Introduction
Mesenchymal stem cells (MSCs) are derived from a
wide range of sources. Human mesenchymal stem cells isolated from
the Wharton’s jelly of the umbilical cord (HUMSCs) are easily
obtained (1). These cells display
the properties of stem cells and stromal cells, including the
expression of matrix receptors [cluster of differentiation 44
(CD44) and CD105], integrins (CD29 and CD51) and stem cell markers
[Src homology 2 (SH2) and SH3], and they may be induced to
differentiate into a variety of mature cells (2). Unlike MSCs isolated from other
sources, such as fetal organs and bone marrow, the generation of
HUMSCs is not invasive and does not evoke ethical issues. In
addition, HUMSCs demonstrate a high proliferation rate and
self-renewal capacity compared with other types of adult stem
cells. Therefore, HUMSCs may be a valuable novel source of cells
for tissue engineering.
Adenoviral vectors are efficiently produced
(3) and are capable of transducing
proliferating and quiescent cells, thus greatly enhancing gene
expression. As the transgene is not integrated into the chromosome,
transduction of genes by adenoviral vectors does not modify the
genome of infected cells. Therefore, adenoviral vectors are
promising gene delivery vehicles (4–6).
Several studies have demonstrated that the
expression of key embryonic transcription factors (TFs) in adult
MSCs induced their differentiation into insulin-expressing cells
(7,8). The pancreatic and duodenal homeobox 1
(pdx1) is expressed in all pancreatic precursor cells during
embryonic development and in β-cells in adults (9). The V-maf musculoaponeurotic
fibrosarcoma oncogene homolog A (mafa), a member of the basic
leucine zipper family, regulates insulin gene expression through
binding to the insulin promoter at the C1-box (10). The class B basic helix-loop-helix
factor neurogenin 3 (ngn3) is the master gene controlling endocrine
cell fate decisions in uncommitted pluripotent pancreatic
progenitor cells (11). Previous
studies have demonstrated that pdx1, mafa and ngn3 are important
for reprogramming MSCs into insulin-producing cells (12).
In the present study, the exogenous expression of
the aforementioned three TFs of mouse origin was observed
individually or in combination, to assess the programming of HUMSCs
into pancreatic endocrine cells. The results demonstrated that the
expression of pdx1, ngn3 and mafa activated the endogenous
promoters of glucagon, pdx1 and nkx2.2 in HUMSCs.
Materials and methods
Cell culture
Ethical approval was obtained from the Institutional
Review Board of Shantou University Medical College (Shantou,
Guangdong, China). The human umbilical cords from consenting
patients undergoing full-term cesarean section were collected
immediately into sterilized 50 ml tubes, washed with
phosphate-buffered saline (PBS) and cut into 2- to 3-cm-long
pieces. Following the removal of the arteries and veins, the
remaining tissue (the Wharton’s jelly) was sectioned into smaller
fragments and transferred to a 75-cm2 flask containing
Dulbecco’s modified Eagle’s medium (DMEM)/F12. This culture media
was supplemented with 10% fetal bovine serum, 100 μg/ml
penicillin/streptomycin, 1 g/ml amphotericin B, 5 ng/ml epidermal
growth factor (EGF; Invitrogen Life Technologies, Carlsbad, CA,
USA) and 5 ng/ml basic fibroblast growth factor (bFGF,
Sigma-Aldrich, St. Louis, MO, USA). The cultures were left
undisturbed for 5–7 days in 5% CO2 at 37ºC to allow the
migration of cells from the explants, at which point the media were
replaced.
Phenotypic characterization of
HUMSCs
Approximately 1×106 HUMSCs at passage 3
were dispersed with trypsin and resuspended in PBS containing
phycoerythrin (PE)-conjugated antibodies against CD29 and CD59 (BD
Biosciences, Franklin Lakes, NJ, USA) for 60 min at 4ºC. Cells were
washed three times with PBS and incubated with PE-conjugated rabbit
anti-mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and fluorescein isothiocyanate-conjugated goat anti-rat IgG
(Santa Cruz Biotechnology, Inc.), respectively, for 30 min at room
temperature. Following three washes, cells were resuspended in 0.5
ml PBS and analyzed by flow cytometry with the use of EPICS XL
(Beckman Coulter, Inc., Brea, CA, USA).
Adenoviral expansion and infection
The E1-deleted adenovirus (serotype 5) carrying the
cytomegalovirus promoter/enhanced green fluorescent protein (EGFP)
hybrid gene was purchased from Vector Gene Technology Company, Ltd.
(Beijing, China). Adenoviral vectors expressing mouse pdx1
(Ad-pdx1), mafa (Ad-mafa) and ngn3 (Ad-ngn3) were obtained from the
Beta Cell Biology Consortium (Nashville, TN, USA). For
amplification of the adenoviruses, 1×108 infectious
units of the viruses were added to a 10-cm dish preseeded with
1×106 Ad293 cells (Stratagene, La Jolla, CA, USA)
overnight. Following incubation for 30–48 h, cells were harvested
by scraping and centrifugation at 12,000 × g for 10 min, while the
supernatant was saved for the next round of virus amplification.
The harvested cells underwent four freeze/thaw cycles and were spun
at 12,000 × g for 10 min to obtain the cell lysates. Serial
dilutions of the supernatant and cell lysates were used to
transduce the Ad293 cells in a 96-well plate preseeded with 5,000
cells overnight. The viral titers were determined by counting the
number of EGFP positive cells under a fluorescence microscope
following 30 h of culturing, as described previously (13). HUMSCs at passage 3–5 were seeded at
a density of 1×105 cells/well in 6-well plates.
Following 24 h of culturing, the media were replaced with 1 ml
serum-free media containing the indicated adenoviruses at a
multiplicity of infection of 50, for 4 h. The media were then
replaced with serum containing media supplemented with 10 ng/ml
bFGF, 10 ng/ml EGF and 10 mmol/l nicotinamide. Media were replaced
on alternate days over the next seven days.
Quantitative reverse
transcription-polymerase chain reaction (qPCR)
Total RNA was isolated from HUMSCs using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. cDNA was prepared using the
Primescript RT Reagent kit (Takara Bio, Inc., Shiga, Japan). cDNA
samples were analyzed by qPCR using the SYBR premix (Takara Bio,
Inc.) in an ABI 7300 system (Applied Biosystems, Foster City, CA,
USA). The primers used for qPCR analyses were as follows: Forward:
5′-ggatgaaatccaccaaagctcac-3′ and reverse:
5′-aagttcaacatcactgccagctc-3′ for mouse pdx1 (NM_008814, 193 bp);
forward: 5′-cacattctggagagcgagaag-3′ and reverse:
5′-tctcgtatttctccttgtacaggtc-3′ for mouse mafa (NM_194350, 109 bp);
forward: 5′-ttcacgagccagtatgaccttcac-3′, and reverse:
5′-gaagacagacctgggatgcaca-3′ for human pdx1 (NM_000209, 148 bp);
forward: 5′-accaaaccgtcccagcgtta-3′ and reverse:
5′-ggctgacaatatcgctactcacaca-3′ for human nkx2.2 (NM_002509, 116
bp); forward: 5′-cagagcttaggacacagagcacatc-3′ and reverse:
5′-acgttgccagctgccttgta-3′ for human glucagon (NM_002054, 161
bp).
Statistical analysis
Data are presented as the mean ± standard deviation
of each group. The Student’s t-test was performed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of HUMSCs isolated from
the Wharton’s jelly of human umbilical cords
HUMSCs were isolated from human umbilical cords
under sterile conditions. These cells grew rapidly in serum
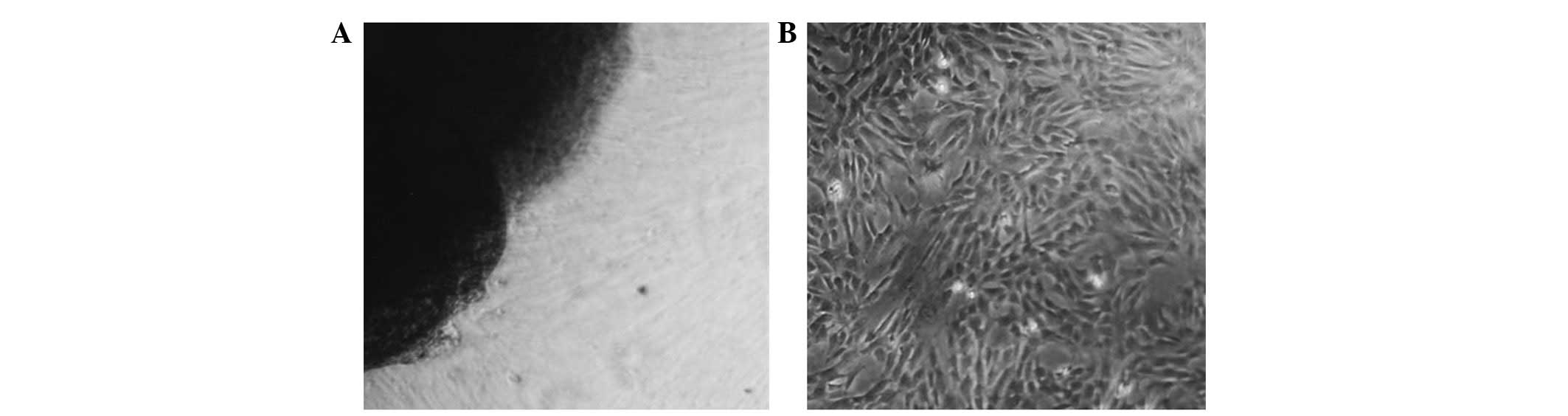
containing DMEM media supplemented with bFGF. As shown in Fig. 1A, spindle-shaped HUMSCs migrated
out from the fragments of Wharton’s jelly following 5–7 days of
culturing. Typically, the primary culture was established within
10–14 days (Fig. 1B), following
which, the cells were split every 3–5 days at a ratio of 1:3. The
cell shape changed from stellar-like to fibroblastic-like over
time.
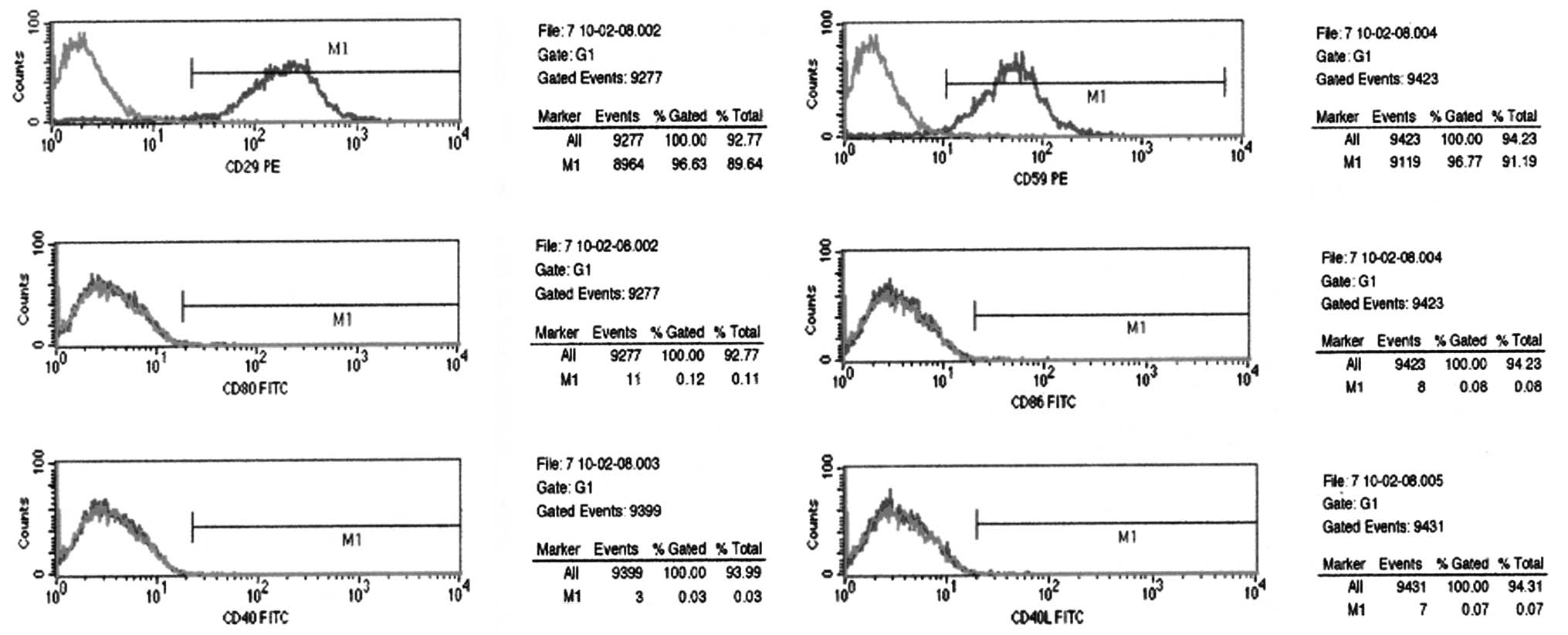
Phenotypic analysis using flow cytometry was
performed on passage 3 HUMSCs. The results showed that HUMSCs
expressed surface antigens, CD29 and CD59, which are associated
with pluripotent adult stem cells. In addition, the expression
levels of CD80, CD86, CD40 and CD40L antigens were low (Fig. 2).
In vitro programming of human umbilical
cord mesenchymal stem cells to promote pancreatic gene
expression
Fluorescence microscopy demonstrated that adenovirus
pdx1, ngn3 and mafa were transfected into HUMSCs (Fig. 3). The morphology of HUMSCs did not
change 3 or 7 days following adenovirus transduction. qPCR analysis
of the genes associated with the pancreas 3 days after transduction
demonstrated that the glucagon gene expression in HUMSCs
transfected with ad-EGFP blank, ad-EGFP and ad-ngn3 individually
did not result in the expression of the neuroD1 gene (Fig. 4).
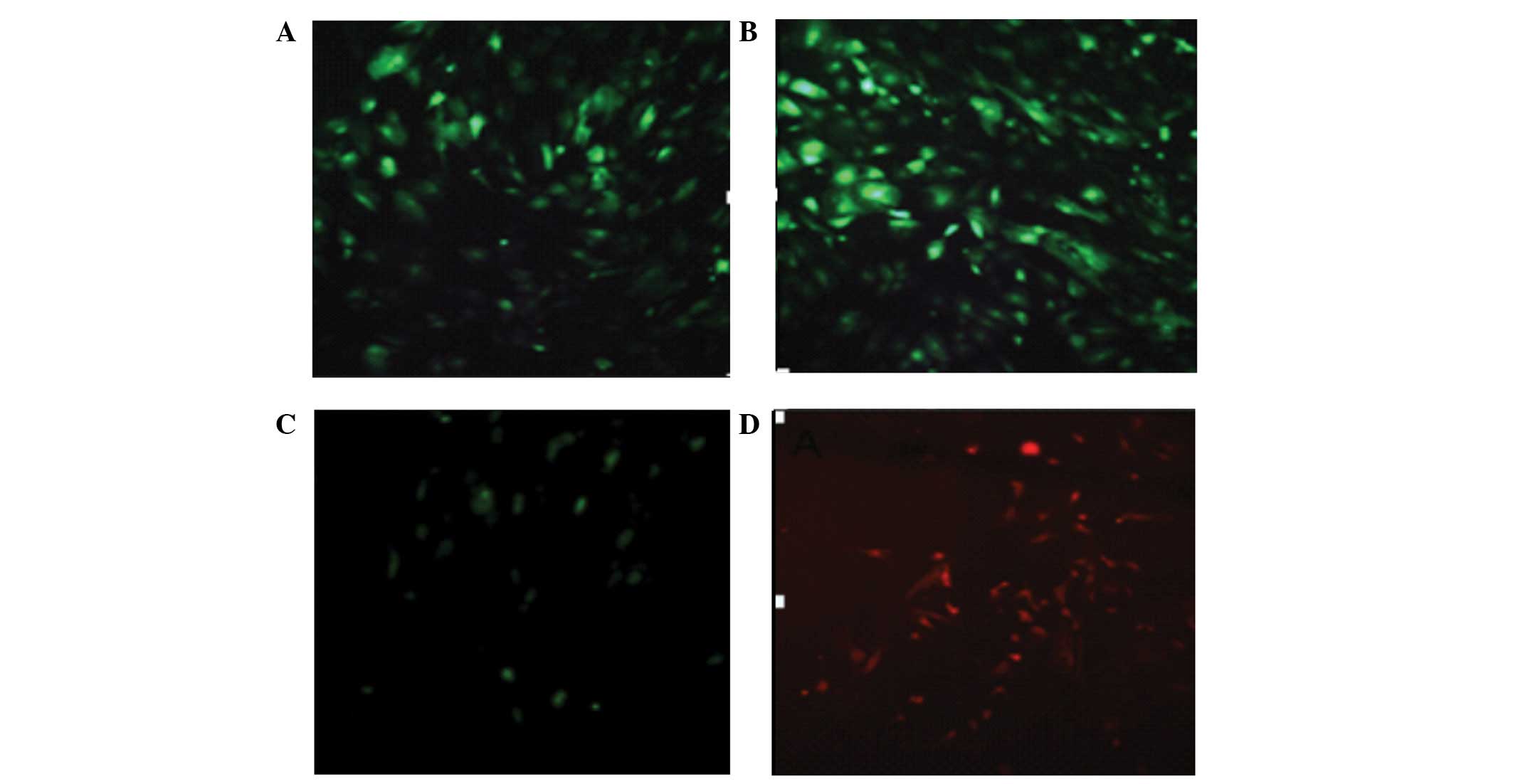 | Figure 3Adenoviruses, including adeno-EGF,
adeno-EGFP-ngn3, adeno-EGFP-pdx1 and adeno-EGFP-mafa were
transfected into HUMSCs. (A and B) Expression of green fluorescent
protein; (C and D) expression of mouse pdx1 and mafa (green and
red; magnification, ×200). EGF, epidermal growth factor; EGFP,
enhanced green fluorescent protein; ngn3, class B basic
helix-loop-helix factor neurogenin 3; pdx1, pancreatic and duodenal
homeobox 1; mafa, V-maf musculoaponeurotic fibrosarcoma oncogene
homolog A; HUMSCs, human umbilical cord mesenchymal stem cells. |
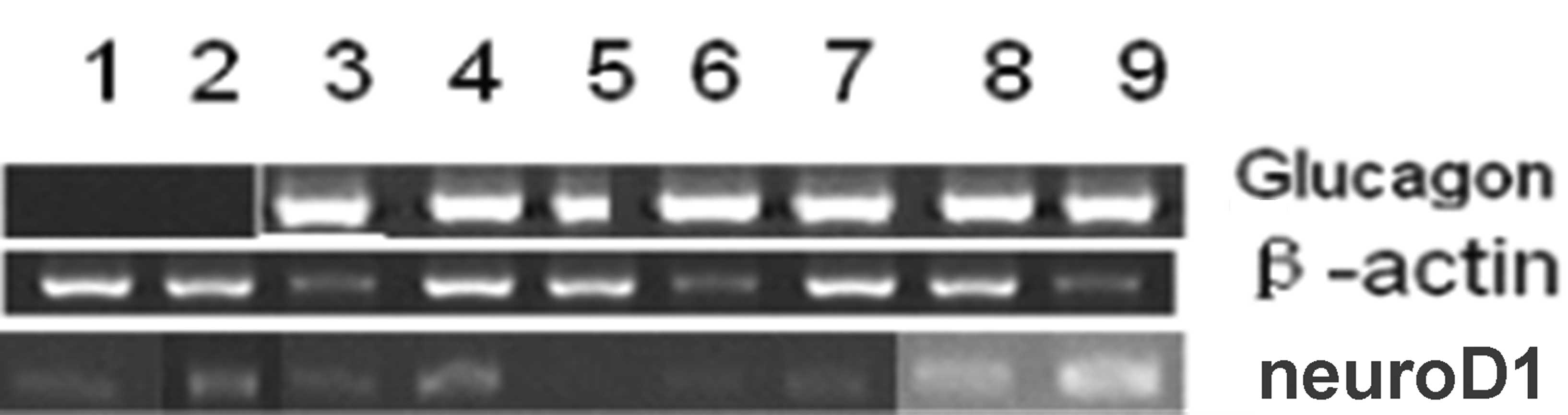 | Figure 4qPCR analysis of the pancreatic genes
in HUMSCs. The glucagon gene was expressed 3 days following
induction. HUMSCs transfected with ad-ngn3 alone did not express
the neuroD1 gene, M DL2 000 marker; β-actin (396 bp); glucagon (384
bp), neuroD1 (508 bp). Lanes were as follows: 1, phosphate-buffered
saline; 2, ad-EGFP; 3, ad-pdx1; 4, ad-mafa; 5, ad-ngn3; 6, ad-pdx1
+ ad-mafa; 7, ad-pdx1 + ngn3; 8, ad-mafa + ngn3; 9, ad-pdx1 + mafa
+ ngn3. qPCR, quantitative reverse transcription polymerase chain
reaction; HUMSCs, human umbilical cord mesenchymal stem cells; Ad,
adenovirus; ngn3, class B basic helix-loop-helix factor neurogenin
3; EGFP, enhanced green fluorescent protein; pdx1, pancreatic and
duodenal homeobox 1; mafa, V-maf musculoaponeurotic fibrosarcoma
oncogene homolog A. |
qPCR
Numerous TFs including pdx1, ngn3 and mafa have been
demonstrated to be essential for the development of β-cells in
vivo and the reprogramming of a variety of other cell types
into insulin-producing cells in vitro. To demonstrate their
potential for generating insulin-producing cells, passage 3–5
HUMSCs were infected with adenoviral vectors carrying mouse pdx1,
ngn3 or mafa. Fig. 5 demonstrates
that following the exogenous expression of pdx1, ngn3 and mafa
individually, only mafa was able to induce glucagon gene
expression. The combination of pdx1 and ngn3 had a synergistic
effect on the glucagon gene expression and the combination of the
three genes demonstrated the greatest effect on glucagon gene
expression (P<0.05). At day 7, the combination of mafa and ngn3
resulted in lower expression levels of glucagon than those with
mafa transfection alone. However, the combination of the three
genes was most effective in inducing the glucagon gene expression
at day 7 (P<0.05). To investigate the possible mechanism
underlying the activation of the glucagon gene, the expression of
endogenous TFs required for pancreatic cell development were
measured. Endogenous pdx1 activation was detected upon the
expression of any of the three exogenous TFs alone at day 3 and day
7. At day 3, the greatest level of pdx1 gene expression was induced
by the combination of the three genes while the combination of any
of the two genes induced a lower pdx1 gene expression than that
induced by the TFs alone (P<0.05) (Fig. 6). At day 7, the combination of mafa
and ngn3 provided the most effective stimulation of the pdx1 gene
(P<0.05). Fig. 7 shows that the
endogenous pdx1 gene level increased over time. Endogenous nkx2.2
was significantly activated by exogenous expression of ngn3 at day
3 only (P<0.05). In addition, nkx2.2 gene expression was higher
at day 7 than at day 3 (P<0.05), with the exception of that when
ngn3 alone was transduced (Fig.
7)
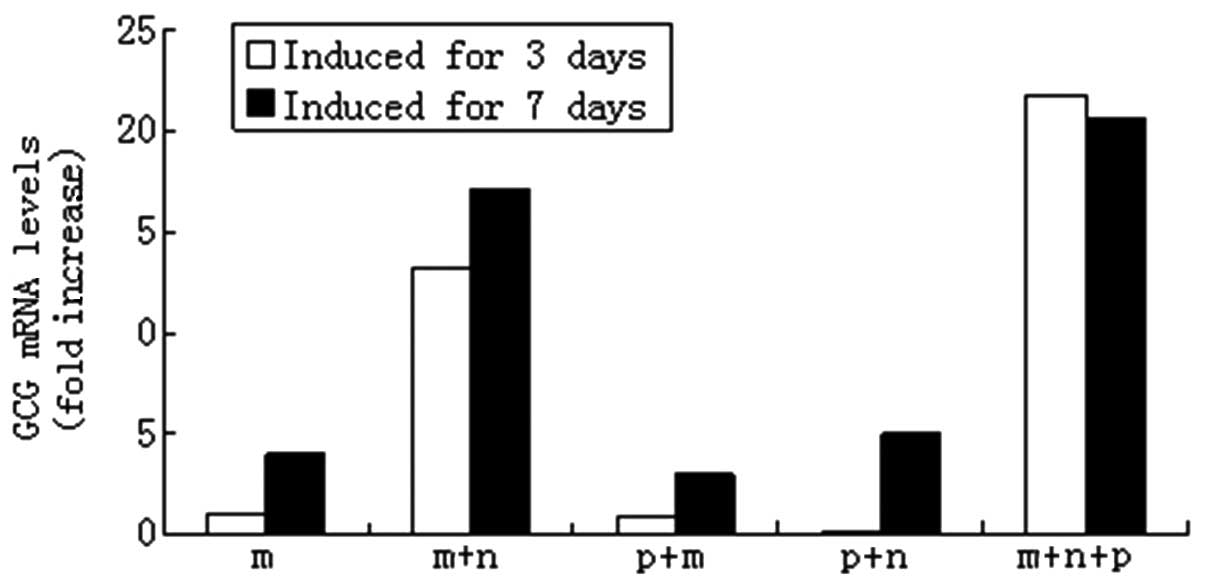 | Figure 5Programming of HUMSCs into pancreatic
cells by the ectopic expression of pdx1, ngn3 and mafa. HUMSCs were
infected with indicated adenoviral vectors at a multiplicity of
infection of 50. After 3 and 7 days of culturing, the gene levels
of glucagon were measured using qPCR. The expression level of
β-actin (Ct = 18) was used for normalization. Transcript levels in
untreated HUMSCs were designated as 1. HUMSCs, human umbilical cord
mesenchymal stem cells; pdx1, pancreatic and duodenal homeobox 1;
ngn3, class B basic helix-loop-helix factor neurogenin 3; mafa,
V-maf musculoaponeurotic fibrosarcoma oncogene homolog A; qPCR,
quantitative reverse transcription polymerase chain reaction; m,
mafa; n, ngn3; p, pdx1; GCG, glucagon. |
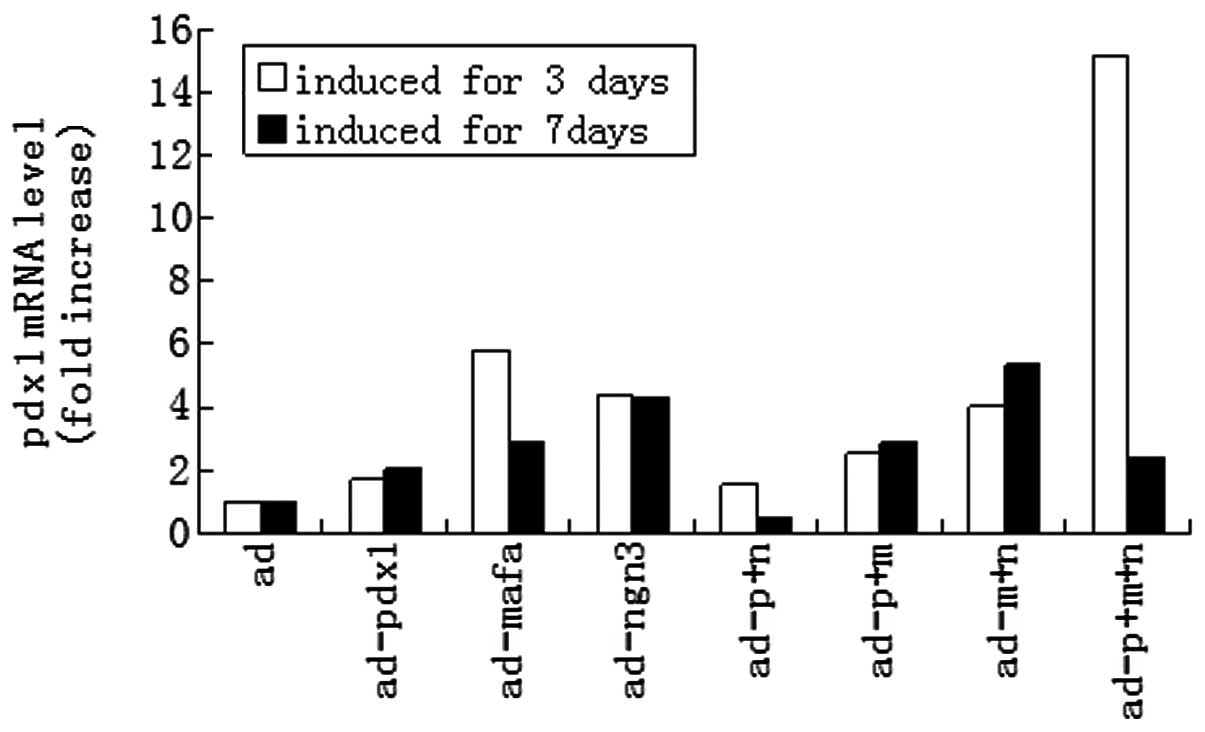 | Figure 6Activation of the endogenous pdx1 gene
by expression of pdx1, ngn3 and mafa. HUMSCs were infected with
indicated adenoviral vectors at a multiplicity of infection of 50.
Following 3 and 7 days of culturing, the relative transcript levels
of the endogenous pdx1 were measured using qPCR. The expression
level of β-actin (Ct = 18) was used for normalization. Transcript
levels in untreated HUMSCs were designated as 1. pdx1, pancreatic
and duodenal homeobox 1; ngn3, class B basic helix-loop-helix
factor neurogenin 3; mafa, V-maf musculoaponeurotic fibrosarcoma
oncogene homolog A; HUMSCs, human umbilical cord mesenchymal stem
cells; qPCR, quantitative reverse transcription polymerase chain
reaction; ad, adenovirus; p, pdx1; n, ngn3; m, mafa. |
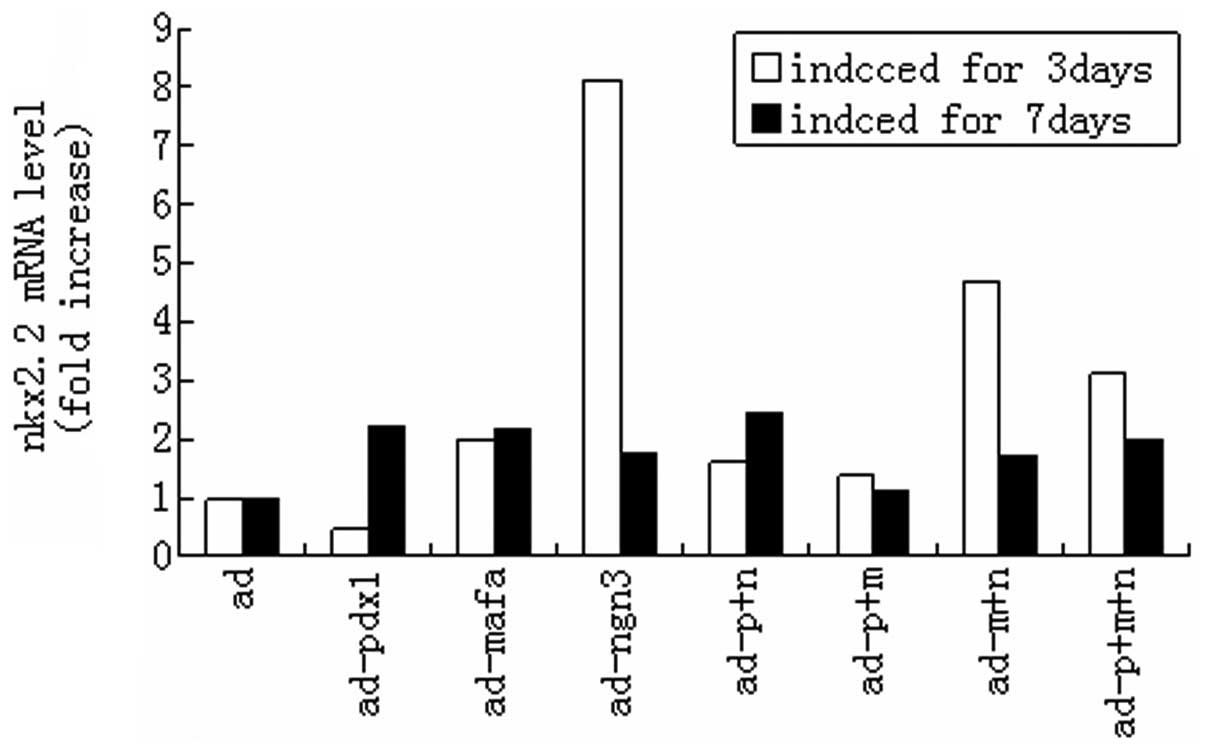 | Figure 7Activation of the endogenous nkx2.2
gene by expression of mouse pdx1, ngn3 and mafa. HUMSCs were
infected with the indicated adenoviral vectors at a multiplicity of
infection of 50. After 3 and 7 days of culturing, the relative
transcript levels of the endogenous nkx2.2 were measured using
qPCR. The expression level of β-actin (Ct = 18) was used for
normalization. Transcript levels in untreated HUMSCs were
designated as 1. nkx2.2, nk2 homeobox 2; pdx1, pancreatic and
duodenal homeobox 1; ngn3, class B basic helix-loop-helix factor
neurogenin 3; mafa, V-maf musculoaponeurotic fibrosarcoma oncogene
homolog A; HUMSCs, human umbilical cord mesenchymal stem cells;
qPCR, quantitative reverse transcription polymerase chain reaction;
ad, adenovirus; p, pdx1; n, ngn3; m, mafa. |
Discussion
MSCs have been considered to be candidates for cell
replacement therapy, as they may be induced to differentiate into
other cell types, such as insulin-producing cells, under certain
conditions (14). In this study,
it was demonstrated that HUMSCs were able to be programmed into
pancreatic endocrine precursors cells by the exogenous expression
of three pancreatic TFs, pdx1, ngn3 and mafa.
Previous studies have shown that pluripotent human
mesenchymal stem cells may be isolated from Wharton’s jelly of
human umbilical cords. These stem cells possess the properties of
MSCs and are able to differentiate into neuron-like cells,
adipocytes, osteocytes and chondrocytes (2,15).
Furthermore, HUMSCs have been observed to express high levels of
MSC markers, such as CD29 and CD59, but not graft-versus-host
disease-related markers, including CD40, CD40L, CD86 and CD80.
Therefore, HUMSCs may be a preferential source for cell replacement
therapy for the treatment of various diseases.
In this study, the differentiation potential of
HUMSCs into pancreatic lineage cells by exogenous expression of
three TFs was investigated. These TFs are important activators of
the insulin gene in mature β-cells. Pdx1 is a master regulator for
the development of all pancreatic cell types, while ngn3 is
critical for the specification of endocrine progenitor cells in the
pancreas. Mafa and mafb are effective activators of the insulin and
glucagon genes, respectively (16,17).
A primary HUMSC culture in media containing
nicotinamide, bFGF and EGF was established. Nicotinamide, a poly
(ADP-ribose) synthetase inhibitor, has been demonstrated to
stimulate β-cell differentiation in cultured human fetal pancreatic
cells (18) and to protect β-cells
from desensitization induced by exposure to high glucose levels
(19). In addition, EGF is
important in the differentiation and proliferation of pdx1-positive
pancreatic progenitor cells (20).
In the present study, to investigate the potential
mechanism underlying the differentiation of HUMSCs induced by the
exogenous TFs, the activation of endogenous glucagon, pdx1 and
nkx2.2 genes was examined. Pdx1 expression is maintained throughout
the development of the pancreas, providing both spatial and
temporal instructions for the commitment of the endoderm to a
pancreatic fate. The expression of pdx1 also activates nkx2.2 in
α-, β- and pancreatic polypeptide-cells (21). In the current study, it was
determined that endogenous glucagon, pdx1 and nkx2.2 were
significantly activated by the expression of mouse pdx1, ngn3 and
mafa.
The effects of the members of the maf family on
insulin and glucagon gene transcription are well understood. Mafa
and mafb are effective activators of insulin and glucagon gene
transcription, respectively (16,17).
Exogenous expression of mouse mafa alone is able to induce glucagon
gene expression due to the high homology among family members
(22).
Previous studies have demonstrated contradictory
results concerning glucagon gene activation by the overexpression
of pdx1 and other TFs. Pdx1 was observed to increase glucagon mRNA
levels in MSCs derived from the human pancreas and bone marrow, as
well as in cultured rat hepatocytes (12,23).
However, the results from studies by Ritz-Laser et
al(24) and Wang et
al(25) identified that
glucagon mRNA levels in a glucagonoma cell line and a rat
insulinoma cell line were decreased by pdx1 expression. The results
of the present study demonstrated that the endogenous glucagon gene
was not activated at day 3 when cells were tranduced by pdx1 or
ngn3 alone. However, the combination of pdx1 and ngn3 had a
synergistic effect on glucagon gene expression, although the
expression levels were minimal and increased over time. The
combination of the three genes significantly increased the glucagon
gene expression levels (by 21-fold) at day 3, which decreased to
the basal level at day 7.
Heterologous expression of pdx1 has been observed to
induce insulin production in human bone marrow MSCs (8,25).
However, the expression levels of insulin were low and the
endogenous pdx1 gene was not activated in these studies. In the
present study, endogenous pdx1 was activated by the heterologous
expression of mouse pdx1, ngn3 and mafa. However, the combination
of any two of these genes induced lower expression levels of pdx1
than those induced by the individual genes alone, suggesting there
are antagonistic activities between the two genes that affect pdx1
expression when transduced into HUMSCs. At day 3, the combination
of all three genes effectively activated pdx1 expression (the
levels increased by 15-fold), which decreased by day 7.
In addition, nkx2.2 also induces endocrine
differentiation and is controlled by alternative promoters at
different cellular stages (26).
The results of the present study demonstrated that the expression
of the nkx2.2 gene was also significantly increased by ngn3 at day
3. Expression of the Nkx2.2 gene was also significantly increased
by Pdx1 at day 7 (Fig. 7).
In conclusion, this study demonstrated that the
expression of pdx1, ngn3 and mafa induced a marked change in the
gene expression profile of HUMSCs towards an early pancreatic
phenotype. The combination of pdx1, ngn3 and mafa activated
glucagon and pdx1 in HUMSCs, with the highest expression at day 3.
In addition, ngn3 alone induced nkx2.2 expression. Different
combinations of the exogenous TFs had either synergistic or
antagonistic effects, and the expression of endogenous genes varied
with time. This study demonstrated that HUMSCs were induced to
express glucagon, the predominant hormone of α-cells. This may
suggest that HUMSCs are able to be induced to differentiate into
pancreatic endocrine cells.
Acknowledgements
This study was supported by the Science and
Technology Planning Project of Guangdong Province, China (grant
nos. 2010B031600273, 2008B030301237 and 2006B36005023); the Science
and Technology Planning Project of Shantou, China [grant nos.
E200900137 and E201100373]; the National Natural Science Foundation
of China (grant no. 81070478); and the Foundation of Department of
Health, Guangdong, China (grant no. B2010223).
References
|
1
|
Weiss ML, Medicetty S, Bledsoe AR, et al:
Human umbilical cord matrix stem cells: preliminary
characterization and effect of transplantation in a rodent model of
Parkinson’s disease. Stem Cells. 24:781–792. 2006.PubMed/NCBI
|
|
2
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton’s jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004.
|
|
3
|
Matsushita T, Elliger S, Elliger C, et al:
Adeno-associated virus vectors can be efficiently produced without
helper virus. Gene Ther. 5:938–945. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McMahon JM, Conroy S, Lyons M, et al: Gene
transfer into rat mesenchymal stem cells: a comparative study of
viral and nonviral vectors. Stem Cells Dev. 15:87–96. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacKenzie KL, Hackett NR, Crystal RG and
Moore MA: Adenoviral vector-mediated gene transfer to primitive
human hematopoietic progenitor cells: assessment of transduction
and toxicity in long-term culture. Blood. 96:100–108.
2000.PubMed/NCBI
|
|
6
|
Brokhman I, Pomp O, Fishman L, et al:
Genetic modification of human embryonic stem cells with adenoviral
vectors: differences of infectability between lines and correlation
of infectability with expression of the coxsackie and adenovirus
receptor. Stem Cells Dev. 18:447–456. 2009. View Article : Google Scholar
|
|
7
|
Heremans Y, Van De Casteele M, in’t Veld
P, et al: Recapitulation of embryonic neuroendocrine
differentiation in adult human pancreatic duct cells expressing
neurogenin 3. J Cell Biol. 159:303–312. 2002. View Article : Google Scholar
|
|
8
|
Karnieli O, Izhar-Prato Y, Bulvik S and
Efrat S: Generation of insulin-producing cells from human bone
marrow mesenchymal stem cells by genetic manipulation. Stem Cells.
25:2837–2844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soyer J, Flasse L, Raffelsberger W, et al:
Rfx6 is an Ngn3-dependent winged helix transcription factor
required for pancreatic islet cell development. Development.
137:203–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Q, Brown J, Kanarek A, et al: In vivo
reprogramming of adult pancreatic exocrine cells to beta-cells.
Nature. 455:627–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guz Y, Montminy MR, Stein R, et al:
Expression of murine STF-1, a putative insulin gene transcription
factor, in beta cells of pancreas, duodenal epithelium and
pancreatic exocrine and endocrine progenitors during ontogeny.
Development. 121:11–18. 1995.
|
|
12
|
Sharma A, Fusco-DeMane D, Henderson E, et
al: The role of the insulin control element and RIPE3b1 activators
in glucose-stimulated transcription of the insulin gene. Mol
Endocrinol. 9:1468–1476. 1995.PubMed/NCBI
|
|
13
|
Wilson LM, Wong SH, Yu N, et al: Insulin
but not glucagon gene is silenced in human pancreas-derived
mesenchymal stem cells. Stem Cells. 27:2703–2711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawasaki H, Mizuguchi T, Oshima H, et al:
Efficient transformation of small hepatocytes into
insulin-expressing cells by forced expression of Pdx1. J
Hepatobiliary Pancreat Surg. 15:403–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma L, Feng XY, Cui BL, et al: Human
umbilical cord Wharton’s Jelly-derived mesenchymal stem cells
differentiation in to nerve-like cells. Chinese Med J (Engl).
118:1987–1993. 2005.
|
|
16
|
Matsuoka TA, Kaneto H, Stein R, et al:
MafA regulates expression of genes important to islet beta-cell
function. Mol Endocrinol. 21:2764–2774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Artner I, Le Lay J, Hang Y, et al: MafB:
an activator of the glucagon gene expressed in developing islet
alpha- and beta-cells. Diabetes. 55:297–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Otonkoski T, Beattie GM, Mally MI, et al:
Nicotinamide is a potent inducer of endocrine differentiation in
cultured human fetal pancreatic cells. J Clin Invest. 92:1459–1466.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohgawara H, Kawamura M, Honda M, et al:
Reversal of glucose insensitivity of pancreatic B-cells due to
prolonged exposure to high glucose in culture: effect of
nicotinamide on pancreatic B-cells. Tohoku J Exp Med. 169:159–166.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Jiang W, Liu M, et al: Highly
efficient differentiation of human ES cells and iPS cells into
mature pancreatic insulin-producing cells. Cell Res. 19:429–438.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sussel L, Kalamaras J, Hartigan-O’Connor
DJ, et al: Mice lacking the homeodomain transcription factor Nkx2.2
have diabetes due to arrested differentiation of pancreatic beta
cells. Development. 125:2213–2221. 1998.PubMed/NCBI
|
|
22
|
Matsuoka TA, Zhao L, Artner I, et al:
Members of the large Maf transcription family regulate insulin gene
transcription in islet beta cells. Mol Cell Biol. 23:6049–6062.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weiss ML, Anderson C, Medicetty S, et al:
Immune properties of human umbilical cord Wharton’s jelly-derived
cells. Stem Cells. 26:2865–2874. 2008.
|
|
24
|
Ritz-Laser B, Gauthier BR, Estreicher A,
et al: Ectopic expression of the beta-cell specific transcription
factor Pdx1 inhibits glucagon gene transcription. Diabetologia.
46:810–821. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Maechler P, Ritz-Laser B, et al:
Pdx1 level defines pancreatic gene expression pattern and cell
lineage differentiation. J Biol Chem. 276:25279–25286. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watada H, Scheel DW, Leung J and German
MS: Distinct gene expression programs function in progenitor and
mature islet cells. J Biol Chem. 278:17130–17140. 2003. View Article : Google Scholar : PubMed/NCBI
|