Introduction
Stroke is a leading cause of impairment and
disability worldwide. The most common impairment resulting from
stroke is motor impairment, which typically affects the control of
movement on one side of the body. Rehabilitation strategies for
stroke patients have primarily focused on the recovery of impaired
movement and its associated motor function (1). Motor imagery (MI) practice has been
used in stroke rehabilitation (2,3) and
has produced positive effects in hand movement, sit-to stand
performance and activities of daily living (4–6).
MI has been defined as the mental representation of
movement without any body movement (7,8). It
is a complex cognitive process that is involved in the reactivation
of specific motor actions within working memory (9,10).
Neuroimaging studies have shown that MI activates more or less the
same brain regions as the actual execution of a movement, including
motor and premotor areas, the prefrontal cortex and the parietal
cortex (10–15). Studies have demonstrated the
benefits of MI in improving motor performance in patients with
neurological diseases (16).
Electroencephalography (EEG), a noninvasive and
convenient method to record brain signals, has been used to study
MI. MI desynchronizes the ongoing EEG activity and this
event-related phenomena may be due to desynchronized activities of
the activated neurons during the processing of cognitive
information or the production of motor behavior, termed
event-related desynchronization (ERD) (17). MI is associated with ERD in EEG
over motor cortical areas, particularly at the contralateral
hemisphere (18). ERD has been
used to analyze the brain activity in stroke patients (19–21).
However, it has been observed that ERD patterns are similar during
MI, motor execution and passive movement (18,22,23).
Failure to detect MI through ERD patterns prevents the effective
evaluation of MI execution during training and functional recovery
following MI training. Therefore, identification of an EEG measure
to distinguish between MI and passive movement will aid in
elucidating an effective method to analyze MI in stroke
patients.
In the present study, the event-related potentials
(ERPs) measured with EEG from stoke patients and healthy controls
performing tasks involving MI, passive movement without MI and
passive movement with MI tasks were investigated. The purpose of
this study was to identify the differences in ERPs for MI and
passive movement in stroke patients and controls.
Subjects and methods
Subjects
This study was approved by the Research Ethics
Committee of Beijing Boai Hospital (Beijing, China) and all
subjects provided their informed consent. Nine stroke patients (5
males and 4 females) were selected from the Neurology Department of
the Capital Medical University (Beijing, China). Patients were
selected according to the following criteria: i) Male or female
patients whose age ranged from 25 to 45 years; ii) cerebral
infarction or hemorrhage was diagnosed according to the report from
the the classification of cerebrovascular diseases III of the
National Institute of Neurological Disorders and Stroke (24); iii) cerebral infarction or
hemorrhage in the basal ganglion confirmed by brain computerized
tomography (CT) or magnetic resonance imaging (MRI); iv) disease
duration of 3–6 months; and v) no history of other neurological
diseases. Patients with the following conditions were excluded: i)
patients with lesions in the cerebellum and cortex observed by MRI
or CT; ii) impaired cognitive function with the Mini-Mental State
Examination (score of <17); iii) patients who were unable or
unwilling to complete the study and; iv) patients with severe
diseases of the heart, liver, kidney, brain or hematopoietic
system, as well as epilepsy, infectious diseases or brain trauma.
Only patients with subcortical stroke were selected in order to
exclude the possible effects of lesions in the cerebellum and
cortex on MI.
Nine age-matched healthy volunteers (5 males and 4
females) were selected according to the following criteria: i) male
or female patients whose age ranged from 25 to 45 years; ii) no
history of neurological or psychiatric disease; iii) normal brain
CT and MRI; iv) no history of trauma or administration of medicine
within 3 months prior to the study; v) no severe diseases that
affected the performance of the experiments; and vi) willingly
cooperated with the examination.
Tasks
Subjects performed the following tasks: MI, passive
movement with no MI and passive movement with MI. The subjects were
seated in front of a 17-inch screen located at a distance of 1 m.
The subject’s hand and forearm were held still and the index
fingers were not in contact with any objects. Only index fingers
were moved by the examiner for passive movement tasks and all the
other fingers rested on the table. Subjects were requested not to
move their eyes prior to and during tasks, but to look at a
fixation cross on the screen. For each subject, the left and right
index fingers were tested. For each finger, the tasks consisted of
12 sessions, with 4 sessions composed of 20 trials for each
experimental condition (MI, passive movement with MI and passive
movement without MI). There were a total of 80 trials per condition
for each finger. Sections were randomized and started with a 10 sec
presentation of a word sign on the screen to instruct the subjects
to prepare for the MI, passive movement with no MI or passive
movement with MI. For MI tasks, subjects were asked to imagine the
movements of the finger (flexion or extension of the index finger)
without actually performing them when they saw an upward arrow or a
downward arrow presented on the computer screen. For passive
movement with no MI task, subjects were asked not to move or
imagine movement. The subjects were not allowed to see the
instructions and the examiner moved the subject’s figures according
to the instructions on the screen. The subjects looked at the
fixation cross on a nearby screen. For passive movement with MI,
subjects were asked to imagine the same movements without actually
performing them when they saw the arrows on the screen. The
examiner moved the subject’s figures according to the instructions
on the screen. Each trial started with a 2 sec presentation of a
fixation cross in the center of the screen and the subjects began
to perform the tasks when they saw the arrow stimulus continuously
presented for 1 sec on the screen. The inter-trial interval was 10
sec. Task switching instructions were presented on the computer
screen for 2 min. During this resting period, the subjects were
asked to stay motionless and relax. An electromyogram (EMG) was
recorded by a dense array 128 channel EEG system 200 (Electrical
Geodesics Inc., Eugene, OR, USA) throughout the experiment to
ensure that subjects did not move their hand during imagery of the
movement.
In a 30 min training period, subjects familiarized
themselves with the stimulus and practiced the timing of the
passive movements and imaging movements. Subjects were trained to
perform imagination without introducing muscular contraction and
were reminded to use kinaesthetic imagery rather than visual
imagery during the training and experimental period. The training
length was a minimum of 30 min and was prolonged until the subjects
were comfortable with the imagination task. EMG was recorded
throughout the training period and the subjects were asked to
practice imagining the movement until they were able to imagine the
movement without an EMG response.
ERP measurement
The EEG was recorded by a dense array 128 channels
EEG system (Electrical Geodesics Inc., Eugene, OR, USA 200). The
data were recorded from 128 scalp electrodes mounted on an elastic
cap (Brain Products, Gilching, Germany) according to the
international 10–20 system (Fig.
1). Vertical and horizontal electrooculograms were recorded
separately. All electrodes were referenced to the mastoid surface
during recording. The electrode impedance was kept below 5 kΩ. Data
were recorded continuously with a band pass of 0.05–40 Hz and a
sampling rate of 500 Hz and stored on a hard disk for off-line
analysis. The EEG recordings were analyzed according to the
stimulus (MI, passive movement with no MI and passive movement with
MI) and the sides of finger movement (left vs. right), and were
averaged in each group separately. The EEG data were averaged for
800 ms, including 100 ms prior to the onset of stimulus for
baseline correction. The artifacts due to eye movement and blinking
were removed from EEG recordings, using independent component
analysis (25). Individual trials
with excessive muscle activity (>100 μV peak-to-peak amplitude)
were excluded. The averaged data were re-referenced to an average
voltage value across all electrodes.
Statistical analysis
Analyses were performed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). All values are presented as the mean ± SD.
One-way analysis of variance was used for comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
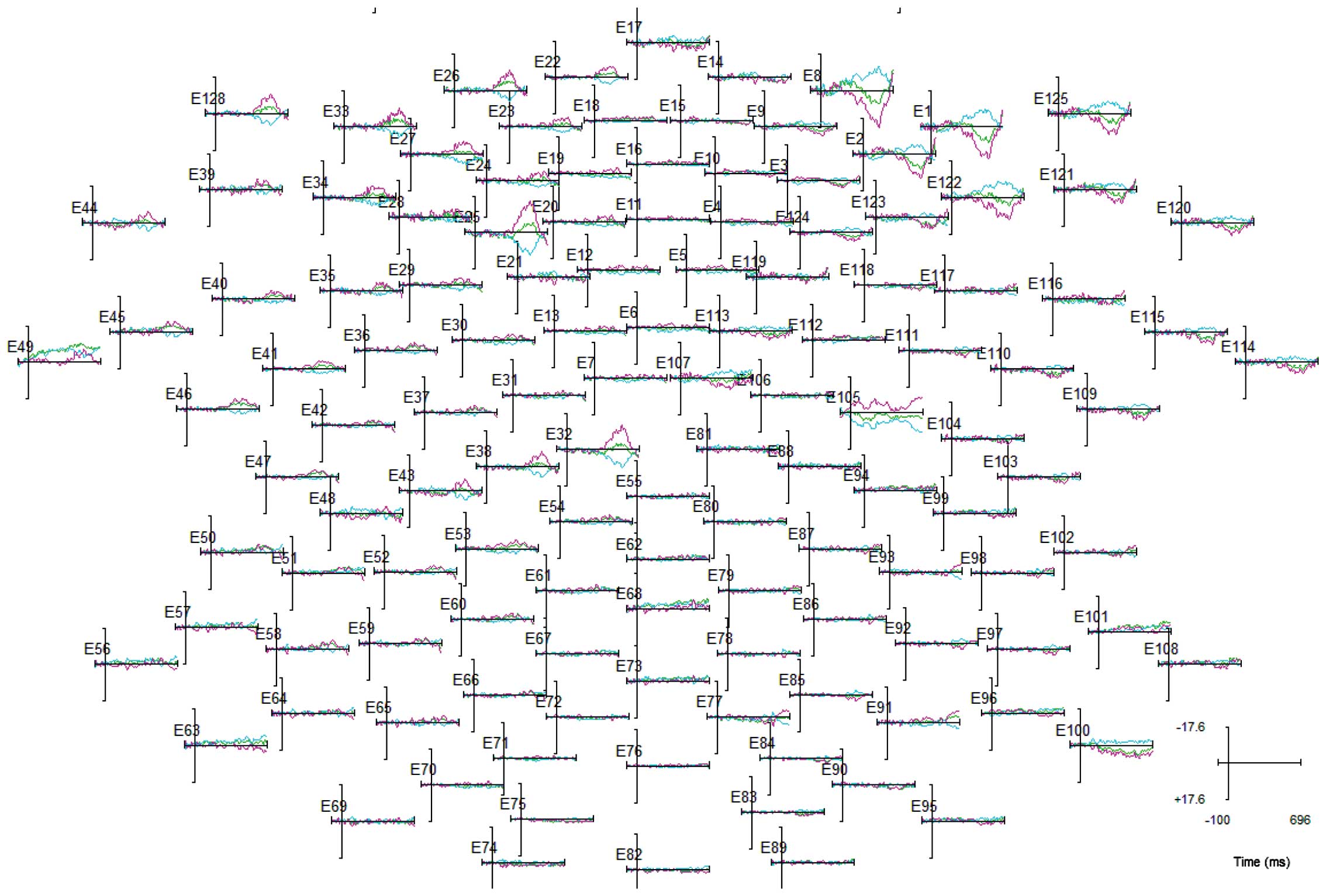
Fig. 2 shows
averaged ERPs evoked by MI in controls at 128 electrode sites over
the scalp surface. The amplitude of ERPs evoked by imagined
movement was distributed maximally over frontal scalp sites. As the
inferior precentral area has been observed to be associated with
the MI induced by the finger movement task (26), ERP data was selected and analyzed
from two electrodes E44 and E120 that were positioned close to F9
and F10 (corresponding to the inferior precentral area) according
to the international 10–20 system.
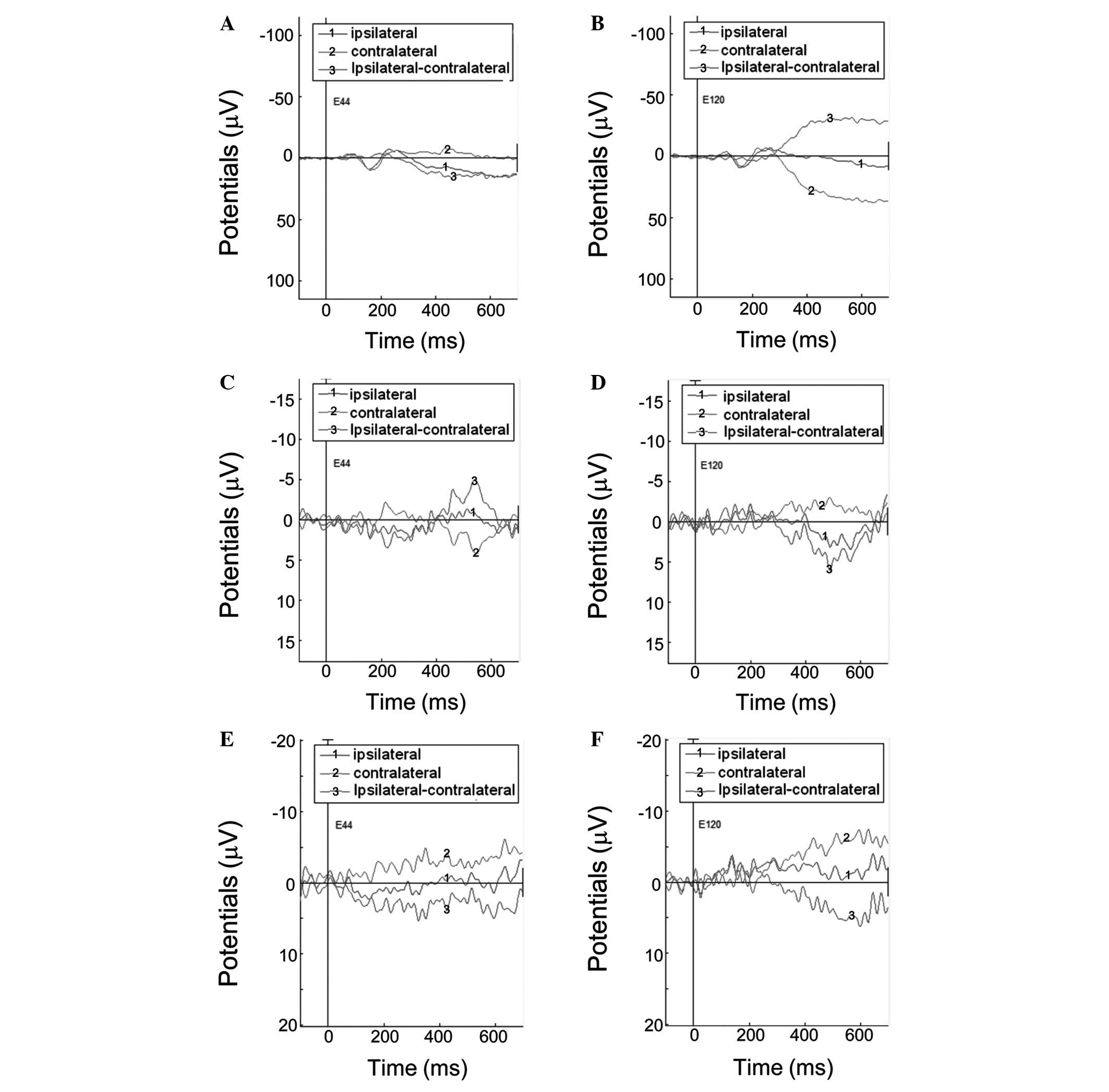
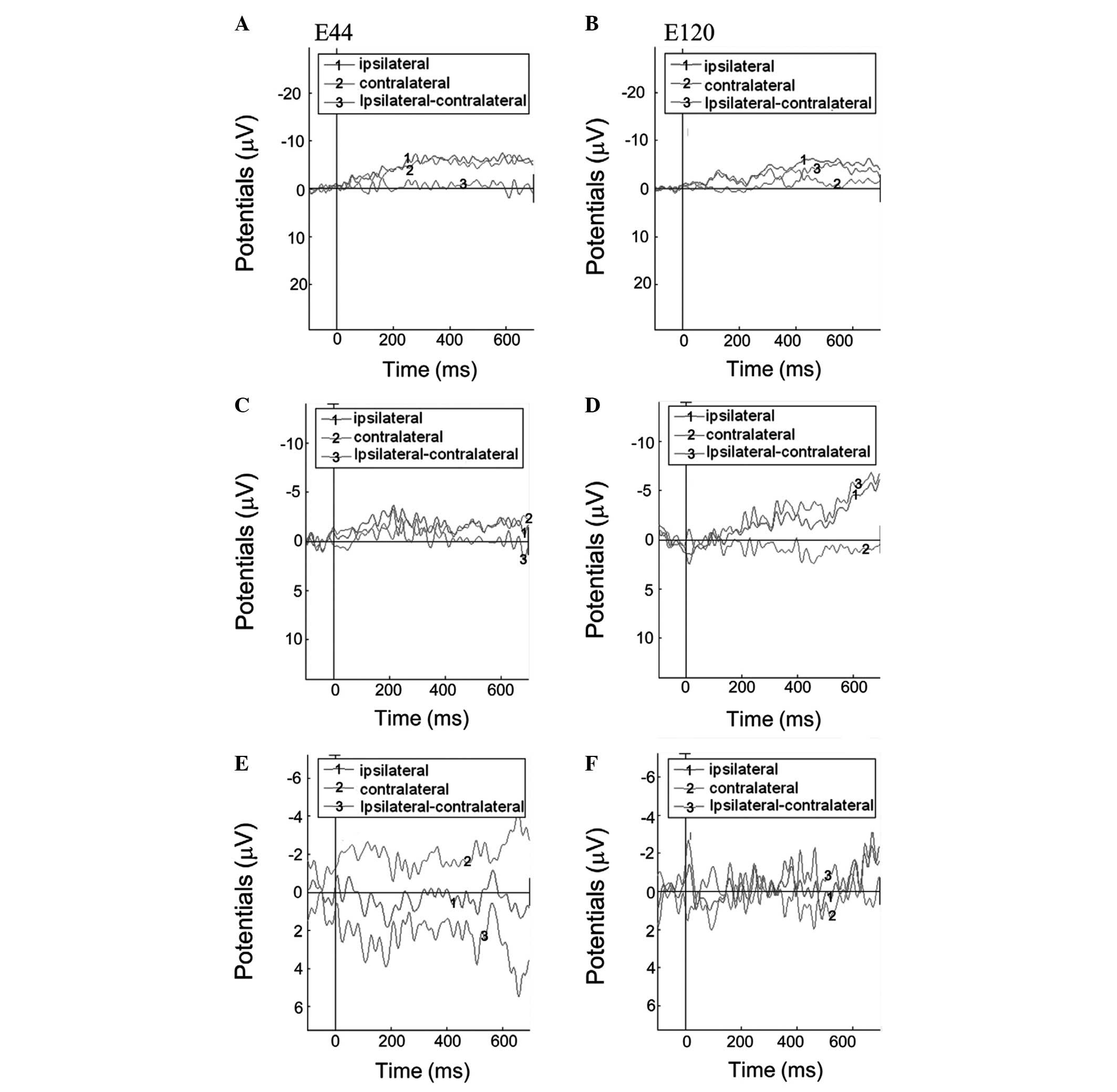
For the controls, the lateralization effect was
observed for ERPs evoked by passive movement with MI (Fig. 3A and B, and Fig. 4). The difference of ERPs was
calculated by subtracting the ERP amplitude of contralateral from
the ipsilateral finger or imaged movements. At the left electrode
E44 (Fig. 3A), the difference
between ERPs was negative during the 0–300 and positive during the
300–700 ms interval. By contrast, at the E120 right electrode
(Fig. 3B), the difference between
the ERPs was positive during the 0–300 and negative during the
300–700 ms interval. A similar effect, but to a lesser extent, was
observed for ERPs evoked by MI alone (Fig. 3C and D). However, no lateralization
effect was observed for ERPs evoked by passive movement with no MI
(Fig. 3E and F). The difference
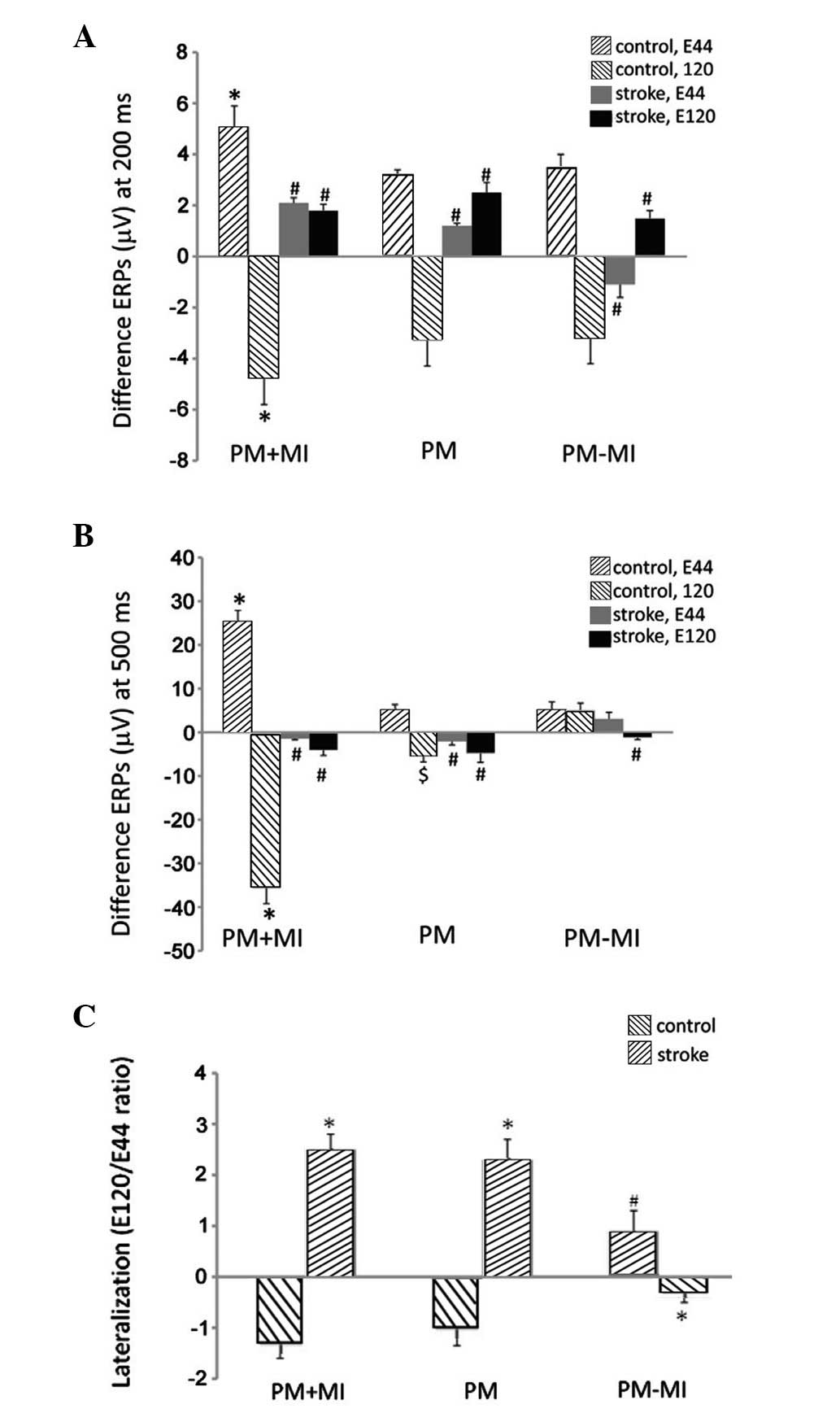
between ERPs at 200 and 500 ms were significantly larger in
patients with passive movement with MI than those with MI alone and
with no MI (P<0.05, Fig. 4A and
B). The lateralization was also calculated by a ratio of the
potential at 500 ms at electrode E120 to that at electrode E44
(Fig. 4C). A lateralization effect
(negative lateralization ratio) was observed for ERPs evoked by
passive movement with MI and MI alone, but not by passive movement
without MI (Fig. 4C). The
lateralization ratio in passive movement without MI was
significantly different from that in passive movement with MI and
MI alone (P<0.05).
For stroke patients, no lateralization effect was
observed for ERPs evoked by passive movement with MI, imagined
movement and passive movement with no MI (Figs. 4 and 5). For passive movement with MI and MI
alone, the difference between ERPs was negative during the 0–700 ms
interval at the E44 and E120 electrodes (Fig. 5A–D). For passive movement without
MI, the difference between ERPs were positive during the 0–700 ms
interval at electrode E44, but negative at the electrode E120
(Fig. 5E and F). The amplitudes of
the difference between ERPs at 200 and 500 ms were significantly
smaller in stroke patients compared with those in the controls
(P<0.05, Fig. 4A and B). By
contrast, the lateralization ratio in the stroke patients was
opposite and significantly different from that of the controls
(P<0.05, Fig. 4C).
Discussion
The present study aimed to investigate the
characteristic features in ERPs evoked by MI, passive movement
without MI and passive movement with MI in healthy controls and
stroke patients. Several predominant results were observed in ERPs
which may be used to distinguish movement-related electrical
activity of the brain between controls and stroke patients. The
movement-related potentials were observed to be distributed
maximally over the frontal cortex. The amplitudes of ERPs in stroke
patients were smaller than those in the controls and for controls,
the difference between ERPs exhibited different directions between
the 0–300 and the 300–700 ms interval. However, this feature was
absent in stroke patients. Furthermore, ERPs evoked by MI and
passive movement with MI exhibited lateralization effects in
controls, but not in stroke patients. This lateralization effect
was specific to MI as ERPs evoked by passive movement without MI
did not exhibit the lateralization effect.
It is generally believed that MI and motor execution
share a common neural substrate, including motor and premotor
cortex (3). Concurrent with this
hypothesis, it was identified that MI produces lateralization
effects similar to passive movement with MI in the controls. The
MI-induced lateralization effect was smaller compared with that
evoked by passive movement with MI. This is concurrent with several
studies that demonstrated that MI induced changes in brain activity
are less pronounced than those induced by motor execution (27,28).
Furthermore, the lateralization effect is unique to MI as passive
movement does not evoke it. Passive movement, similar to MI and
motor execution, has been demonstrated to induce a lateralization
effect in ERD with a stronger ERD contralateral to the movement
(22,23,29),
suggesting that ERD is not a useful measurement to distinguish MI
from passive movement. However, the lateralization effect in ERPs
identified in the present study was unique to MI and therefore, may
be used as a measurement to distinguish MI and passive
movement.
The present study also demonstrated that the
lateralization effect induced by MI or passive movement with MI
observed in controls is not present in stroke patients, suggesting
that MI-induced lateralization of brain activity is damaged in
stroke patients. It has been suggested that the primary motor
cortex (M1) on the side of the stroke affects lateralization during
MI (30,31). However, the lateralization effect
was not identified in the inferior precentral area of either side
of the brains of the stroke patients. This difference may be due to
several reasons. Different studies include distinct patient
populations; in the present study only patients with a disease
duration of 3–6 months were selected, while two other studies
(30,31) included stroke patients with a
disease duration of μ8 months. In addition, different regions of
interest are investigated among studies. It has been suggested that
in normal healthy subjects, the inferior precentral area is
particularly associated with MI and the primary sensory and motor
areas and anterior cerebellum are associated with marginal imagery
activity (26). In the present
study therefore, the inferior precentral area was selected, while
other studies investigated the primary motor cortex (M1) (30,31).
Furthermore, previous functional MRI studies in chronic stroke
patients showed that virtual reality practice only increased
lateralization in the primary sensorimotor cortex, and not in other
brain areas, such as the primary motor cortex (M1), premotor
cortex, supplementary motor area and primary sensory cortex
(32). Whether virtual reality
practice or other interventions improve the MI-induced
lateralization in ERPs in the inferior precentral area requires
further investigation.
There are certain limitations to this study. As with
all studies of MI, the present study did not confirm that
participants actually performed the MI tasks as instructed.
However, the tasks were very simple and all participants were
instructed to tell the examiner of any difficulty in performing the
MI tasks. In addition, the current study did not test ERPs induced
by motor execution in these patients as the purpose of this study
was to identify the characteristic feature of ERPs induced by MI in
the stroke patients, using a simple index finger movement task. Our
future study concerning the difference in ERPs induced by MI and
motor execution in stoke patients is under investigation, using
various complex movement tasks. Moreover, only nine stroke patients
are included in the present study. Thus, a study with a larger
number of participants is required.
The present results implicate the use of MI in
stroke rehabilitation. Several studies have shown that MI practice
improves motor function in stroke patients (4–6).
However, failure to measure MI ability is a predominant limitation
to evaluate MI impairment and recovery following MI training in
stroke patients. The MI-induced lateralization in ERPs observed in
this study may be a useful measure to evaluate the MI in stroke
patients. Future studies are required to determine whether the
MI-induced lateralization in ERPs are recovered in these stroke
patients following MI training.
In conclusion, MI-induced lateralization in stroke
patients and healthy age-matched controls was investigated. It was
demonstrated that MI-induced lateralization observed in healthy
controls was not present in stroke patients. The results suggest
that the MI-induced lateralization effect in ERPs may be a useful
measure for evaluating MI impairment and recovery in stroke
patients.
References
|
1
|
Wade D: Rehabilitation therapy after
stroke. Lancet. 354:176–177. 1999. View Article : Google Scholar
|
|
2
|
Braun SM, Beurskens AJ, Borm PJ, Schack T
and Wade DT: The effects of mental practice in stroke
rehabilitation: a systematic review. Arch Phys Med Rehabil.
87:842–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Vries S and Mulder T: Motor imagery and
stroke rehabilitation: a critical discussion. J Rehabil Med.
39:5–13. 2007.PubMed/NCBI
|
|
4
|
Guttman A, Burstin A, Brown R, Bril S and
Dickstein R: Motor imagery practice for improving sit to stand and
reaching to grasp in individuals with poststroke hemiparesis. Top
Stroke Rehabil. 19:306–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Page SJ, Levine P and Leonard AC: Effects
of mental practice on affected limb use and function in chronic
stroke. Arch Phys Med Rehabil. 86:399–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dijkerman HC, Ietswaart M, Johnston M and
MacWalter RS: Does motor imagery training improve hand function in
chronic stroke patients? A pilot study Clin Rehabil. 18:538–549.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guillot A and Collet C: Contribution from
neurophysiological and psychological methods to the study of motor
imagery. Brain Res Brain Res Rev. 50:387–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grush R: The emulation theory of
representation: motor control, imagery, and perception. Behav Brain
Sci. 27:377–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Annett J: Motor imagery: perception or
action? Neuropsychologia. 33:1395–1417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeannerod M and Decety J: Mental motor
imagery: a window into the representational stages of action. Curr
Opin Neurobiol. 5:727–732. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Decety J, Perani D, Jeannerod M,
Bettinardi V, Tadary B, Woods R, Mazziotta JC and Fazio F: Mapping
motor representations with positron emission tomography. Nature.
371:600–602. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porro CA, Francescato MP, Cettolo V,
Diamond ME, Baraldi P, Zuiani C, Bazzocchi M and di Prampero PE:
Primary motor and sensory cortex activation during motor
performance and motor imagery: a functional magnetic resonance
imaging study. J Neurosci. 16:7688–7698. 1996.PubMed/NCBI
|
|
13
|
Porro CA, Cettolo V, Francescato MP and
Baraldi P: Ipsilateral involvement of primary motor cortex during
motor imagery. Eur J Neurosci. 12:3059–3063. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munzert J and Zentgraf K: Motor imagery
and its implications for understanding the motor system. Prog Brain
Res. 174:219–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malouin F, Richards CL, Jackson PL, Dumas
F and Doyon J: Brain activations during motor imagery of
locomotor-related tasks: a PET study. Hum Brain Mapp. 19:47–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dickstein R and Deutsch JE: Motor imagery
in physical therapist practice. Phys Ther. 87:942–953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pfurtscheller G and Klimesch W: Functional
topography during a visuoverbal judgment task studied with
event-related desynchronization mapping. J Clin Neurophysiol.
9:120–131. 1992. View Article : Google Scholar
|
|
18
|
Pfurtscheller G and Neuper C: Motor
imagery activates primary sensorimotor area in humans. Neurosci
Lett. 239:65–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasad G, Herman P, Coyle D, McDonough S
and Crosbie J: Applying a brain-computer interface to support motor
imagery practice in people with stroke for upper limb recovery: a
feasibility study. J Neuroeng Rehabil. 7:602010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Platz T, Kim IH, Pintschovius H, Winter T,
Kieselbach A, Villringer K, Kurth R and Mauritz KH: Multimodal EEG
analysis in man suggests impairment-specific changes in
movement-related electric brain activity after stroke. Brain.
123:2475–2490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stepień M, Conradi J, Waterstraat G,
Hohlefeld FU, Curio G and Nikulin VV: Event-related
desynchronization of sensorimotor EEG rhythms in hemiparetic
patients with acute stroke. Neurosci Lett. 488:17–21.
2011.PubMed/NCBI
|
|
22
|
Alegre M, Labarga A, Gurtubay IG, Iriarte
J, Malanda A and Artieda J: Beta electroencephalograph changes
during passive movements: sensory afferences contribute to beta
event-related desynchronization in humans. Neurosci Lett.
331:29–32. 2002. View Article : Google Scholar
|
|
23
|
Müller GR, Neuper C, Rupp R, Keinrath C,
Gerner HJ and Pfurtscheller G: Event-related beta EEG changes
during wrist movements induced by functional electrical stimulation
of forearm muscles in man. Neurosci Lett. 340:143–147.
2003.PubMed/NCBI
|
|
24
|
No authors listed. Special report from the
National Institute of Neurological Disorders and Stroke.
Classification of cerebrovascular diseases III. Stroke. 21:637–676.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung TP, Makeig S, Humphries C, Lee TW,
McKeown MJ, Iragui V and Sejnowski TJ: Removing
electroencephalographic artifacts by blind source separation.
Psychophysiology. 37:163–178. 2000.PubMed/NCBI
|
|
26
|
Hanakawa T, Immisch I, Toma K, Dimyan MA,
Van Gelderen P and Hallett M: Functional properties of brain areas
associated with motor execution and imagery. J Neurophysiol.
89:989–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beisteiner R, Höllinger P, Lindinger G,
Lang W and Berthoz A: Mental representations of movements. Brain
potentials associated with imagination of hand movements.
Electroencephalogr Clin Neurophysiol. 96:183–193. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lang W, Cheyne D, Höllinger P, Gerschlager
W and Lindinger G: Electric and magnetic fields of the brain
accompanying internal simulation of movement. Brain Res Cogn Brain
Res. 3:125–129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaiser V, Kreilinger A, Müller-Putz GR and
Neuper C: First steps toward a motor imagery based stroke BCI: new
strategy to set up a classifier. Front Neurosci. 5:862011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stinear CM, Fleming MK, Barber PA and
Byblow WD: Lateralization of motor imagery following stroke. Clin
Neurophysiol. 118:1794–1801. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabaté M, González B and Rodríguez M:
Brain lateralization of motor imagery: motor planning asymmetry as
a cause of movement lateralization. Neuropsychologia. 42:1041–1049.
2004.PubMed/NCBI
|
|
32
|
You SH, Jang SH, Kim YH, Hallett M, Ahn
SH, Kwon YH, Kim JH and Lee MY: Virtual reality-induced cortical
reorganization and associated locomotor recovery in chronic stroke:
an experimenter-blind randomized study. Stroke. 36:1166–1171. 2005.
View Article : Google Scholar : PubMed/NCBI
|