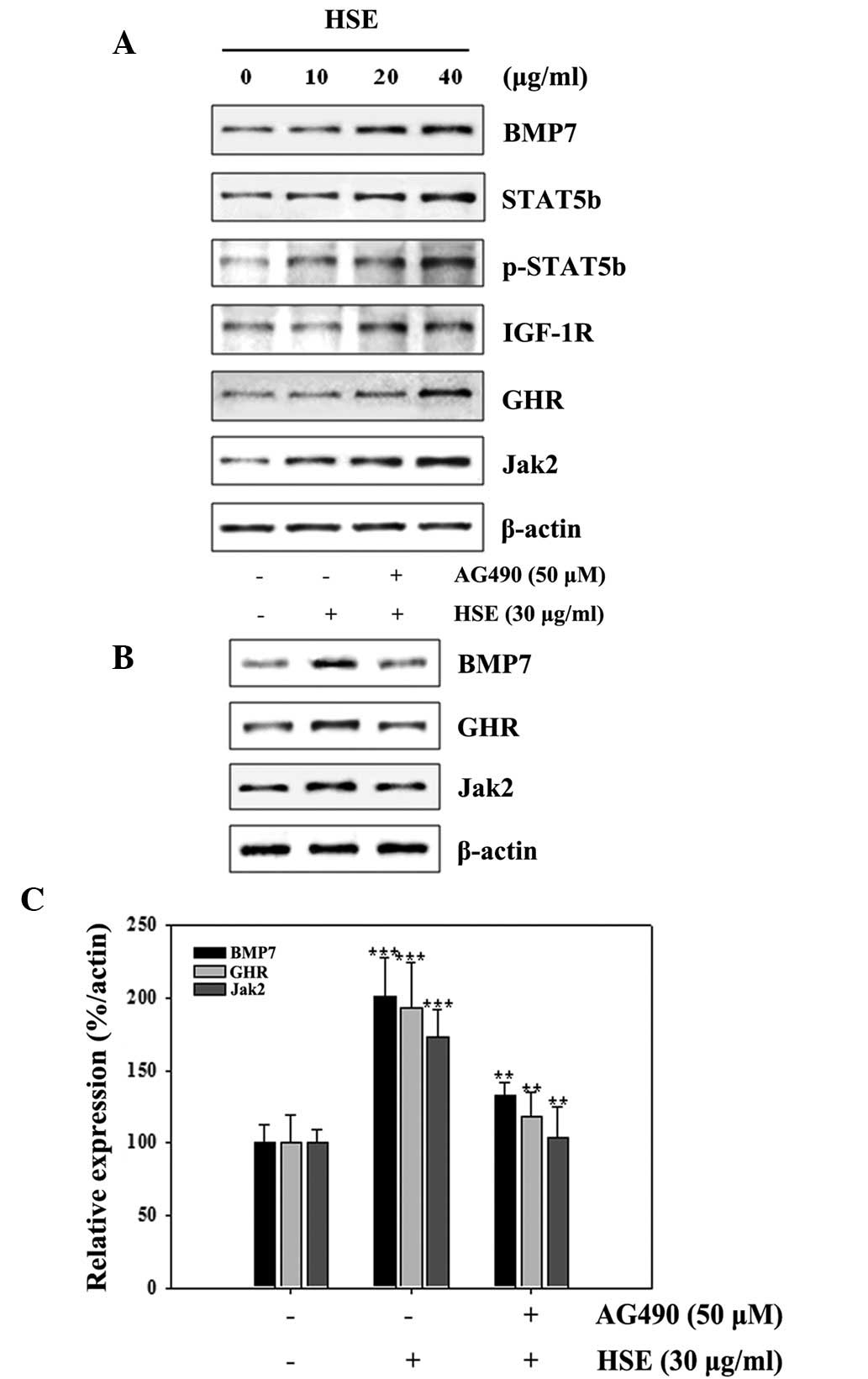
HSE increases GH signaling-related
protein expression in osteoblast-like cells
The expression levels of various proteins involved
in GH signaling were assessed by western blot analysis. GH
initiates signals by binding to the GHR to activate tyrosine
kinase, Jak2 and downstream pathways, including STAT5B, thereby
regulating the expression of genes, including IGF-1. GH exerts
effects directly and via IGF-1, which signals by activating the
IGF-1R. We hypothesized that HSE increases the expression of BMP7,
STAT5B, p-STAT5B, IGF-1R, GHR and Jak2 in osteobast-like cells. As
demonstrated in Fig. 2A, HSE
treatment increased expression of BMP7, IGF-1R, STAT5B, Jak2 and
p-STAT5B in MC3T3-E1 in a dose-dependent manner. These observations
indicate that HSE functions via the Jak2/STAT5B signaling pathway
in MSCs. Next, Jak2 was inhibited using AG490, which led to a
blockade of HSE-induced BMP7 and GHR protein expression.
HSE-induced BMP7 and GHR protein expression was inhibited by AG490
(Fig. 2B). The relative expression
density of protein with respect to actin provided a clear view on
the effect of HSE on MC3T3-E1 cells at AG490 (Fig. 2C). These results indicate that
HSE-induced signaling is similar to GH signaling via the Jak2/STAT5
pathway.
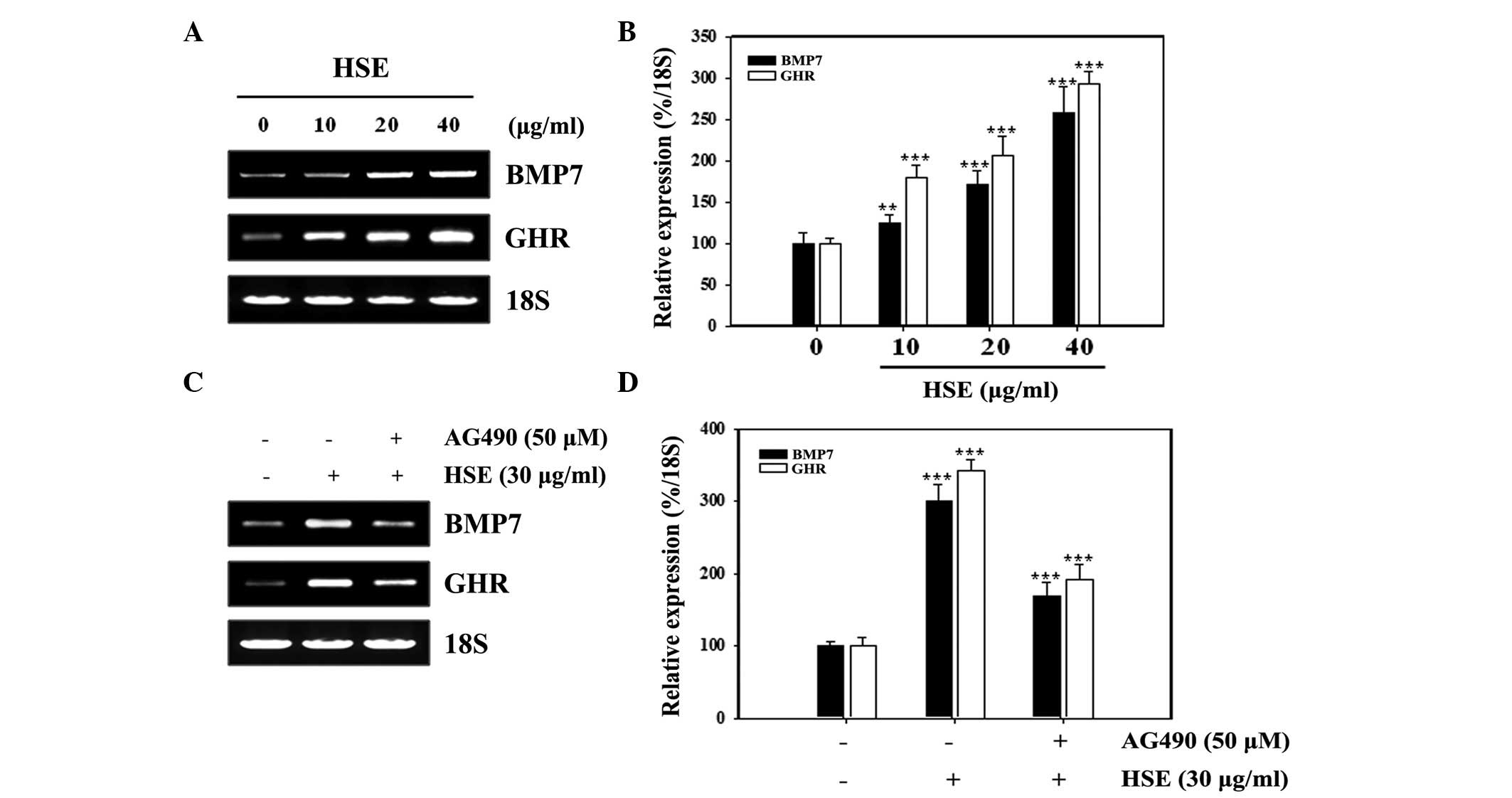
HSE-induced BMP7 and GHR expression
involves Jak2
Next, the role of Jak2 in the enhanced expression of
BMP7 and GHR upon HSE treatment was studied using the commercial
Jak2 inhibitor, AG490. As demonstrated in Fig. 3A, HSE upregulated BMP7 and GHR mRNA
expression in a dose-dependent manner. The relative expression
density of mRNA with respect to 18S revealed the effect of HSE on
MC3T3-E1 cells at various concentration levels (Fig. 3B). The involvement of Jak2 in this
augmented expression was analyzed by inhibition of Jak2 using
AG490. This inhibition led to a reduction of HSE-induced BMP7 and
GHR mRNA expression (Fig. 3C). The
relative expression with respect to 18S revealed a statistically
significant repression in the expression of BMP7 and GHR (Fig. 3D). These results demonstrate that
HSE-induced BMP7 and GHR expression increases via Jak2.
HSE-induced BMP7 and GH signaling
requires STAT5B activation in MC3T3-E1 cells
To investigate whether STAT5B is involved in the
effects of HSE on GH signaling, a siRNA strategy was used. MC3T3-E1
cells were transfected with specific STAT5B siRNA and then exposed
to HSE treatment. STAT5B knockdown decreased the basal levels of
STAT5B protein expression. Knockdown of STAT5B also inhibited
HSE-induced p-STAT5B, BMP7 and IGF-1R expression levels in MC3T3-E1
cells (Fig. 4A). Relative protein
expression levels with respect to actin were analyzed to determine
the effect of STAT5B in enhanced expression of proteins by HSE
(Fig. 4B). These results
demonstrated that STAT5B played an essential role in GH signaling
activation in MC3T3-E1 cells.
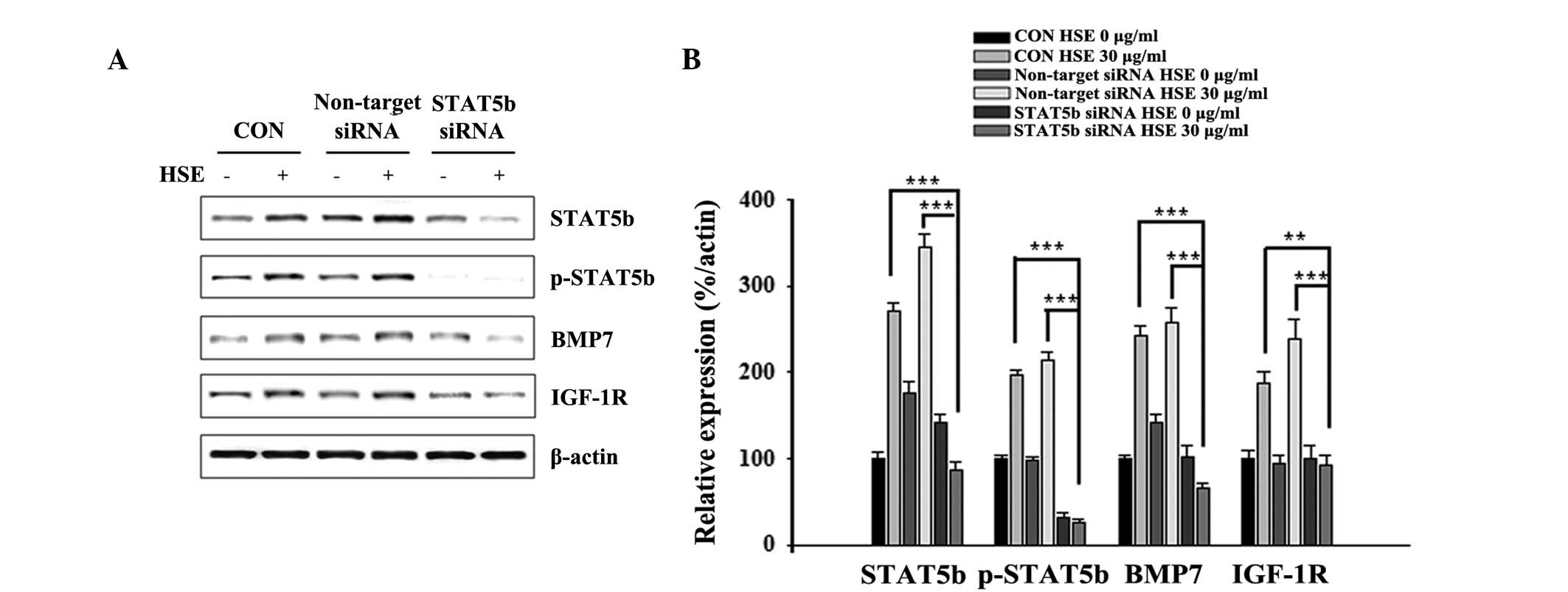 | Figure 4HSE-enhanced BMP7 and GH signaling
requires STAT5B activation in MC3T3-E1 cells. (A) MC3T3-E1 cells
were transfected with siRNA targeting STAT5B or non-targeting siRNA
for 48 h, cells were cultured with serum free medium for 24 h and
then cultured with 30 μg/ml HSE for 24 h. Protein extracts (20 μg)
were separated by 10% SDS-PAGE and western blot analysis was
performed. β-actin was used as a protein loading control. (B) The
relative levels of STAT5B, p-STAT5B, BMP7 and IGF-1R protein were
determined using densitometric analysis and normalized against
β-actin. Data presented are representative of three independent
experiments. (**P<0.01, ***P<0.001, vs.
β-actin). HSE, Hwanggeumchal sorghum extracts; GH, growth
hormone; BMP7, bone morphogenetic protein 7; STAT5B, signal
transducers and activators of transcription 5B; IGF, insulin-like
growth factor; GHR, growth hormone receptor; IGF-1R, insulin-like
growth receptor-1. |
Discussion
GH is a potent regulator of bone formation (1). In addition, growth factors, including
BMPs, play a key role in bone regeneration through growth and
differentiation, whether secreted locally or used as stimulators
(11,24). Unlike the BMP-induced
osteo-inductive Smad pathway, Jak/STAT signaling is frequently
associated with proliferation and migration of numerous primary
cell lineages (25). In a recent
study, we reported that STAT5B is involved in MSM-induced
osteoblastic differentiation of MSCs and plays an essential role in
MSM-induced GH signaling of C3H10T1/2 cells (22).
The present study was designed to examine the
involvement of Jak2/STAT5B signaling in MC3T3-E1 cells following
their treatment with HSE. Analysis of cell proliferation using the
MTT method was performed with HSE at 10, 20 and 40 μg/ml. As
demonstrated in Fig. 1, there was
no significant difference in the OD values in MC3T3-E1 cells. This
observation indicated that there is no notable proliferation
inhibition associated with HSE treatment in osteoblast cells. Our
next aim was to investigate the effects of HSE on the Jak/STAT
pathway by analyzing the expression of BMP7 and GHR in
osteoblast-like cells. HSE increased the expression of GH
signaling-related proteins, including STAT5B, p-STAT5B, IGF-1R, GHR
and Jak2, in MC3T3-E1 cells (Fig.
2A).
Osteoblasts produce a range of growth factors under
a variety of stimuli, including IGF, platelet-derived growth
factor, TGF-β and BMP (26–29).
BMP7 is an important inducer of bone formation in vivo and
in vitro. The ability of recombinant human BMP7 to induce
large volumes of bone makes it an ideal candidate for treating
delayed unions and non-unions (30). In the present study, treatment with
HSE was found to dependently induce the expression of BMP7 in
MC3T3-E1 cells (Figs. 2A and
3A). In addition, inhibition of
Jak2 led to suppression of BMP7 expression at the transcriptional
and translational levels, indicating the importance of Jak2 in the
enhanced expression of BMP7 upon HSE treatment (Figs. 2B and 3B). These results demonstrate that HSE
has the ability to maintain bone homeostasis via enhanced
expression of BMP7.
Osteoblasts express GHR (31,32)
and transmembrane GHR levels are extremely important for GH
signaling (33). In general, the
expression levels of GHR are regulated and fine tuned through the
IGF-1 and IGF binding protein level. Levels of GHR are important in
the autocrine loop of GH/IGF-1 and other factors modulating the
effect of GH in osteoblasts. In our previous study, HSE was found
to dose dependently enhance the expression of GHR in MC3T3-E1
cells. Inhibition of Jak2 and STAT5B revealed a marked decrease in
GHR levels, demonstrating the significance of the Jak2/STAT5B
signaling axis in the modulation of GHR expression. It is already
known that IGF-1R is a necessary factor for maintaining
GH-stimulated IGF-1 and IGFBP-3 expression (31). GH induces a GHR-Jak2-IGF-1R
complex, indicative of a novel function for IGF-1R (2). In the present study, expression of
IGF-1R was also found to increase with HSE treatment (Fig. 2A) and this increase was mediated
through STAT5B (Fig. 4).
Therefore, we hypothesize that, HSE increases GHR signaling by
enhancing the expression of IGF-1R via STAT5B in MC3T3-E1
cells.
GH signals through membrane-associated GHR, which
results in the activation of receptor-associated Jaks. Jak2
activation results in the engagement of several intracellular
signaling pathways, including STAT-1, -3 and -5 (34–36).
The role of Jak2 in osteoblastic cells is well studied. In a recent
study, we reported that inhibition of Jak2 results in the
inhibition of GH mediated osteoblast differentiation (22), providing an insight to the role of
Jak2 in osteoblastic cell lines. In the present study, the
elevation of Jak2 levels using HSE revealed a simultaneous
elevation in BMP7 and GHR levels. However, the inhibition of Jak2,
suppressed the expression of BMP7 as well as GHR levels (Figs. 2B and 3C). The relative expression levels of
these proteins revealed a statistically significant decline in the
expression of BMP7 and GHR in Jak2 inhibited samples (Figs. 2C and 3D). This confirmed the role of Jak2 in
the enhanced expression of BMP7 and GHR in HSE treatment.
STAT5B is an important member of the STAT family for
osteoblast proliferation and differentiation (22), owing to GH signaling primarily
through the STAT5B/IGF-1R axis (5). This signaling axis has its own
importance in osteoblasts, as it modulates the expression of IGF-1,
which is the most important autocrine factor in osteoblasts. In
addition to the role of STAT5B in the regulation of IGF-1 levels,
we found that abolition of STAT5B by specific siRNA, significantly
decreases HSE-induced BMP7 and IGF-1R expression in osteoblast-like
cells, indicating that the STAT5B signaling pathway functions as a
positive modulator of HSE-induced signaling and enhanced expression
of BMP7 and GHR (Figs. 2B and
3A).
We hypothesize that HSE signals in osteoblast-like
cells through the Jak2/STAT5B axis, similar to GH. In the present
study, the ability of HSE to modulate the Jak2/STAT5B cascade in
osteoblastic cells is reported for the first time. This signaling
axis is involved in the somatomedin hypothesis and dual effector
theory of GH/IGF-1 interaction and signaling. HSE signaling
enhances the expression of BMP7, GHR and IGF-1R in MC3T3-E1 cells,
indicating that the HSE signaling mechanism is important for
osteoblast proliferation, differentiation and bone homeostasis.
Acknowledgements
This study was supported by grants from the
Fundamental R&D Program for Technology of World Premier
Materials funded by the Ministry of Trade, Industry & Energy
(no. 2010-0012318) and was partially supported by the Next
Generation BioGreen 21 Programs (no. PJ0081062011), Rural
Development Administration, Republic of Korea.
References
|
1
|
Giustina A, Mazziotti G and Canalis E:
Growth hormone, insulin-like growth factor and the skeleton. Endocr
Rev. 29:535–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stred SE, Stubbart JR, Argetsinger LS, et
al: Stimulation by growth hormone (GH) of GH receptor-associated
tyrosine kinase activity. Endocrinology. 130:1626–1636.
1992.PubMed/NCBI
|
|
3
|
Argetsinger LS, Campbell GS, Yang X, et
al: Identification of JAK2 as a growth hormone receptor-associated
tyrosine kinase. Cell. 74:237–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter-Su C, Argetsinger LS, Campbell GS,
et al: The identification of JAK2 tyrosine kinase as a signaling
molecule for growth hormone. Proc Soc Exp Biol Med. 206:210–215.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrington J, Smit LS, Schwartz J and
Carter-Su C: The role of STAT proteins in growth hormone signaling.
Oncogene. 19:2585–2597. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gan Y, Zhang Y, Digirolamo DJ, et al:
Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces
GH-induced STAT5 signaling. Mol Endocrinol. 24:644–656. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann NY Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastogenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gazzerro E and Canalis E: Bone
morphogenetic proteins and their antagonists. Rev Endocr Metab
Disord. 7:51–65. 2006. View Article : Google Scholar
|
|
10
|
Kawabata M, Imamura T and Miyaxono K:
Signal transduction by bone morphogenetic proteins. Cytokine Growth
Factor Rev. 9:49–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wozney JM, Rosen V, Byrne M, Celeste AJ,
Moutsatsos I and Wang EA: Growth factor influencing bone
development. J Cell Sci Suppl. 13:149–156. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laflamme C, Curt S and Rouabhia M:
Epidermal growth factor and bone morphogenetic proteins upregulate
osteoblast proliferation and osteoblastic markers and inhibit bone
nodule formation. Arch Oral Biol. 55:689–701. 2010. View Article : Google Scholar
|
|
13
|
Sampath TK, Maliakal JC, Hauschka PV, et
al: Recombinant human osteogenic protein-1 (hOP-1) induces new bone
formation in vivo with a specific activity comparable with natural
bovine osteogenic protein and stimulates osteoblast proliferation
and differentiation in vitro. J Biol Chem. 267:20352–20356.
1992.
|
|
14
|
Vukicevic S, Luyten FP and Reddi AH:
Stimulation of the expression of osteogenic and chondrogenic
phenotypes in vitro by osteogenin. Proc Natl Acad Sci USA.
86:8793–8797. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buijs JT, Henriquez NV, van Overveld PG,
et al: Bone morphogenetic protein 7 in the development and
treatment of bone metastases from breast cancer. Cancer Res.
67:8742–8751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SN, Lapage J and Hirschberg R: Loss
of tubular bone morphogenic protein-7 in diabetic nephropathy. J Am
Soc Nephrol. 12:2392–2399. 2001.PubMed/NCBI
|
|
17
|
Kamath VG, Chandrashekar A and Rajini PS:
Antiradical properties of sorghum (Sorghum bicolor L.
Moench) flour extracts. J Cereal Sci. 40:283–288. 2004. View Article : Google Scholar
|
|
18
|
Park JH, Darvin P, Lim EJ, et al:
Hwanggeumchal sorghum induces cell cycle arrest and
suppresses tumor growth and metastasis through Jak2/STAT pathways
in breast cancer xenografts. PLoS One. 7:e405312012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JH, Lee SH, Chung IM and Park Y:
Sorghum extract exerts an anti-diabetic effect by improving insulin
sensitivity PPAR-γ in mice fed a high-fat diet. Nutr Res Pract.
6:322–327. 2012.PubMed/NCBI
|
|
20
|
Audran M: Drug combination strategies for
osteoporosis. Joint Bone Spine. 73:374–378. 2006. View Article : Google Scholar
|
|
21
|
Turner CH: Toward a cure for osteoporosis:
reversal of excessive bone fragility. Osteoporos Int. 2:12–19.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joung YH, Lim EJ, Darvin P, et al: MSM
enhances GH signaling via the Jak2/STAT5B pathway in
osteoblast-like cells and osteoblast differentiation through the
activation of STAT5B in MSCs. PLoS One. 7:e474772012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung IM and Kim SH: Analysis methods of
phenol compounds. Korean Society of Crop Science. 8:5–12. 2004.
|
|
24
|
Salgado AJ, Coutinho OP and Reis RL: Bone
tissue engineering: state of the art and future trends. Macromol
Biosci. 4:743–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aaronson DS and Horvath CM: A road map for
those who don’t know JAK-STAT. Science. 296:1653–1655. 2002.
|
|
26
|
Canalis E, Pash J, Gabbitas B, et al:
Growth factors regulate the synthesis of insulin-like growth
factor-I in bone cell cultures. Endocrinology. 133:33–38.
1993.PubMed/NCBI
|
|
27
|
Rydziel S, Shaikh S and Canalis E:
Platelet-derived growth factor-AA and -BB (PDGF-AA and -BB) enhance
the synthesis of PDGF-AA in bone cell cultures. Endocrinology.
134:2541–2546. 1994.PubMed/NCBI
|
|
28
|
Canalis E, Pash J and Varghese S: Skeletal
growth factors. Crit Rev Eukaryot Gene Expr. 3:155–166. 1993.
|
|
29
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar
|
|
30
|
Boon MR, van der Horst G, van der Pluijm
G, Tamsma JT, Smit JW and Rensen PC: Bone morphogenetic protein 7:
a broad-spectrum growth factor with multiple target therapeutic
potency. Cytokine Growth Factor Rev. 22:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DiGirolamo DJ, Mukherjee A, Fulzele K, et
al: Mode of growth hormone action in osteoblasts. J Biol Chem.
282:31666–31674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nilsson A, Swolin D, Enerback S and
Ohlsson C: Expression of functional growth hormone receptors in
cultured human osteoblast-like cells. J Clin Endocrinol Metab.
80:3483–3488. 1995.PubMed/NCBI
|
|
33
|
Kassem M, Mosekilde L and Eriksen EF:
Growth hormone stimulates proliferation of normal human bone marrow
stromal osteoblast precursor cells in vitro. Growth Regul.
4:131–135. 1994.PubMed/NCBI
|
|
34
|
Huang Y, Kim SO, Yang N, Jiang J and Frank
SJ: Physical and functional interaction of growth hormone and
insulin-like growth factor-1 signaling elements. Mol Endocrinol.
18:1471–1485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joung YH, Lim EJ, Kim MS, et al:
Enhancement of hypoxia-induced apoptosis of human breast cancer
cells via STAT5B by momilactone B. Int J Oncol. 33:477–484.
2008.PubMed/NCBI
|
|
36
|
Park SH, Joung YH, Park JH, et al: Hypoxia
upregulates Hsp90α expression via STAT5B in cancer cells. Int J
Oncol. 41:161–168. 2012.
|