Introduction
Ischemic heart disease is commonly associated with
acute blockage of the coronary artery and remains one of the
leading causes of morbidity and mortality worldwide. Rapid
reperfusion is critical to salvage viable myocardium following
coronary occlusion. This reperfusion is commonly achieved either by
thrombolysis or percutaneous coronary intervention. However,
reperfusion itself may result in cardiac dysfunction termed
myocardial ischemia/reperfusion injury (MI/RI), which has been
shown to affect clinical outcomes. Therefore, it is essential to
investigate the mechanisms of MI/RI to elucidate potential
prevention strategies (1).
Angiotensin receptor blockers (ARBs) have yielded
encouraging results in animal models and a few have been tested in
humans, however, the mechanism(s) of action associated with MI/RI
remain unclear. Previous clinical and experimental studies have
suggested that the cardio-protective effects of ARBs may extend to
the mechanisms beyond lowering blood pressure, including
anti-inflammation, anti-atherosclerosis and target organ protection
(2,3). It has been previously reported that
valsartan may protect the heart from acute I/R via inhibition of
the toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB pathway and
inflammation (4). Sawicki et
al(5) reported that valsartan
improved the balance between tissue inhibitor of metalloproteinase
(TIMP)-3 and matrix metalloproteinase (MMP)-9, which attenuated the
cardiac remodeling following myocardial I/R injury. Iekushi et
al(6) demonstrated that
valsartan significantly attenuated an increase in the levels of
periostin, an extracellular matrix protein, which is important in
left ventricular remodeling. The inhibition of periostin by
valsartan may contribute to its cardioprotection against myocardial
infarction. These studies suggested that the ARBs may exert cardiac
protection via a number of potential mechanism(s), independent of
blood pressure lowering, which remain unclear.
Autophagy is a highly conserved anabolic process and
is implicated as a cell survival/death mechanism, it degrades and
recycles long-lived cytoplasmic proteins and organelles to maintain
cellular homeostasis and allow adaptation to nutrient depletion
(7,8). Autophagy is recognized to be an
important process that may be a key regulator of ischemic heart
disease; therefore, the involvement of autophagy in myocardial
infarction is being investigated (9–13).
Inefficient autophagy or its absence, results in poor performance
of the myocardium and inhibition of starvation-induced autophagy
results in cardiac dysfunction and dilatation (9,10).
Moreover, autophagy has been shown to be an adaptive response of
the heart that protects the myocardium from homodynamic overload
and acute ischemic mortality (11,12).
In addition, it has been previously reported that Chloramphenicol
succinate induces autophagy and protects the swine heart from I/R
injury (13), suggesting that
autophagy facilitates cardiomyocyte survival as a protective
mechanism. In the current study, it was investigated whether
autophagy is involved in valsartan-induced myocardioprotection in
the rat acute I/R model.
Materials and methods
Animals and drug preconditioning
Male Sprague-Dawley rats (weight, 220–250 g) were
purchased from the Medical Experimental Animal Center of Guangdong
Province (Guangzhou, China). The investigation conformed with the
Guide for the Care and Use of Laboratory Animals published by the
United States National Institutes of Health (NIH publication no.
85-23, revised 1996). The study was approved by the Animal Research
Committee, Guangzhou Medical University (Guangzhou, China). The
rats were randomly divided into four groups: (i) Sham (sham, n=8);
(ii) control with ischemia/reperfusion surgery (I/R, n=12); (iii)
I/R plus valsartan preconditioning (I/R+Val, n=12); (iv) I/R plus
valsartan preconditioning plus 3MA (I/R+Val+3MA, n=12); and (v)
sham plus 3MA preconditioning (Sham+3MA, n=8). Valsartan was
supplied by Beijing Novartis Pharma Ltd. (Beijing, China) and
dissolved in distilled water on the day of administration. The
valsartan suspension (30mg/kg/day) was administered
intraperitoneally for seven days continuously prior to I/R surgery.
3MA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was
administered once 30 min prior to left anterior descending coronary
artery (LAD) ligation from the external jugular vein at a dose of 1
mg/kg.
Myocardial I/R surgery
Myocardial I/R was achieved by temporarily occluding
the LAD and then releasing the occlusion, similar to the method
described previously (14).
Briefly, the rats were anesthetized with pentobarbital (30 mg/kg
intraperitoneally) and the LAD was ligated using a 6-0 silk suture
(Ningbo Medical Needle Co., Ltd., Ningbo, China). The
electrocardiogram (Fujifilm VisuaSonics, Inc., Toronto, ON, Canada)
was monitored for changes in the ST-T segment resulting from
tightening or loosing of the ligature. Following 30 min ischemia,
the myocardium was reperfused by restoring the coronary circulation
for 2 h. The sham control group underwent the same procedures, with
the exception of LAD ligation.
Heart functional measurements
A catheter (1.4F SPR-671, Millar Instruments,
Houston, TX, USA) was connected to a pressure transducer (Power
Lab, AD Instruments, Colorado Springs, CO, USA) and was directly
inserted into the left ventricle (LV) to measure left ventricular
systolic pressure (LVSP), left ventricular end-diastolic pressure
(LVEDP) and heart rate (HR). The maximum increase rate of left
ventricular pressure (+dP/dt)max and maximum decrease rate of left
ventricular pressure (−dP/dt)max were directly calculated from the
selected stable recordings.
Determination of infarct size
Upon completion of the experiment, the ventricles
were collected and sliced transversely into 2-mm-thick slices. The
slices were incubated in 1% 2,3,5-triphenyl tetrazolium chloride
(TTC; pH 7.4) for 20 min at 37°C. The infarcted area was shown as
the area unstained by TTC, measured by Image-Pro Plus 5.0 (Media
Cybernetics Inc., Rockville, MD, USA). Infarct size was expressed
as a percentage of left ventricular volume (%=infarct size/left
ventricle area).
Histological examination
Specimens of the ventricle tissue were fixed,
sectioned and stained with hematoxylin and eosin (H&E) as
described previously (15).
Electron microscopy
Fractions (1 mm3) from fresh ventricles
were pre-fixed in a solution of 2.5% glutaraldehyde and 1% osmium
tetroxide, post-fixed in 1% osmium tetroxide, dehydrated in an
ascending series of alcohols and embedded in epoxy resin. Ultrathin
sections were stained with uranyl acetate and lead citrate. Samples
were viewed under a transmission electron microscope (H-600;
HITACHI, Tokyo, Japan) and analyzed using the IBAS 2.0 Image
Analysis system (Kontron, Deggendorf, German).
Western blot analysis
Western blotting was performed as described
previously (16). The primary
antibodies used were as follows: Anti-LC3B (1:1000; ab51520; Abcam,
Cambridge, MA, USA), anti Beclin 1 (1:1000; #3738),
anti-Phospho-AKT ser473 (1:1000; #4060), anti-AKT (1:1000; #9272),
anti-phospho-mTOR ser2448 (1:1000; #5536) anti-mTOR (1:1000;
#2972), anti-phospho-4E-BP1 thr37/46 (1:1000; #2855), anti-4E-BP1
(1:1000; #9644) and anti-β actin (1:1000) (Cell Signaling
Technology, Inc., Danvers, MA, USA). The intensity of protein bands
was analyzed by Labworks software (ADInstruments Pty Ltd., Bella
Vista, Australia).
Statistical analysis
All data were analyzed with the statistical software
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and
are expressed as the mean ± SEM. The differences between two groups
were analyzed using Student’s unpaired t-test and differences
between three or more groups were evaluated using a one-way
analysis of variance with Bonferroni correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
Valsartan preconditioning improves
cardiac hemodynamics and decreases infarct size in rats subjected
to I/R injury
It has been previously shown that valsartan
treatment may protect against I/R injury in isolated rat hearts
(4,5). In the current study, the ability of
valsartan to protect the heart against I/R injury in an in
vivo rat model was investigated. The left coronary artery was
occluded for 30 min followed by 2 h of reperfusion. As shown in
Table I, valsartan pretreatment
significantly improved the functional recovery with a markedly
improved HR, LVSP and ±dP/dtmax compared with the I/R group at the
different time points of reperfusion. No infarction was detected in
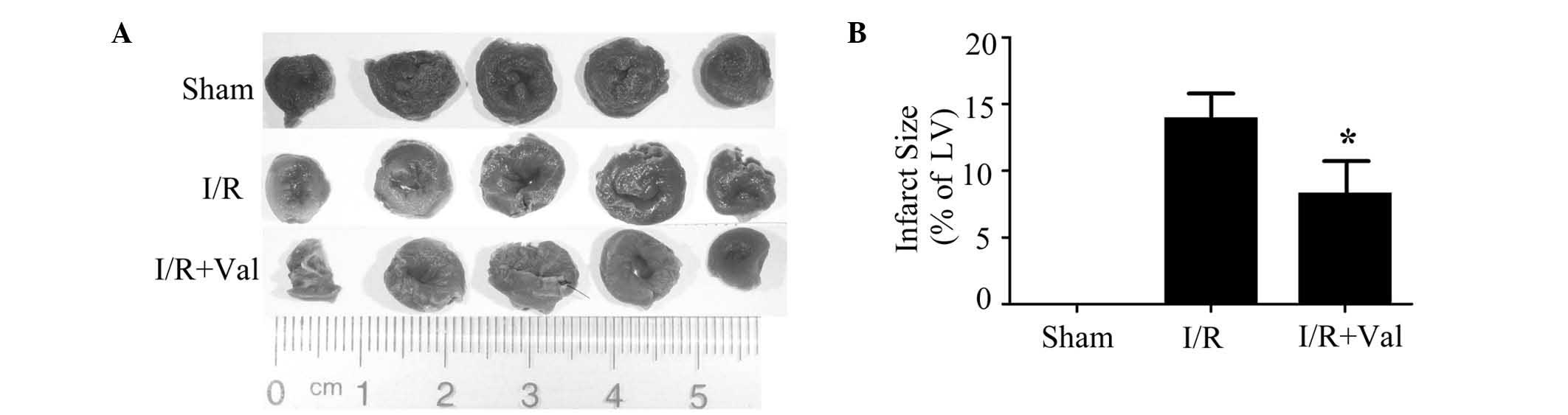
the sham hearts. Compared with control rats in the I/R group,
valsartan preconditioning limited the infarct size significantly as
shown in Fig. 1. These data
demonstrated that valsartan pretreatment protects the heart against
I/R injury in vivo.
 | Table ICardiac hemodynamics in rats subjected
to ischemia-reperfusion. |
Table I
Cardiac hemodynamics in rats subjected
to ischemia-reperfusion.
| A, Baseline |
|---|
|
|---|
| LVSP, mmHg | LVEDP, mmHg | (+dp/dt)max,
mmHg/sec | (−dp/dt)max,
mmHg/sec | HR, beats/min |
|---|
| Basline |
| Sham | 134±3 | 19±5 | 6513±556 | −5708±450 | 435±22 |
| I/R | 129±4 | 19±3 | 6114±530 | −5174±318 | 408±12 |
| I/R+Val | 129±5 | 18±2 | 6038±540 | −5364±314 | 422±6 |
| I/R+Val+3MA | 129±7 | 16±2 | 6060±186 | −5559±326 | 413±19 |
| Sham+3MA | 130±4 | 18±4 | 6421±645 | −5629±338 | 421±20 |
|
| B, Reperfusion |
|
| LVSP, mmHg | LVEDP, mmHg | (+dp/dt)max,
mmHg/sec | (−dp/dt)max,
mmHg/sec | HR, beats/min |
|
| 0 min |
| Sham | 135±5 | 16±3 | 6026±577 | −4917±501 | 435±25 |
| I/R | 102±4 | 19±2 | 3929±474 | −3346±334 | 336±26 |
| I/R+Val | 124±4a | 16±2 | 5269±336a | −3822±231 | 436±16a |
| I/R+Val+3MA | 111±2b | 16±2 | 5208±116 | −3990±137 | 421±14 |
| Sham+3MA | 129±4 | 17±3 | 6083±494 | −4823±459 | 428±22 |
| 30 min |
| Sham | 134±8 | 13±2 | 5860±637 | −4782±536 | 415±19 |
| I/R | 100±5 | 20±2 | 3637±349 | −2557±287 | 305±27 |
| I/R+Val | 119±4a | 13±2a | 5376±266a | −4019±213a | 419±15a |
| I/R+Val+3MA | 116±4 | 16±1 | 3554±456b | −3230±107 | 409±13 |
| Sham+3MA | 129±7 | 15±2 | 5689±567 | −4682±521 | 422±20 |
| 60 min |
| Sham | 124±7 | 14±2 | 5739±472 | −4548±477 | 391±17 |
| I/R | 110±4 | 20±2 | 3712±323 | −2748±326 | 290±32 |
| I/R+Val | 121±5a | 13±1a | 5099±264a | −3726±236a | 414±15a |
| I/R+Val+3MA | 103±5b | 16±1 | 3598±410b | −3037±119 | 369±10b |
| Sham+3MA | 127±7 | 15±3 | 5690±452 | −4620±521 | 417±18 |
| 120 min |
| Sham | 118±5 | 14±1 | 4926±146 | −4127±183 | 397±23 |
| I/R | 92±7 | 19±1 | 3316±341 | −2446±288 | 284±37 |
| I/R+Val | 112±4a | 11±1a | 4495±211a | −3792±181a | 393±18a |
| I/R+Val+3MA | 94±5b | 16±2b | 3030±114b | −2817±840b | 343±14b |
| Sham+3MA | 124±5 | 15±3 | 5243±325 | −4512±367 | 402±20 |
Valsartan preconditioning induces
autophagy in the rat hearts subjected to I/R injury
To investigate the molecular mechanisms by which
valsartan protects the rat heart against I/R injury, the potential
involvement of prosurvival autophagy was observed. The
electrographic assay showed that autophagic vacuoles were
significantly increased in the rats of the I/R+Val group compared
with the I/R group (Fig. 2A). The
conversion of LC3-I to LC3-II was used as a specific marker of
autophagy. As shown in Fig. 2B,
valsartan preconditioning significantly increased the ratio of
LC3-II/LC3-I proteins in the hearts of the I/R+Val group compared
with I/R alone. These data suggest that valsartan preconditioning
induced autophagy activation in the rat hearts subjected to I/R
injury.
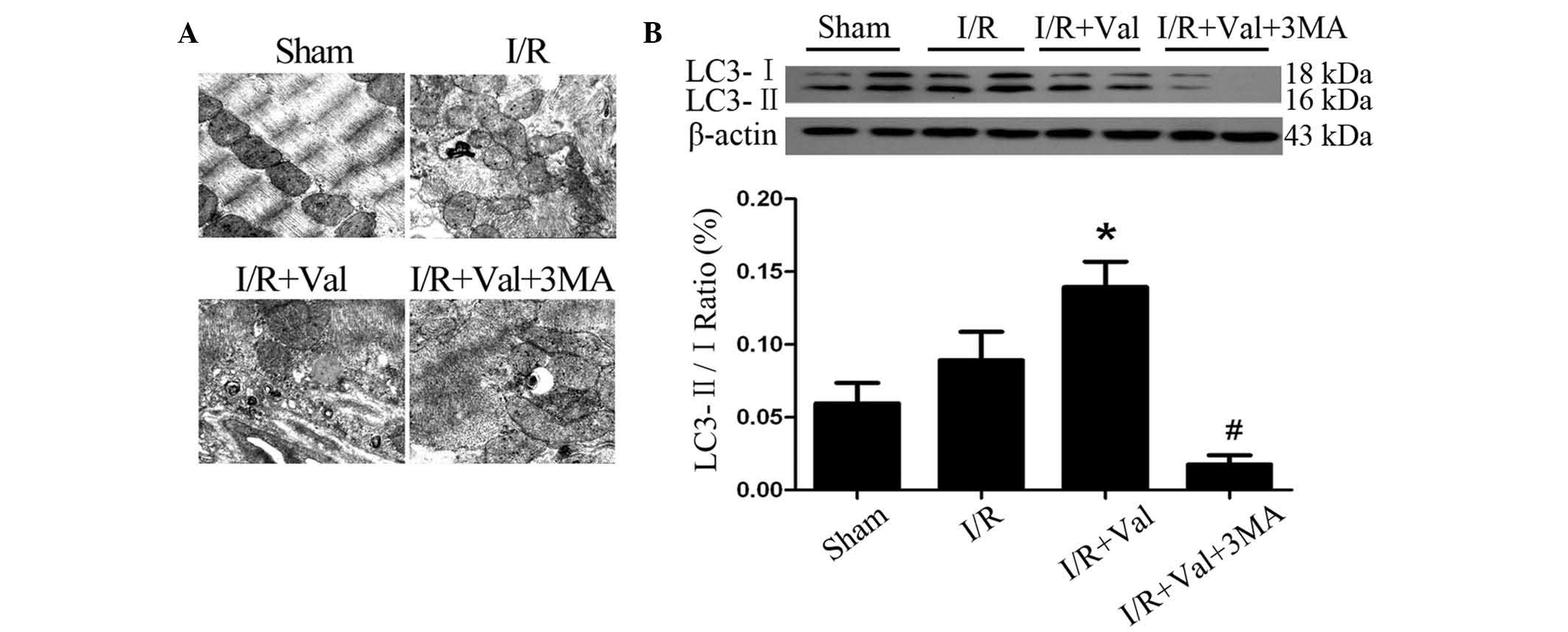 | Figure 2Valsartan preconditioning induced
autophagy in the rat hearts subjected to I/R injury. (A) Electron
micrographs (magnification, ×20,000) of the infarct area in the
sham, I/R, I/R+Val and I/R+Val+3MA groups. (B) Western blot
analysis of the autophagy-associated protein, LC3, in the ventricle
tissue of the aforementioned groups. Values are presented as the
mean ± SEM. *P<0.05, vs. I/R and
#P<0.05, vs. I/R+Val. I/R, ischemia/reperfusion; Val,
Valsartan; 3-MA, 3-methyladenine. |
Autophagy inhibition weakens the effect
of valsartan on the improvement of hemodynamics and histology in
the rats subjected to I/R injury
To investigate the potential involvement of
prosurvival autophagy in valsartan-induced cardioprotection, 3MA, a
specific inhibitor of autophagy, was administrated intravenously on
the day of surgery, 30 min prior to LAD ligation and following
valsartan preconditioning. The hemodynamic assessment showed that
the parameters of cardiac function were significantly attenuated by
the 3MA pre-treatment at the 120 min reperfusion time point in the
I/R+Val+3MA rats compared with the I/R+Val group (P<0.05, n=8;
Table I).
As shown in Fig. 3,
the infarct size in the rats of the I/R+Val+3MA group significantly
increased compared with that of the I/R+Val group (12.35±1.32 vs.
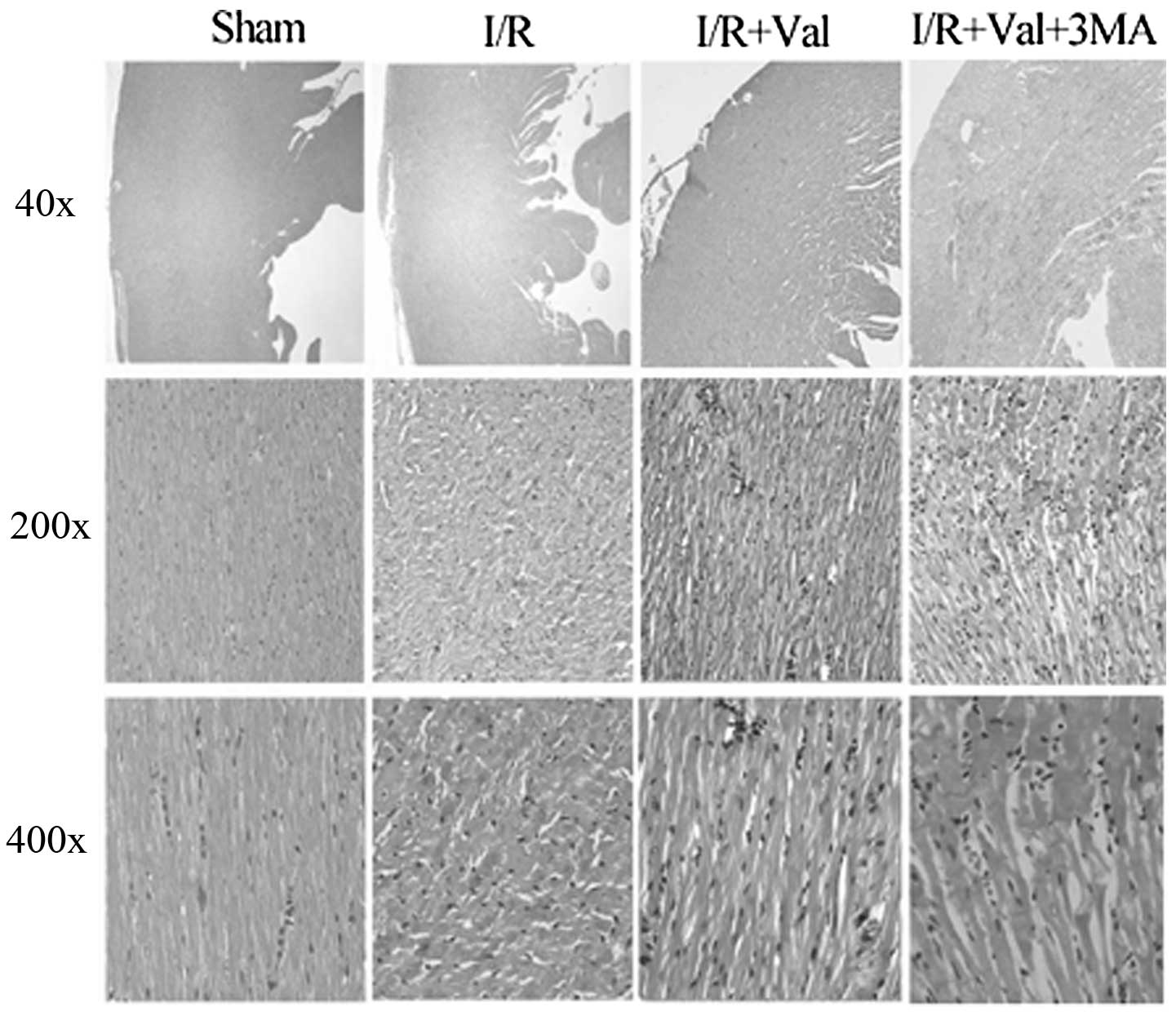
9.17±4.68%, P<0.05, n=6). H&E staining revealed that
valsartan pretreatment significantly improved the cardiac
morphology compared with the I/R control and this effect was
eliminated by 3-MA (Fig. 4).
Cardiac muscle fibers were lined up in order and transverse
striation was clear and well-distributed in the rats of the sham
group. In the heart sections of the rats subjected to I/R injury,
cardiomyocyte damage was characterized by wavy fibers, irregular
transverse striation and edema with neutrophil infiltration, which
was attenuated by valsartan pretreatment. Notably, the hearts of
rats in the I/R+Val+3MA group exhibited increased injury compared
with those of the I/R+Val group. These data suggested that
valsartan-mediated cardiac protection was weakened by 3-MA.
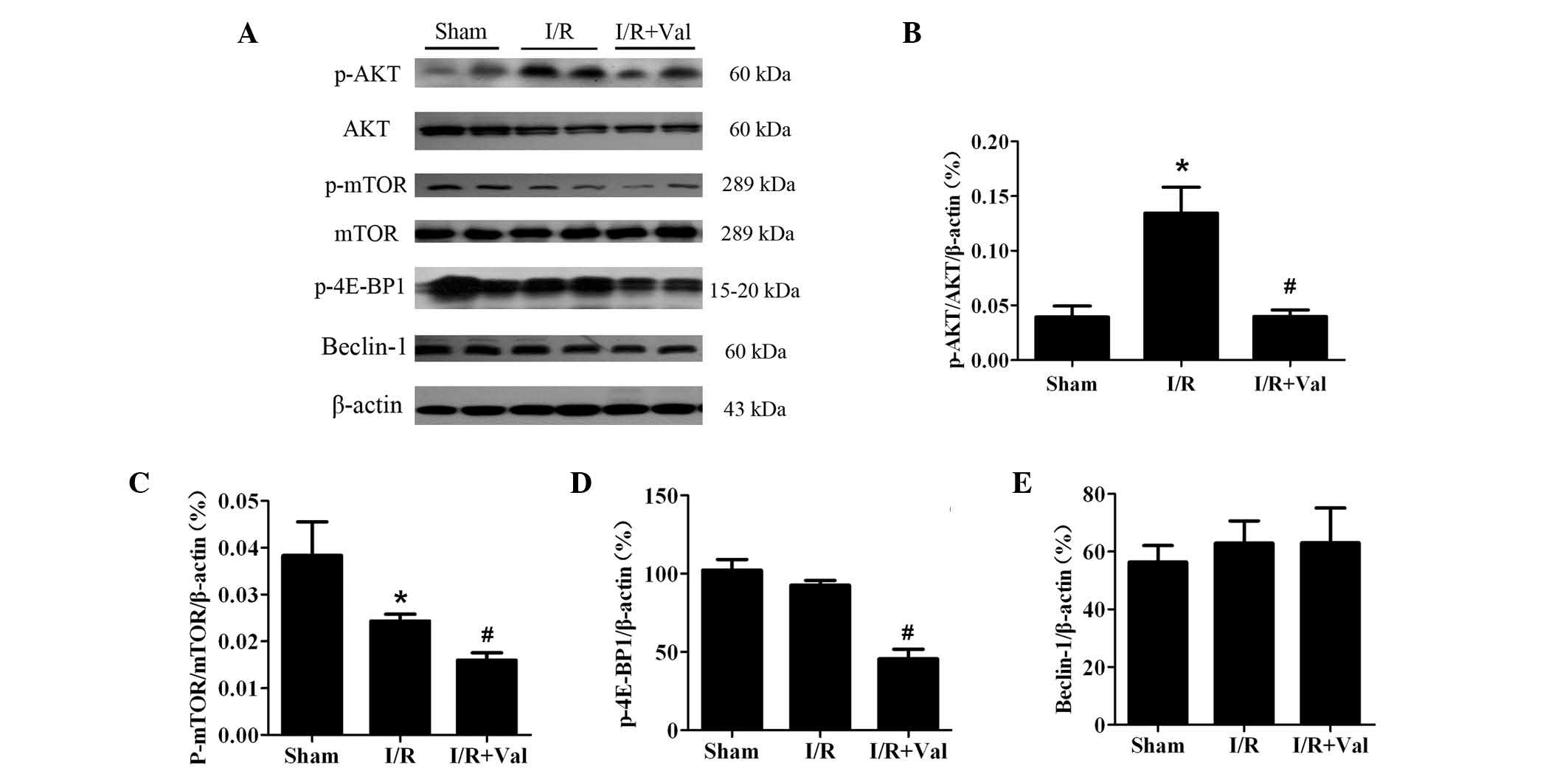
Valsartan induces autophagy via the
AKT/mTOR/S6K signaling pathway
To corroborate the mechanism by which valsartan
pretreatment induced autophagy, western blot analysis was performed
to examine the two most well-understood pathways that regulate
autophagy, the AKT/mTOR and Beclin-1 pathways. As shown in Fig. 5, AKT was phosphorylated in the
hearts of I/R controls and this effect was reversed with valsartan
pretreatment. In addition, phosphorylation of 4E-BP1 was
significantly attenuated by valsartan pretreatment compared with
I/R only. By contrast, Beclin-1 was not changed. The results
indicated that valsartan pretreatment induced autophagy by means of
the AKT/mTOR pathway, without modifying Beclin-1 levels.
Discussion
This study showed that valsartan, administered as a
pretreatment, provided resistance for the rat heart against I/R
injury. Moreover, the results suggested that myocardial protection
of valsartan is associated with increased autophagy. The underlying
mechanism of valsartan-induced autophagy was observed to occur via
the AKT/mTOR signaling pathway.
Autophagy is an essential cellular mechanism that
mediates continuous recycling of intracellular components. As a
response to stress conditions, such as I/R, autophagy digests
cytoplasmic materials to generate essential metabolic substrates
and energy to maintain live cells (8,17).
Autophagy is recognized to be a potent cell survival mechanism and
has been shown to be involved in the ischemic tolerance observed in
the heart and neurons (10–12,18,19).
However, whether autophagy is the key mechanism of the cardiac
protection conferred by valsartan preconditioning in heart I/R
injuries remains unknown. Therefore, the aim of the current study
was to determine whether autophagy mediates the protective effects
of valsartan preconditioning in the heart. In the current study:
(i) autophagy was observed to be activated in the hearts subjected
to I/R injury, which was enhanced by valsartan pretreatment; (ii)
the protective effects of valsartan preconditioning were eliminated
with autophagy inhibition in vivo. These results revealed
that the myocardial protection of valsartan against I/R injury
depends, at least in part, on the activation of autophagy. It has
been reported that valsartan exerted protection on the heart
against I/R injury via an Angiotensin II-independent mechanism
(4–6). Iekushi et al(6) showed that a novel mechanism of
valsartan on myocardial infarction occurs via inhibition of the
antiadhesion molecule, periostin. Two individual studies reported
that valsartan protects the heart from acute I/R via inhibition of
the TLR4/NF-κB pathway or by improving the balance between
TIMP-3/MMP-9 (4,5). To the best of our knowledge, the
current study is the first to investigate valsartan induced
autophagy, which is associated with its protection against
myocardial I/R injury.
The effect and mechanism of autophagy in ischemic
disease has been widely investigated, however, it remains unclear
and controversial (9–13). The current results indicate that
autophagy was upregulated in the rat heart subjected to I/R injury,
which was enhanced by valsartan preconditioning. Matsui et
al(20) reported that
autophagy was implicated in cell death with the increased infarct
size upon reperfusion of the ischemic myocardium. By contrast, it
was previously reported that Chloramphenicol succinate induced
autophagy and protected the swine heart from I/R injury (13), suggesting that autophagy exerts a
protective mechanism against I/R injury. Ma et al(10) reported that I/R injury upregulated
cardiomyocyte autophagy as a stress-response mechanism; however,
the autophagosome clearance is impaired. This study explained why
autophagy has different roles in I/R. In the present study the
results indicated that valsartan-induced autophagy exerted
protection against myocardial I/R injury.
The current results indicate that valsartan induced
autophagy, at least in part, through the suppression of the
AKT/mTOR/p70S6K signaling pathway but not Beclin-1. Consistent with
the present study, Kondo et al(21,22)
have shown that inhibition of the AKT/mTOR/p70S6K pathway by
curcumin led to the induction of autophagy in malignant gliomas.
Autophagy activation is complex and is not completely understood,
and leads to the activation of a wide range of signaling pathways
(23–26). The best understood pathway for the
regulation of autophagy is the target of rapamycin complex 1
(TORC1), which directly phosphorylates and negatively regulates the
Atg1 complex (23,24,27,28).
TORC1 acts as a central component of a number stress pathways,
linking them to the autophagic machinery and its activity may be
inferred by the phosphorylation levels of its target protein,
ribosomal S6 kinase and eukaryotic translation initiation factor
4E-bingding protein 1. Upstream, TORC activity and a number of
stress responses may be regulated via AKT. AKT was phosphorylated
in the hearts of I/R controls and this effect was reversed by
valsartan pretreatment. In addition, phosphorylation of 4E-BP1,
which is downstream of mTOR, was significantly attenuated by
valsartan pretreatment compared with I/R alone. Another pathway
that positively regulates autophagy is Beclin-1, which is markedly
associated with autophagic cell death (29–31).
No change of Beclin-1 was observed. Thus, Figure 5 indicated that valsartan was
shown to preserve AKT/mTOR/S6K phosphoralation and did not alter
the Beclin-1 level. These findings indicated that valsartan-induced
autophagy was AKT/mTOR dependent.
In conclusion, the current study demonstrated that
preconditioning with the AT1R antagonist, valsartan, protected
against I/R injury via autophagy, which is mTOR dependent. Further
studies are required to elucidate the effect of valsartan on
autophagy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81001436 to Dr. X
Wu, grant no. 81000353 to Dr. G Zhang and grant no. 81173062 to Dr.
J Luo), the Education Administration Research Foundation of
Guangdong Province(grant no. LYM10110 to Dr. X Wu) and the
Education Administration Research Foundation of Guangzhou City
(grant nos. 10A018G and 10A157 to Dr. X Wu).
References
|
1
|
Tritto I, Zuchi C, Vitale S and Ambrosio
G: Therapy against reperfusion-induced microvascular injury. Curr
Pharm Des. 19:4586–4596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cianchetti S, Del Fiorentino A, Colognato
R, Di Stefano R, Franzoni F and Pedrinelli R: Anti-inflammatory and
anti-oxidant properties of telmisartan in cultured human umbilical
vein endothelial cells. Atherosclerosis. 198:22–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varagic J, Frohlich ED, Susic D, et al:
AT1 receptor antagonism attenuates target organ effects of salt
excess in SHRs without affecting pressure. Am J Physiol Heart Circ
Physiol. 294:H853–H858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Jiang H, Yang J, Ding JW, Chen LH,
Li S and Zhang XD: Valsartan preconditioning protects against
myocardial ischemia-reperfusion injury through TLR4/NF-kappaB
signaling pathway. Mol Cell Biochem. 330:39–46. 2009. View Article : Google Scholar
|
|
5
|
Sawicki G, Menon V and Jugdutt BI:
Improved balance between TIMP-3 and MMP-9 after regional myocardial
ischemia-reperfusion during AT1 receptor blockade. J Card Fail.
10:442–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iekushi K, Taniyama Y, Azuma J, et al:
Novel mechanisms of valsartan on the treatment of acute myocardial
infarction through inhibition of the antiadhesion molecule
periostin. Hypertension. 49:1409–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kundu M and Thompson CB: Autophagy: basic
principles and relevance to disease. Annu Rev Pathol. 3:427–455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oka T, Hikoso S, Yamaguchi O, et al:
Mitochondrial DNA that escapes from autophagy causes inflammation
and heart failure. Nature. 485:251–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Liu H, Foyil SR, et al: Impaired
autophagosome clearance contributes to cardiomyocyte death in
ischemia/reperfusion injury. Circulation. 125:3170–3181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buss SJ, Muenz S, Riffel JH, et al:
Beneficial effects of Mammalian target of rapamycin inhibition on
left ventricular remodeling after myocardial infarction. J Am Coll
Cardiol. 54:2435–2446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marin TM, Keith K, Davies B, et al:
Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of
LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest.
121:1026–1043. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sala-Mercado JA, Wider J, Undyala VV, et
al: Profound cardioprotection with chloramphenicol succinate in the
swine model of myocardial ischemia-reperfusion injury. Circulation.
122:S179–S184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhai P, Sciarretta S, Galeotti J, Volpe M
and Sadoshima J: Differential roles of GSK-3β during myocardial
ischemia and ischemia/reperfusion. Circ Res. 109:502–511. 2011.
|
|
15
|
Wu X, Cheng J, Li P, et al:
Mechano-sensitive transcriptional factor Egr-1 regulates
insulin-like growth factor-1 receptor expression and contributes to
neointima formation in vein grafts. Arterioscler Thromb Vasc Biol.
30:471–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Huang H, Tang F, Le K, Xu S and Liu
P: Regulated expression of endothelial lipase in atherosclerosis.
Mol Cell Endocrinol. 315:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N: Autophagy: process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar
|
|
18
|
Wang P, Guan YF, Du H, Zhai QW, Su DF and
Miao CY: Induction of autophagy contributes to the neuroprotection
of nicotinamide phosphoribosyltransferase in cerebral ischemia.
Autophagy. 8:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gustafsson AB and Gottlieb RA: Autophagy
in ischemic heart disease. Circ Res. 104:150–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui Y, Takagi H, Qu X, et al: Distinct
roles of autophagy in the heart during ischemia and reperfusion:
roles of AMP-activated protein kinase and Beclin 1 in mediating
autophagy. Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ganley IG, Lam du H, Wang J, Ding X, Chen
S and Jiang X: ULK1. ATG13FIP200 complex mediates mTOR signaling
and is essential for autophagy. J Biol Chem. 284:12297–12305. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egan DF, Shackelford DB, Mihaylova MM, et
al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase
connects energy sensing to mitophagy. Science. 331:456–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stephan JS, Yeh YY, Ramachandran V,
Deminoff SJ and Herman PK: The Tor and PKA signaling pathways
independently target the Atg1/Atg13 protein kinase complex to
control autophagy. Proc Natl Acad Sci USA. 106:17049–17054. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicklin P, Bergman P, Zhang B, et al:
Bidirectional transport of amino acids regulates mTOR and
autophagy. Cell. 136:521–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu L, McPhee CK, Zheng L, et al:
Termination of autophagy and reformation of lysosomes regulated by
mTOR. Nature. 465:942–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou M, Lu N, Hu C, et al: Beclin
1-mediated autophagy in hepatocellular carcinoma cells: implication
in anticancer efficiency of oroxylin A via inhibition of mTOR
signaling. Cell Signal. 24:1722–1732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zou Z, Wu L, Ding H, et al: MicroRNA-30a
sensitizes tumor cells to cis-platinum via suppressing beclin
1-mediated autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ugland H, Naderi S, Brech A, Collas P and
Blomhoff HK: cAMP induces autophagy via a novel pathway involving
ERK, cyclin E and Beclin 1. Autophagy. 7:1199–1211. 2011.
View Article : Google Scholar : PubMed/NCBI
|