Introduction
Melanin is important in protecting the skin from the
harmful effects of ultraviolet irradiation and absorbing toxic
drugs and chemicals. Melanin is synthesized in two predominant
forms, black-brown pigments (eumelanin) and red-yellow pigments
(pheomelanin), within the melanosomes of melanocytes by at least
three melanogenic enzymes. Tyrosinase (EC1.14.18.1) is one of the
predominant enzymes mediated by the melanin production process in
melanosomes (1). The enzyme
catalyzes the rate-limiting step of the hydroxylation of tyrosine
to dihydroxyphenylalanine (DOPA) and its further oxidation to
DOPA-quinone (2). Melanin
synthesis is maintained by a number of regulatory processes that
act at various steps during the synthesis of the protein in the
endoplasmic reticulum (ER), Golgi body and melanosomes, and
disorders in any of these processes lead to abnormal
pigmentation.
Malignant melanoma (MM) cells are derived from
epidermal melanocytes and nevi (3), and numerous cultured melanoma cell
lines have been established from human and mouse MMs. The majority
of MMs produce melanin, and the degree of melanin synthesis differs
for each type of cultured cell line (4–6). For
example, MNT-1 cells were demonstrated to be highly pigmented MMs
(4); however, SK-Mel-28 cells were
not able to produce melanin due to a mutation in the endocytic
pathway that affected the endosomal trafficking of tyrosinase
(5). In addition, B16F10 cells
were pigmented; whereas amelanotic melanoma was not pigmented, due
to the increased expression of proteasome subunit p27 (6).
In this study, three cultured cell lines, MNT-1,
HM3KO and G-361, were observed. These cell lines differed in their
degrees of melanin synthesis, and thus, the determinants of the
degree of melanin synthesis were investigated.
Materials and methods
Cell culture
The MNT-1 (from Dr VJ Hearing, Laboratory of Cell
Biology, National Cancer Institute, National Institutes of Health,
Bethesda, MD, USA), HM3KO (7,8) and
G-361 (9) (supplied by the Health
Science Research Resources Bank, Osaka, Japan) cell lines were
cultured in Dulbecco’s modified Eagle’s medium (Life Technologies,
Carlsbad, CA, USA) or McCoy’s 5A medium (Sigma-Aldrich, St. Louis,
MO, USA) containing 10% fetal bovine serum, 100 U/ml penicillin, 50
μg/ml streptomycin, 50 μg/ml kanamycin and 2.5 μg/ml amphotericin B
(10). The study was approved by
the Ethics Committee of Hirosaki University, Hirosaki, Japan
Measurement of melanin production and
tyrosinase activity
Melanin production in each melanoma cell line was
measured according to the method proposed by Ando et
al(11) with certain
modifications. Approximately 106 cells were collected by
centrifugation at 8,000 × g for 5 min and washed twice with
phosphate-buffered saline (PBS), and then centrifuged at
2,000 xg for 5 min. The precipitate was then solubilized by
treatment with 1 ml 2 M NaOH in 10% dimethyl sulfoxide for 30 min
at 80°C in a capped test tube. The absorbance of melanin in the
supernatant was measured at 450 nm. Tyrosinase activity was
measured as the DOPA oxidase activity (12) with certain modifications.
Approximately 107 cells were collected by centrifugation
and washed twice with PBS. Following centrifugation, the
supernatant was decanted. Cells were suspended in ice-cold 0.1 M
PBS containing 1% Triton X-100 and 0.5 mM phenylmethylsulfonyl
fluoride (PMSF), and homogenized. The suspension was centrifuged
for 5 min at 4°C and 1,200 × g, and the supernatant was collected.
Tyrosinase activity was analyzed spectrophotometrically by
following the oxidation of DOPA to dopachrome at 475 nm.
Supernatant (90 μl) containing 100 μg crude protein was applied to
a 96-well microplate and 10 μl 0.1 M L-DOPA was added. Following
incubation for 1 h at 37°C, the absorbance was measured.
Western blot analysis
Each cultured cell line was suspended in extraction
buffer consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM
EDTA, 1% Triton X-100 and protease inhibitors (2 mM
N-ethylmaleimide, 50 mg/ml aprotinin, 50 mg/ml leupeptin and 50
mg/ml pepstatin), and homogenized. Following centrifugation, the
soluble protein in the crude extract was quantified according to
the method described by Asryants (13). Proteins were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis using 10% gels,
and blotted onto polyvinylidene fluoride membranes. Primary
antibodies, anti-tyrosinase and anti-Hsp70 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), as well as anti-actin
(Thermo Fisher Scientific, Waltham, MA, USA), were diluted at
1:1000 in 0.5% bovine serum albumin/Tris-buffered saline and Tween
20, respectively. The horseradish-peroxidase-conjugated secondary
antibody (Santa Cruz Biotechnology, Inc.) was used and
antigen-antibody complexes were detected by the chemiluminescence
method, using an enhanced chemiluminescence kit (GE Healthcare UK,
Ltd., Buckinghamshire, UK) and X-ray film (FujiFilm Corp., Ltd.,
Tokyo, Japan).
Treatment of melanoma by glycosylation
inhibitors, 1-deoxynojirimycin (DNJ) and D-mannojirimycin
(DMJ)
A total of 106 cells of MM were incubated
as described previously. After 24 h, DNJ and DMJ (14) (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA) were added to the culture at final
concentrations of 1 mM and incubated for an additional 4 days. The
treated cells were harvested and the cell-free extracts were
prepared for analysis.
Immunoprecipitation with anti-tyrosinase
antibody
A total of 100 mg cells of each cultured line was
suspended in 1 ml TNE buffer and homogenized for 30 sec at 4°C.
Protein-A Sepharose 4 Fast Flow (20 μl; GE Healthcare UK, Ltd.) was
added to 200 μl supernatant and the mixture was mixed briefly for 1
h at 4°C. The suspension was centrifuged at 19,000 × g for 10 min
and the supernatant was incubated with 10 μl anti-tyrosinase at 4°C
for ~20 h. Protein-A Sepharose 4 Fast Flow (20 μl) was added to the
mixture and mixed briefly at 4°C for 2 h. After 2 h, the mixture
was centrifuged at 14,000 rpm for 30 sec and the precipitate was
collected. The precipitate was washed five times with TNE buffer
[10 mM Tris-HCl (pH 7.8), 0.15 M NaCl, 1 mM EDTA, 1% NP-40 and 1 mM
PMSF] and the resulting precipitate was subjected to western blot
analysis with Hsp70 antibody. To compare the quantity of Hsp70
associated with anti-tyrosinase among the three cultured cell
lines, the signal intensity was analyzed using Scion image software
(Scion Corp., National Institutes of Health, Frederick, MD, USA)
and the ratios of each numerical value measured in the sample with
the immunoprecipitation treatment to that without the treatment was
determined.
Results
Melanin production and tyrosinase
activity in the three melanoma cell lines
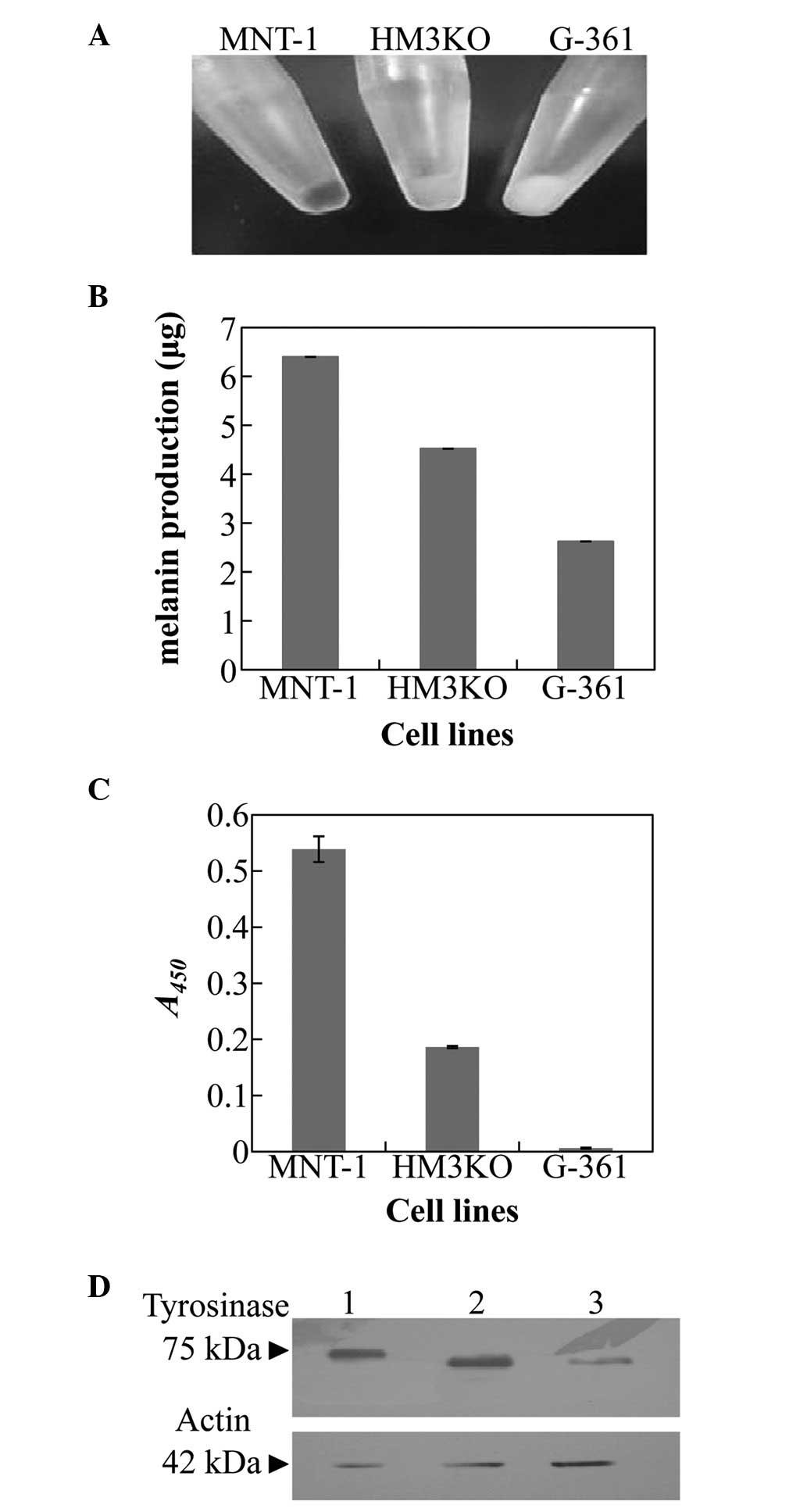
The external appearance of each harvested cell line
is shown in Fig. 1A. The melanin
production and tyrosinase activity was demonstrated from the data
for each harvested cell line and were greatest in the MNT-1 cells,
lower in the HM3KO cells and lowest in the G-361 cells (Fig. 1B and C). Western blot analysis
revealed that the molecular masses of HM3KO- and G-361-derived
tyrosinase were slightly lower than that derived from MNT-1 cells,
and the production level of tyrosinase was greatest in the MNT-1
cells, lower in the HM3KO cells and lowest in the G-361 cells
(Fig. 1D).
Treatment of melanoma cell lines by
glycosylation inhibitors, DNJ and DMJ
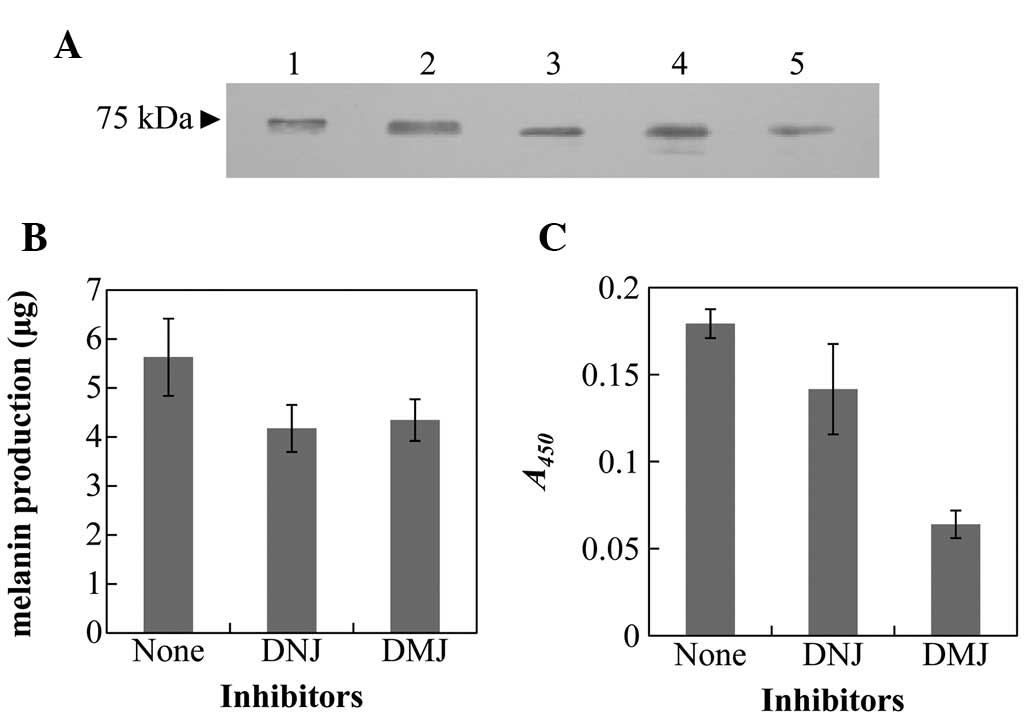
A melanoma cell line, MNT-1, was treated with DNJ
and DMJ, which are protein glycosylation inhibitors. Western blot
analysis using tyrosinase antibodies demonstrated differences in
the molecular masses of the tyrosinase treated with each inhibitor
(Fig. 2A). The molecular mass of
the tyrosinase treated with DMJ was approximately that of the HM3KO
and G-361 cells. The melanin production and tyrosinase activity of
those cells significantly decreased following the treatments, and
were lower than those of untreated MNT-1 cells (Fig. 3B and C).
Immunoprecipitation of Hsp70 with
tyrosinase antibodies
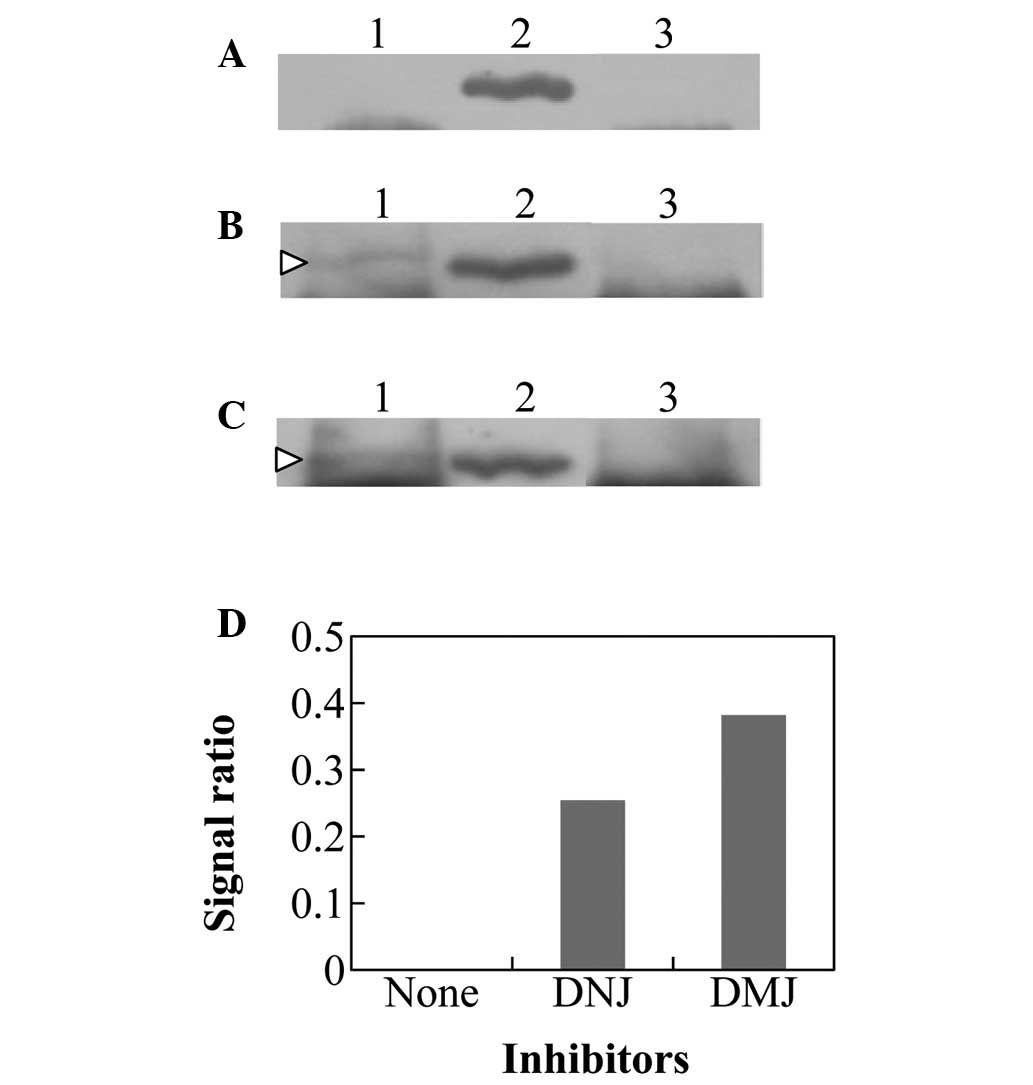
The crude extracts of MNT-1 treated with or without
the glycosylated inhibitors were immunoprecipitated with tyrosinase
antibody and subjected to western blot analysis with Hsp70 antibody
(Fig. 3). In the untreated MNT-1
cells, the reaction signal was almost unidentifiable (Fig. 3A and D). Conversely, the same
fractions in DNJ- and DMJ-treated MNT-1 cells were indicated to be
present in the immunoprecipitated complex of Hsp70 and tyrosinase
(Fig. 3B–D). These data suggested
that the misfolded tyrosinase resulting from the immature
glycosylation in DNJ- and DMJ-treated MNT-1 cells was transported
for other processes via Hsp70 and was not transported to the
melanosomes for melanin production.
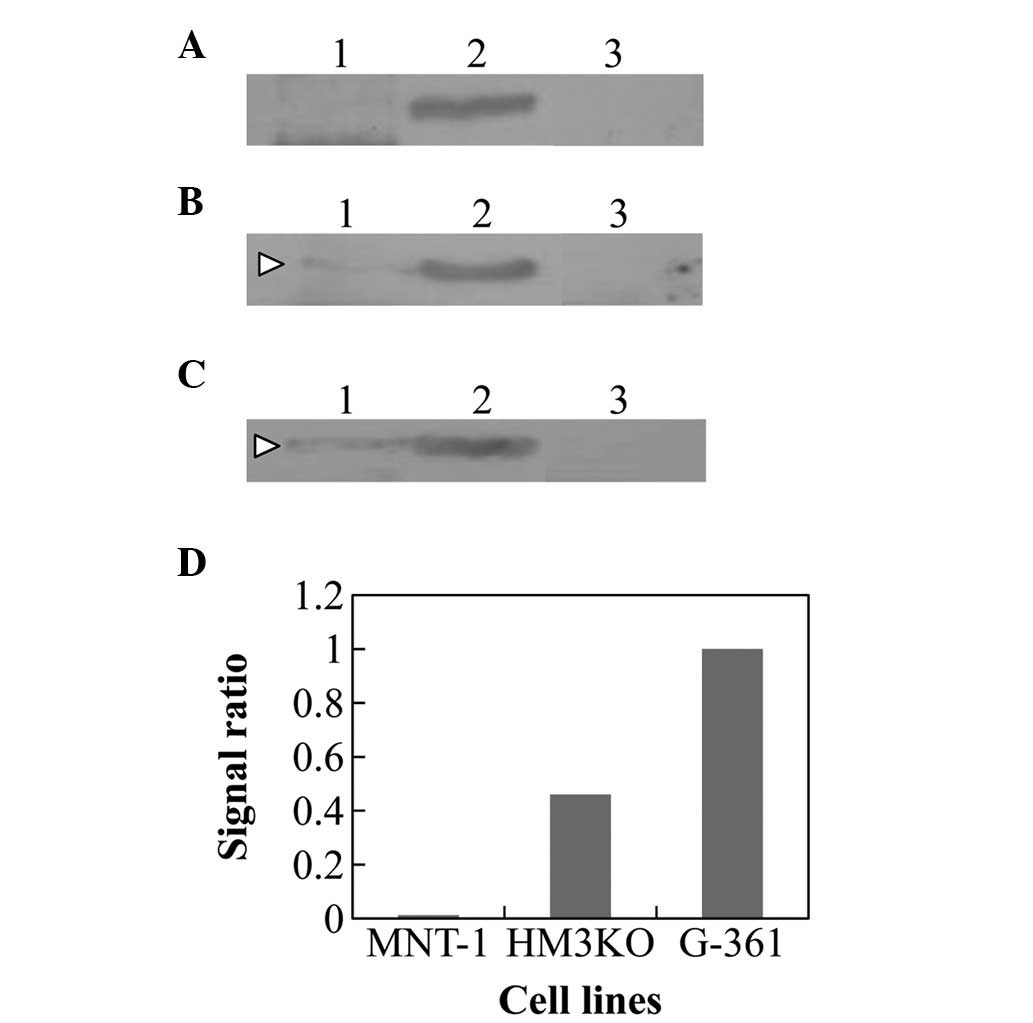
The Hsp70 immunoprecipitation in the extracts of
HM3KO and G-361 cells were examined using the tyrosinase antibody.
Although the reaction product was not recognized in MNT-1 cells,
the presence of the immunoprecipitated complex of Hsp70 and
tyrosinase was detected in the HM3KO and G-361 cells (Fig. 4B–D). The intensity of the
interaction was greater than that observed in the inhibitor-treated
MNT-1 cells (Fig. 3D and 4D).
Discussion
In the present study, three types of cultured MM
cell lines (MNT-1, HM3KO and G-361) were examined. It was
demonstrated that their melanin production levels differed from
each other, and a positive correlation between the melanin
production and tyrosinase activity was observed (Fig. 1A–C). The quantities of tyrosinase
were correlated with the melanin production and the tyrosinase
activity (Fig. 1D). The
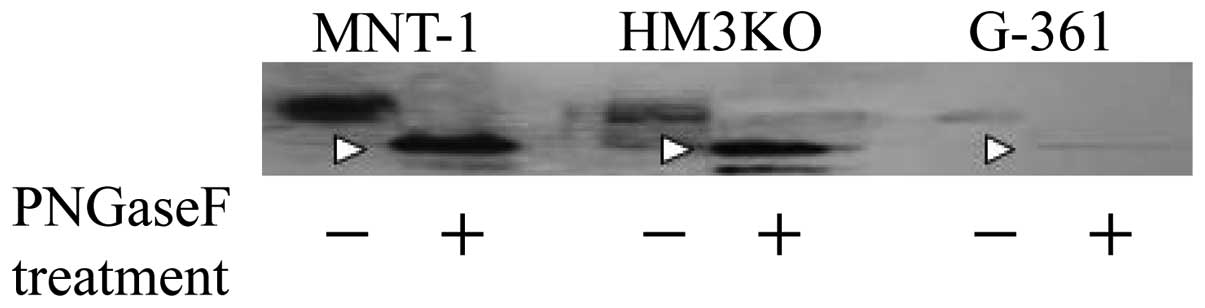
differences among the molecular masses of tyrosinases (Fig. 1D) almost disappeared as a result of
digestion with PNGase F (Fig. 5).
In addition, there were no significant differences in the mRNA
levels of tyrosinase among these cell lines (data not shown) and,
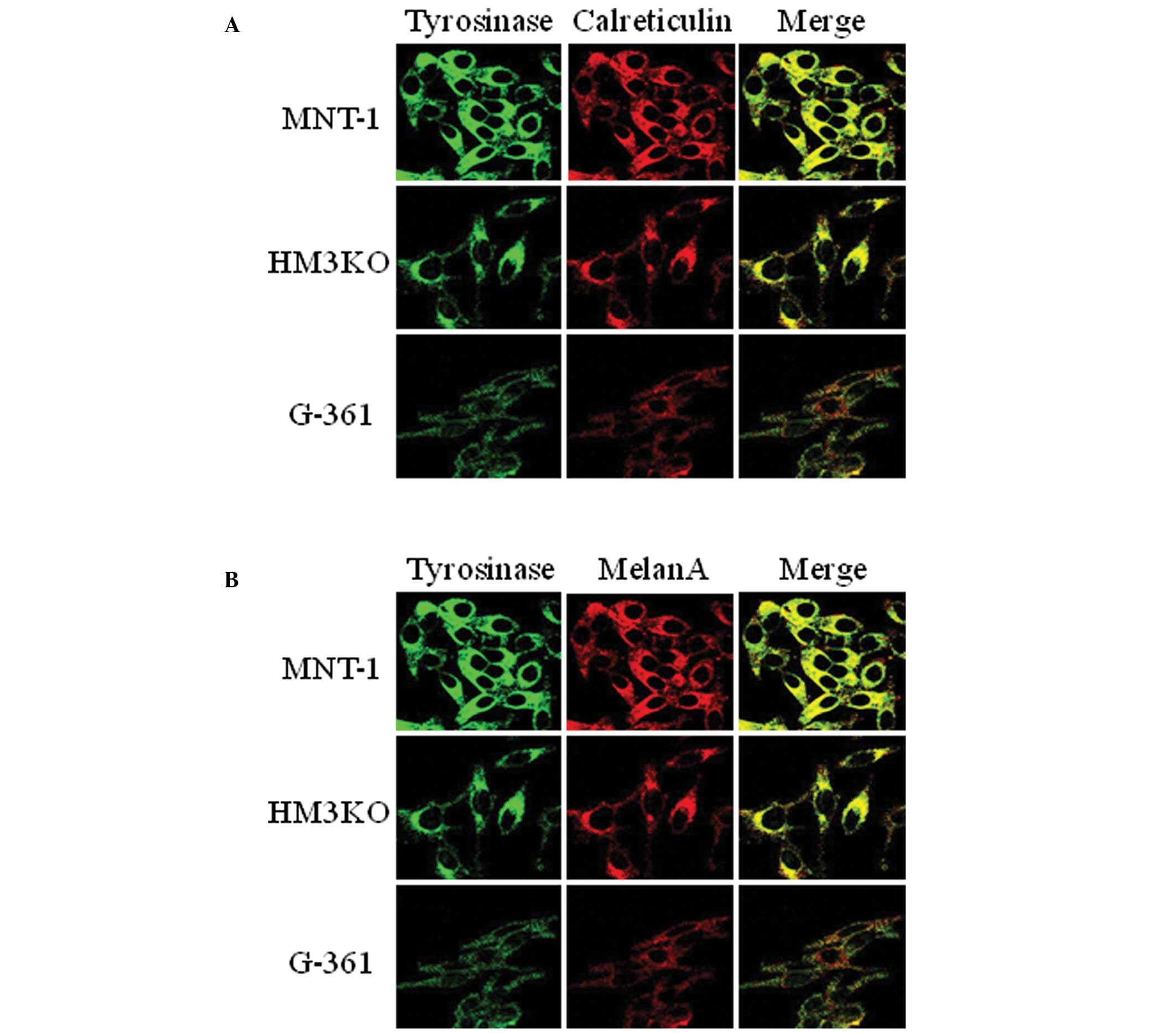
in each cell line, tyrosinase was present at the ER and in the
melanosome (Fig. 6). These results
suggested that the differences in the melanin production and the
tyrosinase activity among these cell lines were due to the
glycosylation of tyrosinase at a post-translational level. However,
the nucleotide sequence of cDNA encoding tyrosinase from G-361
cells (accession no. AB775901) had a nucleotide substitution that
was demonstrated to have a critical affect on pigmentation
(15). No mutations were observed
to be significantly correlated with melanin production and
tyrosinase activity in the nucleotide sequences of cDNA encoding
tyrosinase from MNT-1 (AB775899) and HM3KO (AB775900) cells. It is
possible that the differences measured in the melanin production
and the tyrosinase activity between MNT-1 and HM3KO cells were
mainly due to disorders in the glycosylation process of tyrosinase.
Although the decreases in melanin production and tyrosinase
activity in G-361 cells were caused by a mutation in its nucleotide
sequence, the decrease of the molecular mass of tyrosinase may have
been due to an abnormal glycosylation at a post-transcriptional
level.
Therefore, we evaluated the influence of the
abnormal glycosylation in tyrosinase production on the activity and
melanin production of MNT-1 cells using two glycosylation
inhibitors, DNJ and DMJ. The tyrosinases treated with each
inhibitor demonstrated differences in molecular mass. Furthermore,
melanin production and tyrosinase activity decreased due to DNJ and
DMJ treatment. These data suggested that it is difficult for MNT-1
to synthesize melanin with immature glycosylated tyrosinase. The
endogenous chaperone proteins and lectin-like proteins were
demonstrated to be important in maintaining accurate protein
maturation and degrading misfolded protein by translocation into
proteolytic pathways, such as the ubiquitin/proteasome system and
the ER-associated protein degradation pathway (16,17).
Therefore, in this study, it was further investigated whether the
chaperone protein was associated with the immature glycosylated
tyrosinase, using an immunoprecipitation assay. The extracts of
cells treated with each glycosylation inhibitor were
immunoprecipitated with anti-tyrosinase antibody and analyzed by
western blot analysis with anti-Hsp70 antibody (Fig. 4). In the non-treated MNT-1 cells,
the reaction signal was almost undetectable; however, the same
fractions in DNJ- and DMJ-treated MNT-1 cells were present in the
immunoprecipitated complex of Hsp70 and tyrosinase (Fig. 3). These data indicated that the
immature glycosylated tyrosinase reaction with the chaperone
protein, HSP70, and may have been transported into the proteolytic
pathways to maintain protein quality. In addition, each extract
from the HM3KO and G-361 cells was immunoprecipitated with
anti-tyrosinase antibody, and the reactions with anti-HSP70
antibody were also confirmed in the HM3KO and G-361 cells (Fig. 4). These tyrosinases associated with
Hsp70 were translocated to the proteolytic pathways, leading to
decreased activity and melanin production.
In this study, it was demonstrated that the
glycosylation of tyrosinase is a determinant of melanin production
in at least three types of cultured melanoma cells. Tyrosinase
expressed in MNT-1 cells exhibited accurate glycosylation, and
demonstrated enzyme activity and melanin production. HM3KO and
G-361 cells also expressed tyrosinase; however, the tyrosinase was
not well maintained in the active form.
Acknowledgements
This study was supported in part by a grant from
Hirosaki University (Hirosaki, Japan) for the promotion of
International Scientific Research.
References
|
1
|
Ando H, Kondoh H, Ichihashi M and Hearing
VJ: Approaches to identify inhibitors of melanin biosynthesis via
the quality control of tyrosinase. J Invest Dermatol. 127:751–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang N and Hebert DN: Tyrosinase
maturation through the mammalian secretory pathway: bringing color
to life. Pigment Cell Res. 19:3–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uong A and Zon LI: Melanocytes in
development and cancer. J Cell Physiol. 222:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kushimoto T, Basrur V, Valencia J,
Matsunaga J, Vieira WD, Ferrans VJ, Muller J, Appella E and Hearing
VJ: A model for melanosome biogenesis based on the purification and
analysis of early melanosomes. Proc Natl Acad Sci USA.
98:10698–10703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watabe H, Valencia JC, Le Pape E,
Yamaguchi Y, Nakamura M, Rouzaud F, Hoashi T, Kawa Y, Mizoguchi M
and Hearing VJ: Involvement of dynein and spectrin with early
melanosome transport and melanosomal protein trafficking. J Invest
Dermatol. 128:162–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Godbole D, Mojamdar M and Pal JK:
Increased level of p27 subunit of proteasomes and its
co-localization with tyrosinase in amelanotic melanoma cells
indicate its direct role in the regulation of melanin biosynthesis.
Cell Biol Int. 30:895–902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohashi A, Funasaka Y, Ueda M and Ichihashi
M: c-KIT receptor expression in cutaneous malignant melanoma and
benign melanotic naevi. Melanoma Res. 6:25–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohshima Y, Yajima I, Takeda K, Iida M,
Kumasaka M, Matsumoto Y and Kato M: c-RET molecule in malignant
melanoma from oncogenic RET-carrying transgenic mice and human cell
lines. PLoS One. 5:e102792010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peebles PT, Trisch T and Papageorge AG:
Isolation of four unusual pediatric solid tumor cell lines. Pediat
Res. 12:4851978. View Article : Google Scholar
|
|
10
|
Katagata Y and Hirayama T: Unexpected
expression of Hsp47, a replacement of one amino acid (Val 7 Leu) in
the amino terminal region, in cultured human tumorigenic cell
lines. J Dermatol Sci. 49:33–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ando H, Funasaka Y, Oka M, Ohashi A,
Furumura M, Matsunaga J, Matsunaga N, Hearing VJ and Ichihashi M:
Possible involvement of proteolytic degradation of tyrosinase in
the regulatory effect of fatty acids on melanogenesis. J Lipid Res.
40:1312–1316. 1999.PubMed/NCBI
|
|
12
|
Branza-Nichita N, Negroiu G, Petrescu AJ,
Garman EF, Platt FM, Wormald MR, Dwek RA and Petrescu SM: Mutations
at critical N-glycosylation sites reduce tyrosinase activity by
altering folding and quality control. J Biol Chem. 275:8169–8175.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asryants RA, Duszenkova IV and Nagradova
NK: Determination of Sepharose-bound protein with Coomassie
brilliant blue G-250. Anal Biochem. 151:571–574. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi H, Ahn S, Chang H, Cho NS, Joo K, Lee
BG, Chang I and Hwang JS: Influence of N-glycan processing
disruption on tyrosinase and melanin synthesis in HM3KO melanoma
cells. Exp Dermatol. 16:110–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ibarrola-Villava M, Hu HH, Guedj M,
Fernandez LP, Descamps V, Basset-Seguin N, Bagot M, Benssussan A,
Saiag P, Fargnoli MC, et al: MC1R, SLC45A2 and TYR genetic variants
involved in melanoma susceptibility in southern European
populations: results from a meta-analysis. Eur J Cancer.
48:2183–2191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chien V, Aitken JF, Zhang S, Buchanan CM,
Hickey A, Brittain T, Cooper GJ and Loomes KM: The chaperone
proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and
formation of β-sheet-containing aggregates by human amylin: a
potential role for defective chaperone biology in Type 2 diabetes.
Biochem J. 432:113–121. 2010.PubMed/NCBI
|
|
17
|
Mikami K, Yamaguchi D, Tateno H, Hu D, Qin
SY, Kawasaki N, Yamada M, Matsumoto N, Hirabayashi J, Ito Y and
Yamamoto K: The sugar-binding ability of human OS-9 and its
involvement in ER-associated degradation. Glycobiology. 20:310–321.
2010. View Article : Google Scholar : PubMed/NCBI
|