Introduction
Ketamine is a classical anesthetic agent that is
widely used in clinical surgery. Recently, it has been validated by
mounting studies demonstrating that ketamine has a robust
therapeutic effect for the treatment of depression (1–5).
However, the underlying mechanisms involved remain to be
elucidated.
At present, a large number of theories regarding the
underlying mechanisms of depression have been reported, but no
study has elucidated the mechanisms clearly. It has been shown that
the expression of pro-inflammatory cytokines in the plasma of
depressed patients is significantly increased as compared with
controls (6–8). Moreover, several lines of evidence
have indicated that the expression of pro-inflammatory cytokines in
the peripheral blood of patients tends to return to normal levels
after treatment with antidepressant agents (9,10).
Several lines of evidence have indicated that a single dose of
lipopolysaccharide (LPS) administered intraperitoneally in rodents
is capable of eliciting depressive-like behavioral deficits
(11,12). Collectively, these findings
indicate that inflammatory cytokines are most likely implicated in
the pathogenesis of depression.
Ketamine is often recommended for use in surgery in
sepsis patients due to its anti-inflammatory effects (13,14).
However, to date, no information has been available in the
literature on the association between the antidepressant effects of
ketamine and the expression of inflammatory cytokines in brain
tissue. Therefore, based on this point, we considered whether the
modulation of inflammatory cytokines is the underlying mechanism
involved in exerting the antidepressant effects of ketamine, and
furthermore hypothesized that the changes in inflammatory cytokines
were most likely implicated in the underlying mechanisms of
ketamine-elicited therapeutic effects for depression (15). Thus, in the present study, we
observed the effects of ketamine on LPS-induced depressive-like
behavior, and determined the levels of IL-1β, IL-6 and IL-10 in the
prefrontal cortex in a rat model.
Materials and methods
Animals
Thirty male Wistar rats (200–300 g body weight) were
purchased from the Shanghai Animal Center, Shanghai, China. The
animals were housed 5 per cage with food and water available ad
libitum, and were maintained on a 12 h light/dark cycle (lights
on at 7:00 am). Animals were involved in this experiment in
accordance with the Guide for Care and Use of Laboratory Animals of
Nanjing University (Nanjing, Jiangsu, China). The study was
approved by the Ethics Committee of The Third Affiliated Hospital
of Soochow University (Changzhou, China).
Drugs and interventions
Ketamine was purchased from Gutian Pharmaceutical
Co. (Fujian, China). LPS was purchased from Sigma Co. (St. Louis,
MO, USA). Rats were randomly divided into three groups (n=10 each):
saline group (S group), LPS only group (L group) and LPS plus
ketamine group (LK group). All rats in the three groups were forced
to swim for 15 min on the first day. Twenty-two hours later, rats
were intraperitoneally injected with saline or LPS (1 mg/kg). One
hour later, rats were intraperitoneally injected with saline or
ketamine (10 mg/kg) in the same volume. Another 1 h later, the
forced swimming test (FST) was carried out for 5 min and the
immobility time was recorded. Immediately after the FST, the rats
were sacrificed and the prefrontal cortex was dissected and stored
at −80°C for biochemical analyses.
FST
In accordance with a previous study (16), this test included two individual
stimuli to a cylindrical tank with water in which the rats cannot
touch the bottom of the tank. The volume of the tank was 60 cm
tall, 30 cm in diameter, and was filled with water to a depth of 40
cm. Water in the tank was changed after the testing of every rat.
All animal behavioral observation and other procedures were carried
out between 9:00 am and 15:00 pm. First, rats were placed in the
water for 15 min without exposure to any drug. Twenty-four hours
later, rats were treated again as in the first test for a 5 min
session, and the immobility time was recorded. The definition of
immobility in the FST was that the rat remained floating in the
water without struggling and only made movements necessary to keep
its head above the water. Assessment of immobility time was carried
out by the same trained observer.
Testing IL-1β levels
The prefrontal cortex was dissected and homogenized
in a solution containing 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM
EDTA, 1X protease inhibitor cocktail, 5 mM NaF and 1 mM sodium
vanadate. The homogenate was centrifuged for 10 min at 870 × g at
4°C. The pellet (nuclear fraction) contained nuclei and large cell
debris. The supernatant was centrifuged at 16,000 × g for 10 min.
After centrifugation, the supernatant (cytosolic fraction) was
removed and the pellet (crude synaptosomal fraction) was
resuspended and sonicated in protein lysis buffer [50 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM
NaVO3, 5 mM NaF and 1X protease inhibitor cocktail].
Protein concentration was determined by ABC protein assay. For
western blotting, equal amounts of proteins (10–20 μg) for each
sample were loaded into 10–15% SDS PAGE gels for electrophoresis.
Polyvinylidene difluoride (PVDF) membranes (Nanjing Jiancheng
Bioengineering Company, Nanjing, China) with transferred proteins
were blocked with 2% BSA (Equitech-Bio, Inc., Kerrville, TX, USA)
in PBST phosphate buffer solution [PBS (Sigma-Aldrich Canada,
Oakville, ON, Canada) + 0.1% Tween-20] for 1 h and kept with
primary antibodies overnight at 4°C. The primary antibody
(anti-IL-1β) was used. The next day, blots were washed three times
in PBST and incubated with horseradish peroxidase-conjugated
anti-mouse or anti-rabbit secondary antibody (1:5,000–1:10,000) for
1 h. After three final washes with PBST, bands were detected using
enhanced chemiluminescence (ECL; Beyotime Company, Nantong, China).
The blots were then incubated in stripping buffer (2% SDS, 100 mM
β-mercaptoethanol, 50 mM Tris pH 6.8) for 30 min at 50–55°C
followed by three washes with PBST. The stripped blots were kept in
blocking solution for 1 h and incubated with the primary antibody
directed against total levels of β-actin as the loading control.
Densitometric analysis of immunoreactivity for each protein was
conducted using Image J software (National Institutes of Health,
Bethesda, MD, USA).
Testing IL-6 and IL-10 levels
IL-6 and IL-10 levels in the prefrontal cortex were
individually measured by anti-IL-6 and anti-IL-10 sandwich-ELISA
according to the manufacturer’s instructions (Chemicon, Billerica,
MA, USA). Rat hippocampus was homogenized in PBS with 1 mM
phenylmethylsulfonyl fluoride (PMSF) and 1 mM EGTA. Microtiter
plates (48-well flat-bottom) were coated for 24 h with the samples
diluted 1:2 in sample diluent and the standard curve ranged from
7.8 to 500 pg/ml of IL-6 and IL-10. The plates were then washed
four times with sample diluent and a monoclonal anti-IL-6 and
anti-IL-10 rabbit antibody diluted 1:1,000 in sample diluent was
added to each well and incubated for 3 h at room temperature. After
washing, a peroxidase-conjugated anti-rabbit antibody (diluted
1:1,000) was added to each well and incubated at room temperature
for 1 h. After addition of streptavidin-enzyme, substrate and stop
solution, the amount of IL-6 and IL-10 were determined by
absorbance at 450 nm, respectively. The standard curve demonstrates
a direct relationship between optical density (OD) and IL-6 or
IL-10 concentration. Total protein was measured by Lowry’s method
using bovine serum albumin as a standard.
Statistical analysis
Data are expressed as the means ± SD. Statistical
analyses were made by one-way analysis of variance (ANOVA) and post
hoc analyses were performed by least significant difference (LSD)
tests. These statistical analyses were conducted by Statistical
Product for Social Sciences (SPSS version 17.0; SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant result.
Results
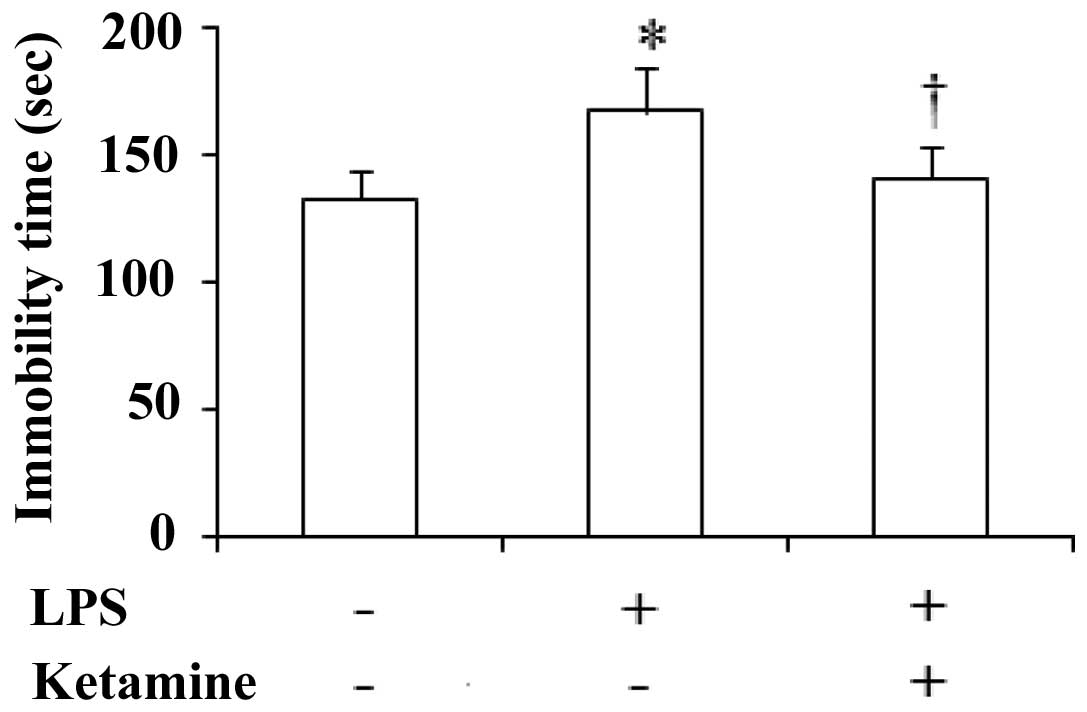
Immobility time of rats in the FST
The immobility time of the rats during the FST
showed significant differences among the three groups
[F(2,27)=16.44, P<0.05]. Administration of LPS only showed a
significant increase in the immobility time as compared with saline
only (P<0.05). Moreover, administration of LPS plus ketamine
significantly increased the immobility time as compared with LPS
only (P<0.05; Fig. 1).
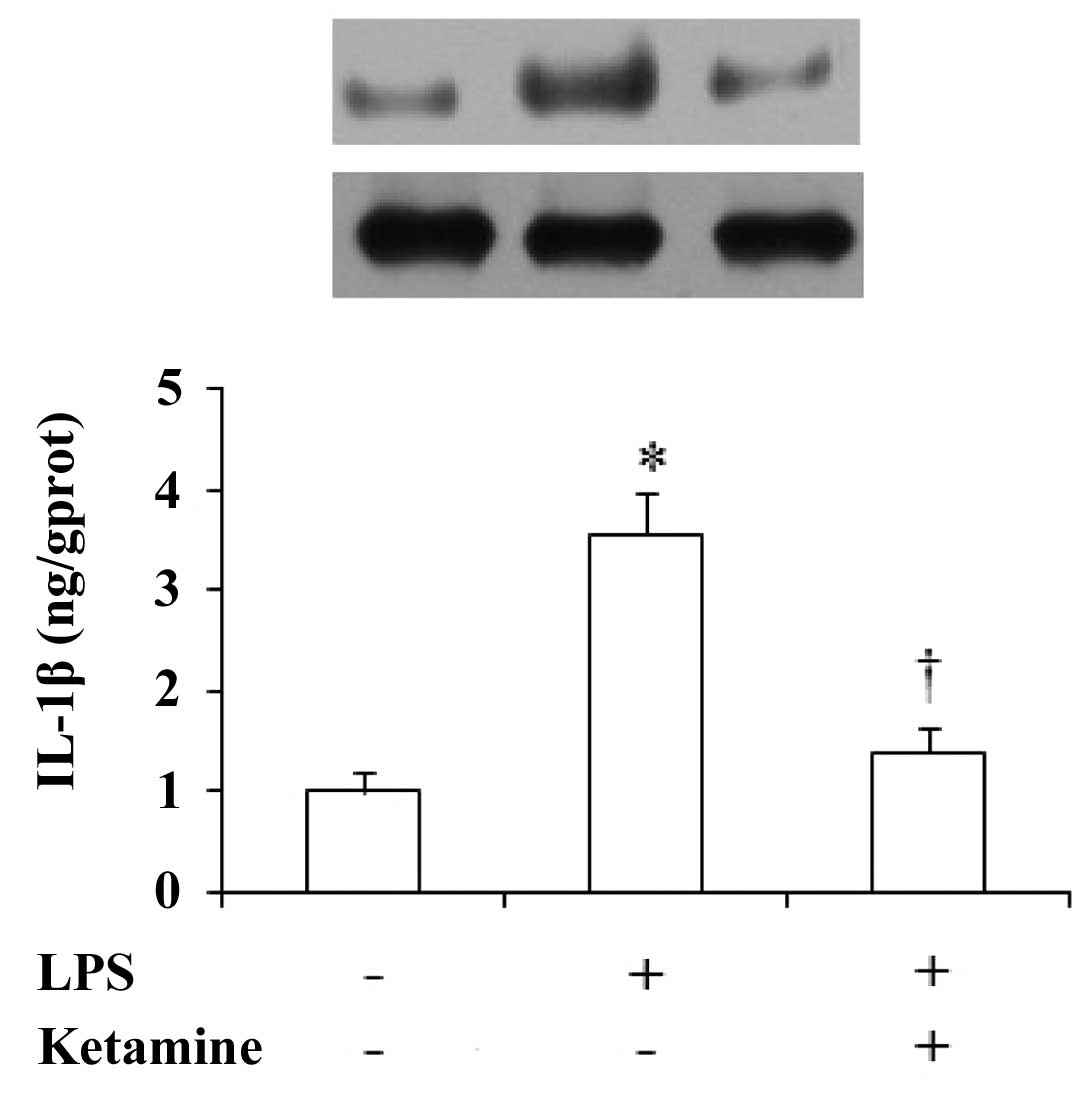
Expression of IL-1β in the rat prefrontal
cortex
The expression of IL-1β in the rat prefrontal cortex
showed significant differences among these groups [F(2,27)=32.13,
P<0.01]. Compared with saline only, administration of LPS only
showed a significant increase in the IL-1β levels (P<0.01),
while administration of LPS plus ketamine significantly decreased
the expression of IL-1β as compared with LPS only (P<0.01;
Fig. 2).
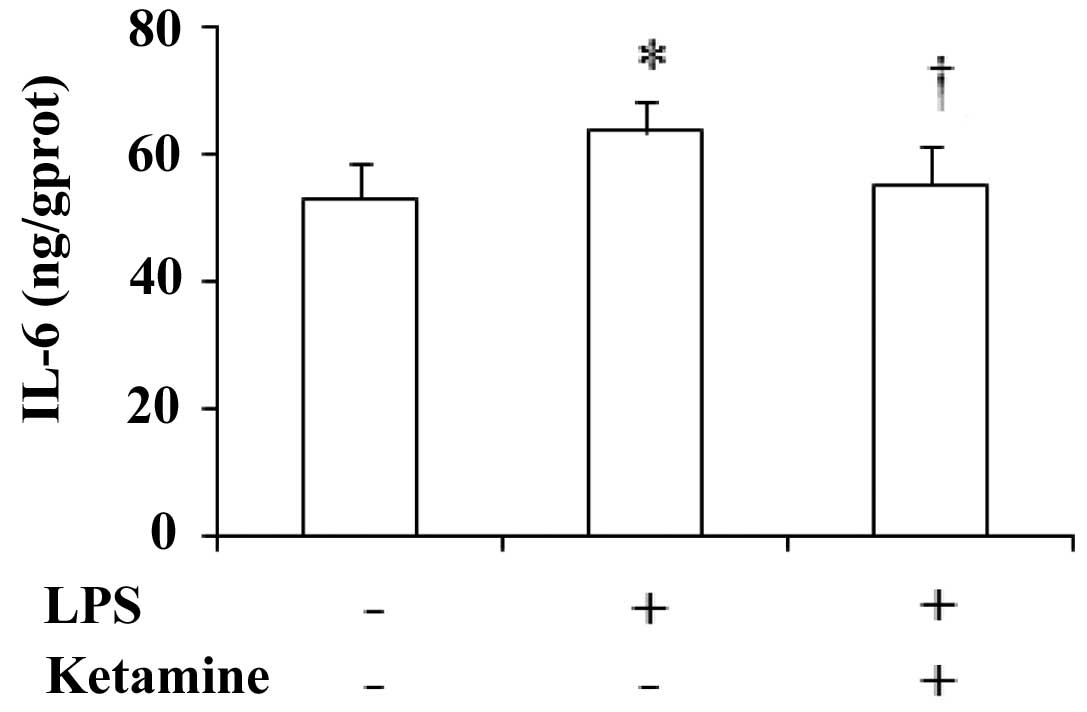
Expression of IL-6 in the rat prefrontal
cortex
The expression of IL-6 in the rat prefrontal cortex
showed significant differences among these groups [F(2,27)=8.36,
P<0.05]. Compared with saline only, administration of LPS only
showed a significant increase in the IL-6 levels (P<0.05).
Compared with LPS only, administration of LPS plus ketamine
significantly decreased the IL-6 levels (P<0.05; Fig. 3).
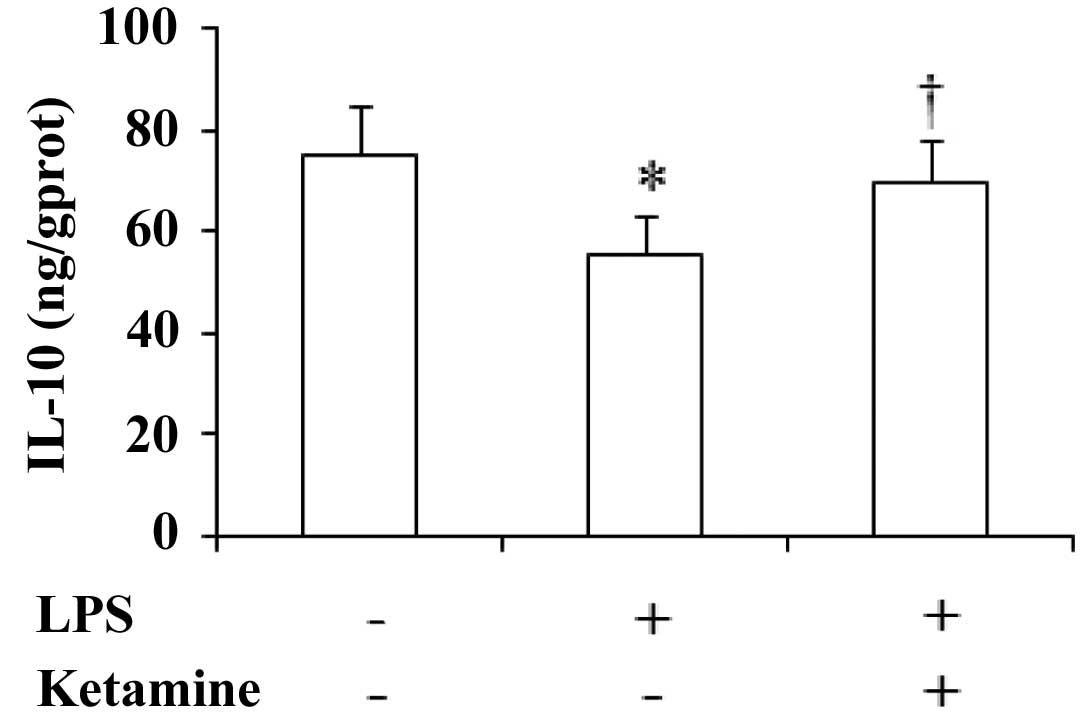
Expression of IL-10 in the rat prefrontal
cortex
The expression of IL-10 in the rat prefrontal cortex
showed significant differences among the three groups
[F(2,27)=6.83, P<0.05]. Compared with saline only,
administration of LPS only showed a significant decrease in the
IL-10 levels (P<0.05), while administration of LPS plus ketamine
significantly increased the IL-10 levels as compared with LPS only
(P<0.05; Fig. 4).
Discussion
The results of the present study demonstrated that
ketamine significantly attenuated the LPS-induced increase of the
immobility time of rats during the FST, and significantly decreased
the expression of IL-1β and IL-6 in the rat prefrontal cortex. The
results also showed downregulated expression of IL-10 in the rat
prefrontal cortex. These findings indicate that ketamine has
antidepressant effects in the rat model of LPS-induced
depressive-like behavior. Li et al(5) have reported that ketamine
administered at a sub-anesthetic dose of 10 mg/kg exerts
antidepressant effects in rats during the FST. Therefore, we used
ketamine at a dose of 10 mg/kg to observe its antidepressant effect
in the present study, and the result was consistent with the
previous findings (5). The only
difference in the present study is that we adopted a novel
depressive-like rat model, which was induced by intraperitoneal
injection of LPS. Although the antidepressant effect of ketamine
has been reported and validated in previous studies, the present
study is the first to elucidate its effect for the treatment of
depression in the LPS-induced depressive-like rat model (1,3,4).
LPS is an element in the Gram-negative bacterial
wall that may cause fever, microcirculation disturbance, shock and
disseminated intravascular coagulation (17,18).
It has been shown that LPS has the potential to induce
depressive-like behavior in a rat model (19). In this study, we observed that LPS
administered at a dose of 1 mg/kg significantly increased the
immobility time of rats during the FST, which suggested that the
depressive-like rat model was successfully constructed. However,
Kang et al(20) have
indicated that mice exhibit depressive-like manifestations after
intraperitoneal injection of LPS at the dose of 0.8 mg/kg. The
discrepancy between the administration doses of LPS is most likely
due to the species difference. Moreover, another study carried out
by Hosseini et al(11)
using LPS at the dose of 100 μg/kg induced depressive-like
behavioral deficits in Wistar rats, but we did not get the same
result in our pilot experiment (data not shown).
Ketamine is widely used for surgery in patients with
sepsis due to its anti-inflammatory effect. A previous study has
reported that ketamine has a fast-acting anti-inflammatory effect
(21). Moreover, ketamine exerts a
rapid antidepressant effect within 1–2 h after administration. The
coincidence of the fast-acting antidepressant and anti-inflammatory
effects suggests that modulating inflammatory cytokines are likely
to participate in the antidepressant effect of ketamine. As
mentioned previously, pro-inflammatory cytokines were reversed to
normal levels after treatment with antidepressant agents (9,10).
Therefore, we demonstrated that LPS-induced behavioral deficits and
changes in expression of inflammatory cytokines were reversed after
ketamine administration. Consequently, the hypothesis we proposed
was verified in the present study.
Given the preclinical and clinical data indicating
elevations in IL-1β and IL-6 levels in plasma and/or the brain of
patients or animals with depression (8,19),
we were intrigued to evaluate IL-1β and IL-6 protein levels in the
prefrontal cortex in a rat model demonstrating depressive-like
behaviors following LPS exposure. Notably, analysis of IL-1β and
IL-6 levels after ketamine treatment alleviated LPS-induced
depressive-like behavior and was associated with the downregulation
of IL-1β and IL-6 levels. These results as well as the changed
expression of IL-10 revealed that these three important
inflammatory cytokines were implicated in the mechanisms involved
in the antidepressant effect of ketamine. Furthermore, the
mediation of the inflammatory pathway in the pathogenesis and the
therapeutic mechanisms of depression has been validated again in
this study.
However, a limitation for this study is that we did
not conduct a ketamine only group, since ketamine only was not
suitable for the study design and the result has been observed in
our previous studies (22,23). In conclusion, the antidepressant
effect of ketamine was associated with the modulation of
inflammatory cytokines. Future studies are required to further
elucidate the relationship between the inflammation pathway and the
antidepressant effect of ketamine.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30872424).
References
|
1
|
Aan Het Rot M, Zarate CA Jr, Charney DS
and Mathew SJ: Ketamine for depression: where do we go from here?
Biol Psychiatry. 72:537–547. 2012.PubMed/NCBI
|
|
2
|
Mathew SJ, Shah A, Lapidus K, et al:
Ketamine for treatment-resistant unipolar depression: current
evidence. CNS Drugs. 26:189–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machado-Vieira R, Salvadore G,
Diazgranados N and Zarate CA Jr: Ketamine and the next generation
of antidepressants with a rapid onset of action. Pharmacol Ther.
123:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krystal JH: Ketamine and the potential
role for rapid-acting antidepressant medications. Swiss Med Wkly.
137:215–216. 2007.PubMed/NCBI
|
|
5
|
Li N, Lee B, Liu RJ, et al: mTOR-dependent
synapse formation underlies the rapid antidepressant effects of
NMDA antagonists. Science. 329:959–964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catena-Dell’Osso M, Bellantuono C, Consoli
G, Baroni S, Rotella F and Marazziti D: Inflammatory and
neurodegenerative pathways in depression: a new avenue for
antidepressant development? Curr Med Chem. 18:245–255.
2011.PubMed/NCBI
|
|
7
|
Hayley S, Poulter MO, Merali Z and Anisman
H: The pathogenesis of clinical depression: stressor- and
cytokine-induced alterations of neuroplasticity. Neuroscience.
135:659–678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raedler TJ: Inflammatory mechanisms in
major depressive disorder. Curr Opin Psychiatry. 24:519–525.
2011.PubMed/NCBI
|
|
9
|
De Berardis D, Conti CM, Serroni N, et al:
The effect of newer serotonin-noradrenalin antidepressants on
cytokine production: a review of the current literature. Int J
Immunopathol Pharmacol. 23:417–422. 2010.PubMed/NCBI
|
|
10
|
Song C and Wang H: Cytokines mediated
inflammation and decreased neurogenesis in animal models of
depression. Prog Neuropsychopharmacol Biol Psychiatry. 35:760–768.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hosseini M, Zakeri S, Khoshdast S, et al:
The effects of Nigella sativa hydro-alcoholic extract and
thymoquinone on lipopolysaccharide-induced depression like behavior
in rats. J Pharm Bioallied Sci. 4:219–225. 2012.
|
|
12
|
Park SE, Lawson M, Dantzer R, Kelley KW
and McCusker RH: Insulin-like growth factor-I peptides act
centrally to decrease depression-like behavior of mice treated
intraperitoneally with lipopolysaccharide. J Neuroinflammation.
8:1792011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loix S, De Kock M and Henin P: The
anti-inflammatory effects of ketamine: state of the art. Acta
Anaesthesiol Belg. 62:47–58. 2011.PubMed/NCBI
|
|
14
|
Sun J, Zhou ZQ, Lv R, Li WY and Xu JG:
Ketamine inhibits LPS-induced calcium elevation and NF-kappa B
activation in monocytes. Inflamm Res. 53:304–308. 2004.PubMed/NCBI
|
|
15
|
Yang JJ, Zhou ZQ and Yang C: Letter to the
editor: does ketamine exert a fast-acting antidepressant effect via
inhibition of pro-inflammatory cytokines? Psychol Med. 41:17872011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porsolt RD, Le Pichon M and Jalfre M:
Depression: a new animal model sensitive to antidepressant
treatments. Nature. 266:730–732. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herzum I and Renz H: Inflammatory markers
in SIRS, sepsis and septic shock. Curr Med Chem. 15:581–587. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biswas SK and Lopez-Collazo E: Endotoxin
tolerance: new mechanisms, molecules and clinical significance.
Trends Immunol. 30:475–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dunn AJ, Swiergiel AH and de Beaurepaire
R: Cytokines as mediators of depression: what can we learn from
animal studies? Neurosci Biobehav Rev. 29:891–909. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang A, Hao H, Zheng X, et al: Peripheral
anti-inflammatory effects explain the ginsenosides paradox between
poor brain distribution and anti-depression efficacy. J
Neuroinflammation. 8:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniguchi T and Yamamoto K:
Anti-inflammatory effects of intravenous anesthetics on
endotoxemia. Mini Rev Med Chem. 5:241–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang C, Li WY, Yu HY, et al: Tramadol
pretreatment enhances ketamine-induced antidepressant effects and
increases mammalian target of rapamycin in rat hippocampus and
prefrontal cortex. J Biomed Biotechnol. 2012:1756192012. View Article : Google Scholar
|
|
23
|
Yang C, Hong T, Shen J, et al: Ketamine
exerts antidepressant effects and reduces IL-1β and IL-6 levels in
rat prefrontal cortex and hippocampus. Exp Ther Med. 5:1093–1096.
2013.PubMed/NCBI
|