Introduction
Human gliomas are one of the main types of
malignancy in the central nervous system. Gliomas are the most
aggressive form of brain tumor and are associated with high rates
of morbidity and mortality (1).
Despite the recent advances in surgery, radiotherapy and
chemotherapy, the survival chances of patients with glioma remain
poor. The median overall survival of patients with malignant glioma
is <1 year and local recurrence occurs in >90% of patients
(2). As the survival rates of
cancer patients improve, the incidence of brain metastases is
rising (3). Transforming growth
factor β (TGF-β) promotes cancer metastases (4).
TGF-β is a multifunctional cytokine involved in the
regulation of cell proliferation, differentiation and survival or
apoptosis of numerous types of cell. It induces epithelial
mesenchymal transition (EMT) through the activation of downstream
signaling pathways, including Smad and non-Smad signaling pathways.
TGF-β induces Akt activation through PI3K during EMT in various
cell types. Once activated, Akt initiates the mTOR signaling
pathway which is involved in cell survival, growth, migration and
invasion (5). In the brain, TGF-β
is normally expressed at a very low level, which increases markedly
following injury. Increased mRNA levels of the three TGF-β isoforms
(5) correlate with the degree of
malignancy of human gliomas (6).
Blocking the action of TGF-β inhibits tumor viability, migration
and metastases in mammary cancer, melanoma and prostate cancer
models.
MicroRNAs (miRNAs), a class of small noncoding RNAs,
are a novel type of gene expression regulator as they target mRNAs
for translational repression or cleavage. Deregulation of miRNAs
has been demonstrated in a variety of tumors, including breast
cancer, leukemia, lung cancer and colon cancer, which indicates a
significant correlation between miRNAs and human tumor malignancy
(7). miRNAs are targets for
anticancer therapies as their activity is efficiently blocked by
sequence-specific oligonucleotides or other antisense approaches
(8). miR10a and miR10b have been
demonstrated to be upregulated in glioblastomas and anaplastic
astrocytomas, reaching >100-fold overexpression in certain cases
(9,10). The miRNAs miR10a and miR10b are
close homologs, differing by a single central nucleotide. In the
mouse embryo, miR10a is mainly expressed in a region of the
posterior trunk (11), whereas
miR10a in adult mice is broadly expressed, with the highest levels
identified in the kidney, muscle, lung and liver. The miR10a
homolog miR10b is highly overexpressed in several tumor types and
is reportedly involved in the progression of cancer (12).
miR10a regulates the metastatic properties of
hepatocellular cancer (HCC) by directly targeting EphA4 (13) and is involved in the metastatic
behavior of pancreatic cancer (14). The homolog of miR10a, miR10b, has
been suggested to enhance the migration and invasion of metastatic
breast cancer cells by repressing the translation of HoxD10
(15–17). In addition, it is reported that
miR-10b is expressed in glioma tumors but not in normal brain
cells, neural progenitor cells or mature glia or neurons (18–20).
Therefore, miR10a/10b is considered a target for anti-glioma
therapy (21).
The present study investigated whether miR10a/10b
expression is associated with TGF-β expression levels in brain
tumor tissues. It has previously been reported that TGF-β induces
miR10a expression in Treg cells (22). Furthermore, the present study
evaluated the hypothesis that the 3′ untranslated region (3′UTR) of
phosphatase and tensin homolog deleted on chromosome 10 (PTEN)
contains binding sequences for miR10a and miR10b. Tumor suppressor
PTEN is a dual-specific phosphatase that is a negative regulator of
the PI3K-AKT-mTOR signaling pathway, thus controlling a variety of
processes associated with cell survival, proliferation and growth
(23). Therefore, the theory that
miR10a and miR10b are induced by TGF-β and involved in
TGF-β-induced metastasis by suppressing PTEN expression in brain
tumors was evaluated.
Materials and methods
Human tissue samples
Fresh, frozen human brain tumor samples were
obtained from the Department of Neurosurgery at the Integrated
Chinese and Western Medicine Hospital of Zhejiang Province
(Hangzhou, China). The study protocol was approved by the
institutional Ethics Committee of the Integrated Chinese and
Western Medicine Hospital of Zhejiang Province and informed consent
was obtained from all patients or patients’ families.
Cell cultures and treatment
Human glioma U251 and SHG-44 cells were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The U251 and SHG-44 cells were cultured in RPMI-1640 medium
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal calf
serum (PAA Laboratories GmbH, Linz, Austria) at 37°C under 5%
humidified CO2 and 100 μg/ml each of streptomycin and
penicillin G was added (Amresco, Solon, OH, USA). Cells were
treated with TGF-β (5 ng/ml) and were transfected with miR10a
and/or 10b mimics, miR10a and/or 10b inhibitors (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) or the PTEN expression plasmid
pCDNA3.1-PTEN.
RNA isolation and quantitative PCR
(qPCR)
RNA was extracted using TRIzol (Invitrogen,
Carlsbad, CA, USA). Total RNA (1μg) was reverse-transcribed using a
RevertAid First Strand cDNA Synthesis kit (Fermentas, Waltham, MA,
USA). miR10a/10b, U6, TGF-β, PTEN and β-actin expression levels
were measured using SYBR-Green (Roche, Mannheim, Germany). The
expression of each target gene was determined by triplicates from
three to six separate experiments and normalized using β-actin and
miR10a/10b normalized using U6. qPCR Assays-on-Demand were
performed using the ABI PRISM 7300 Sequence Detection system 2.1
(PE Applied Biosystems, Foster City, CA, USA) using relative
quantification. Analysis and fold differences were determined using
the comparative cycle threshold (CT) method. Fold change was
calculated from the ΔΔCT values with the formula
2−ΔΔCT.
Western blot analysis
Anti-PTEN and anti-GAPDH rabbit monoclonal
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Protein (100 μg) was subjected to 12% SDS-PAGE
and transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked for 1 h
in PBST (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween-20)
containing 2% nonfat dried milk. Subsequently, Anti PTEN (1:1,000)
and anti GAPDH (1:1,000) rabbit monoclonal antibodies were
incubated with the membranes. After 2 h, the primary antibodies
were washed and the mouse anti-rabbit secondary antibodies (Cell
Signaling Technology) were incubated with the membranes. Protein
bands were detected by an enhanced chemiluminescence reaction (ECL
Detection; Millipore).
Migration assay
The migration assay was performed using 24-well
transwell chambers (8 μm; Millipore). The U251 cells transfected
with miR10a/10b mimics, miR10a/10b inhibitors or pCDNA3.1-PTEN were
suspended in RPMI-1640 medium without serum and 2×104
cells were seeded onto Matrigel™ inserts in triplicate. They were
then placed into a 24-well culture plate containing 500 μl
RPMI-1640 medium with or without 5 ng/ml TGF-β. Following culture
for a total of 48 h, cells on the upper side of the filters were
removed with cotton-tipped swabs and the filters were washed with
PBS. Cells on the underside of the filters were examined and
counted under a microscope. Images were obtained with four randomly
selected fields from each insert The number of cells in each field
was counted and averaged. Migration is expressed as fold increase
compared with the control.
Constructs for luciferase reporter
assays
Primers were designed to the region of the PTEN
3′UTR believed to contain miR10a/b binding sequences using Primer
Premier 5 (Premier Biosoft, Palo Alto, CA, USA). The primers were
as follows: forward, AATGGCAATAGGACATTGTG and reverse,
TACATGACACAGCTACACAA. The PTEN 3′UTR fragment was cloned from human
genomic DNA into the pGL3-control-luciferase vector (Promega,
Madison, WI, USA). Constructed plasmids were confirmed by
sequencing.
Luciferase assays
Cells were plated in 24-well plates at a density of
1.5×104 cells per well. Each well received 250 ng of a
pGL3-pro-luciferase reporter and 5 ng of a Renilla
luciferase reporter (Promega). The cells were harvested using
Passive Lysis buffer (Promega) following co-transfection with PTEN
3′UTR constructs and miR10a/10b or their inhibitor for 24 h.
Luciferase and Renilla luciferase activities were determined
using a Dual-Luciferase Reporter assay system (Promega) in a Plate
Chameleon luminometer (BioScan, Inc., Washington, DC, USA). Firefly
luciferase was normalized by Renilla luciferase to correct
for transfection efficiency. Fold induction was determined by
dividing the averaged normalized values from each treatment by the
control value for each transfection condition within that
experiment. Values were averaged from multiple experiments, as
indicated in the figure legends.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.01 software, version (GraphPad Software, San Diego, CA,
USA). Two-tailed Student’s t-tests were used for the pair-wise
comparison of experimental groups. Statistical significance was
defined at the ≥95% confidence interval or P≤0.05. In each figure,
asterisks indicate significant differences from the controls
(P<0.05). Bar graphs represent the mean ± standard error of the
mean of the number of independent experiments indicated in each
figure legend. In addition, the analysis of the correlations among
miR10a/10b, TGF-β and PTEN expression in human glioma patients was
conducted by bivariate analysis using Spearman’s correlation
coefficient.
Results
miR10a/10b expression is associated with
TGF-β expression levels in human glioma samples
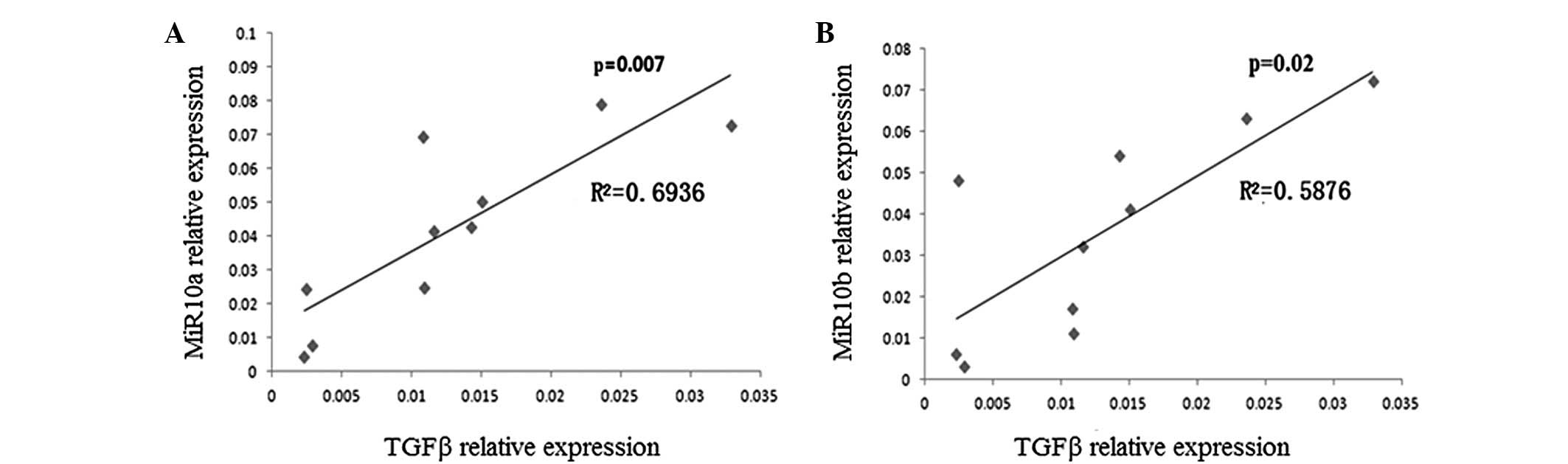
The expression of TGF-β and miR10a/10b was measured
in the tissues of 10 patients with brain tumors using qPCR. The
correlation between TGF-β and miR10a or miR10b expression was
analyzed according to the method described in Materials and
methods. As shown in Fig. 1, there
was a significant association between TGF-β and miR10a expression
(r2=0.6936, P=0.007) and between TGF-β and miR10b expression
(r2=0.5876, P=0.02) in the tissues of the patients with brain
tumors.
TGF-β promotes migration and miR10a/10b
expression in glioma cells
TGF-β is believed to promote cell EMT and migration.
As miR10a/10b expression is associated with the expression levels
of TGF-β in brain tumor patients and it is reported that miR10a/b
also promotes cell migration, further investigations were carried
out to determine whether miR10a/10b is the direct target gene of
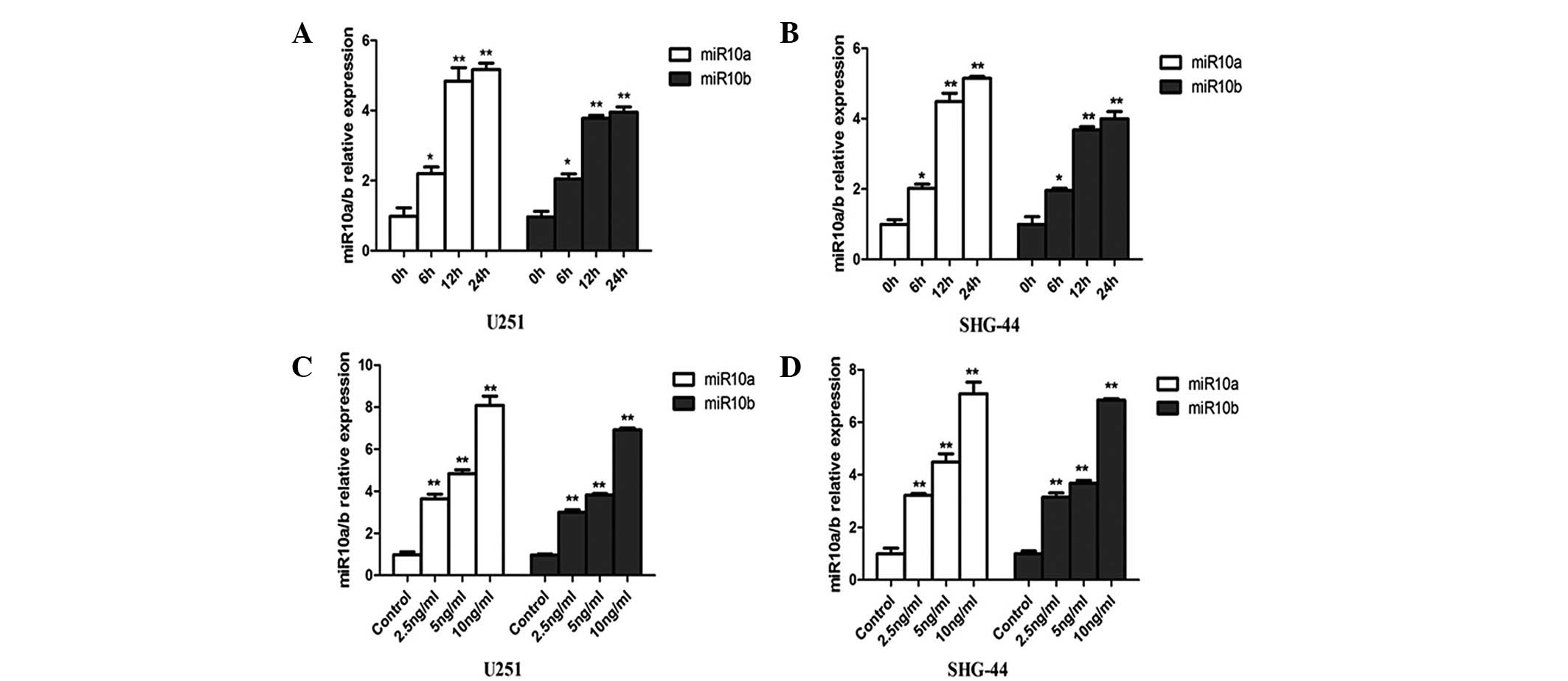
TGF-β. U251 and SHG-44 cells were treated with TGF-β, and the
levels of miR10a/10b expression were measured by qPCR (Fig. 2). The miR10a and miR10b expression
levels were significantly upregulated by TGF-β in the two cell
lines (Fig. 2A and B).
Concentration-response experiments demonstrated a maximal
miR10a/10b induction with 10 ng/ml TGF-β after 12 h of treatment in
U251 and SHG-44 cells, which was ~5–8 fold above that in the
control groups (Fig. 2C and D).
Since 5 ng/ml TGF-β is close to the physiological concentration
(24), this concentration was used
in the following experiments.
TGF-β-induced miR10a/10b expression
strengthens glioma cell migration
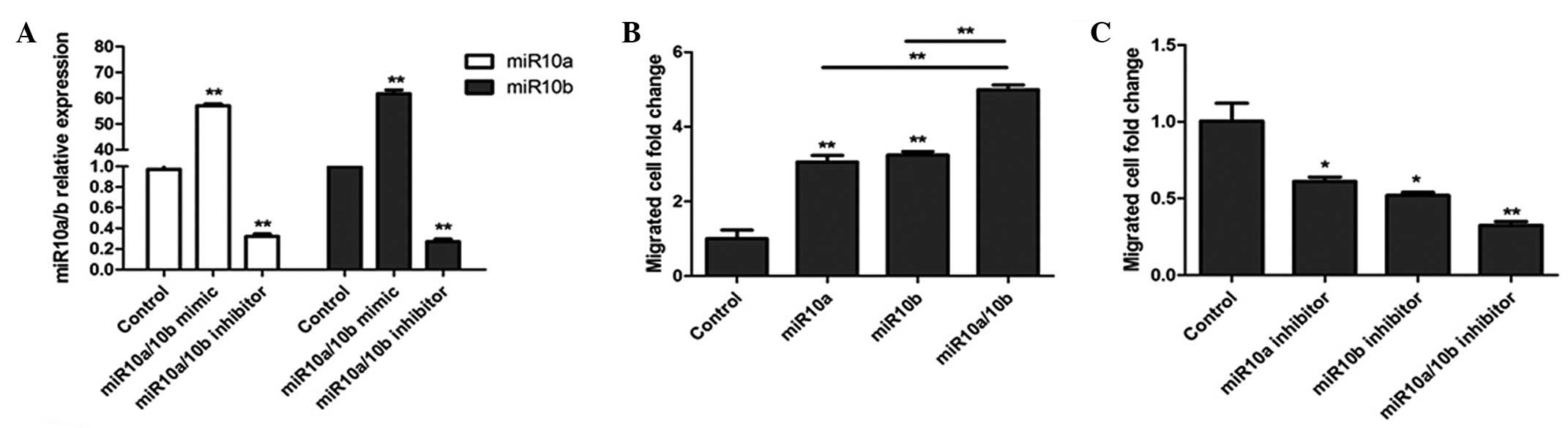
To define the functional impact of TGF-β-induced
miR10a/10b expression, a migration assay was conducted as described
in Materials and methods. miR10a and miR10b mimics were transfected
into U251 cells and after 24 h, miR10a and miR10b expression was
evaluated by qPCR. As shown in Fig.
3A, the miR10a and miR10b mimics significantly increased the
expression levels of miR10a/10b and their inhibitors significantly
decreased the expression levels of miR10a/10b compared with the
control levels. Following transfection with miR10a/10b mimics or
inhibitors, U251 cell migration was assessed. As shown in Fig. 3B, treatment of U251 cells with
miR10a/10b mimics resulted in brain tumor cell migration. However,
in cells pretreated with miR10a/10b inhibitor, cell migration was
inhibited (Fig. 3C).
TGF-β-induced miR10a/10b expression
promotes glioma cell migration through targeting PTEN
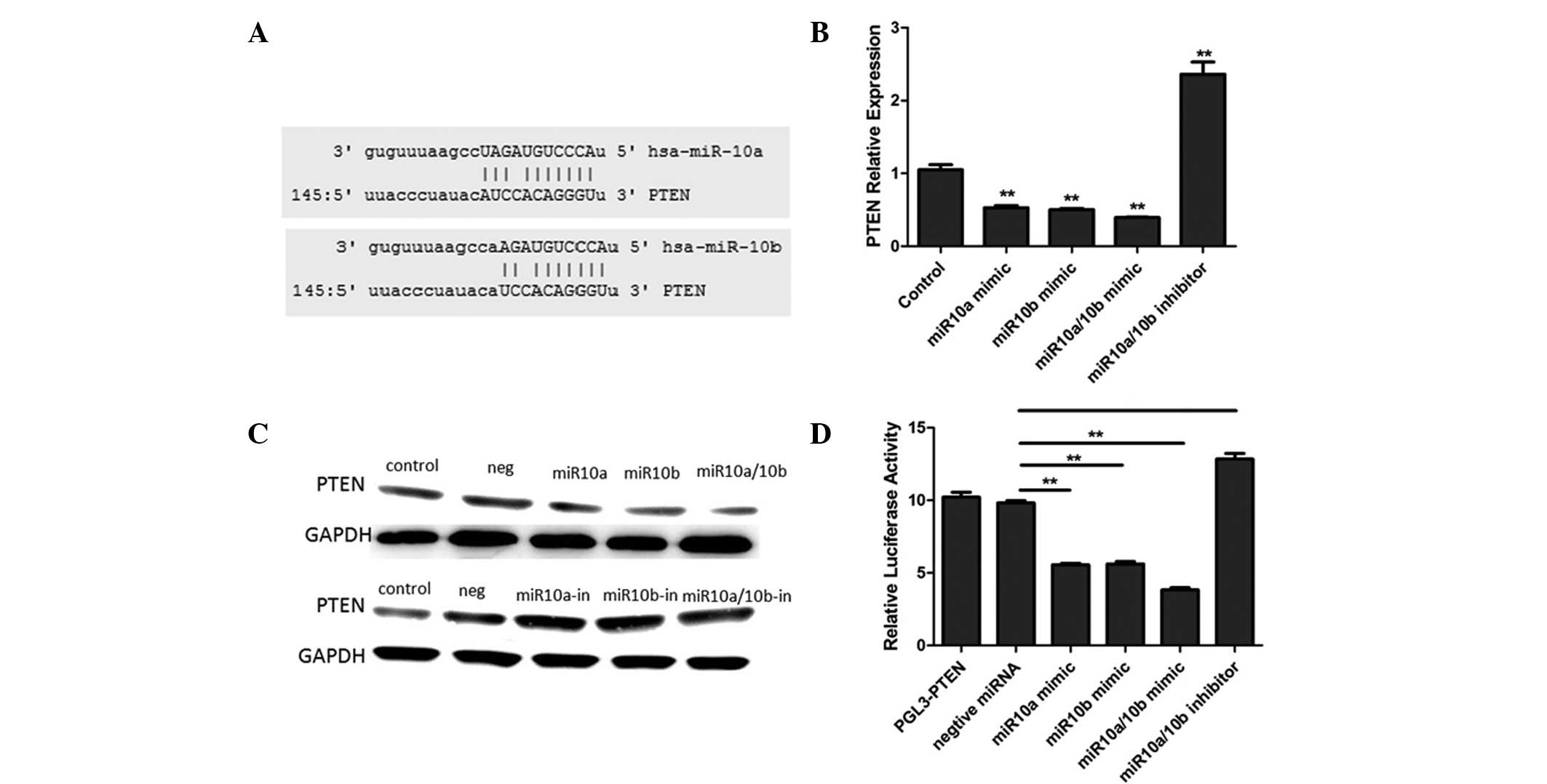
PTEN as a tumor suppressor inhibits cell
proliferation and migration by blocking PI3K-AKT signaling. Through
online software [miRanda (http://diana.cslab.ece.ntua.gr/microT/), TargetScan
(http://www.targetscan.org/) and PicTar
(http://pictar.mdc-berlin.de/)], it was
identified that the PTEN 3′UTR contains binding sequences for
miR10a and miR10b (Fig. 4A). To
further establish that miR10a/10b targets PTEN, miR10a and miR10b
mimics and inhibitors were transfected into U251 cells. The results
demonstrated that miR10a and miR10b mimics downregulated PTEN
expression levels and their inhibitors upregulated PTEN expression
levels (Fig. 4B and C).
Furthermore, the luciferase reporter analysis results identified
that miR10a and miR10b mimics significantly suppressed the
expression of a luciferase reporter gene fused to the 3′UTR region
of PTEN, which was reversed by the further introduction of a
miR10a/10b inhibitor in U251 cells (Fig. 4D). These results confirmed that
miR10a/10b suppresses PTEN expression through binding to its 3′UTR.
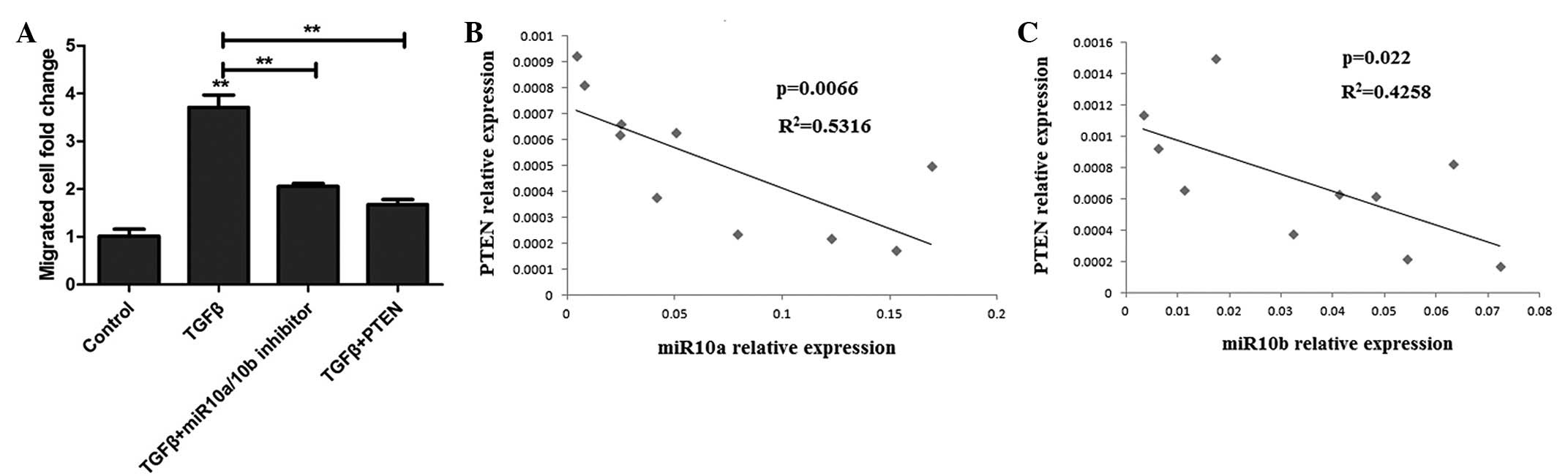
To further investigate whether TGF-β-induced cell migration occurs
through miR10a/10b targeting PTEN, U251 cells were transfected with
miR10a/10b inhibitors or the pCDNA3.1-PTEN plasmid prior to TGF-β
treatment. After 48 h, the migrated cells were analyzed and the
results demonstrated that the miR10a/10b inhibitor inhibits
TGF-β-induced cell migration and that the PTEN overexpression
plasmid has a similar effect to the inhibitor (Fig. 5A). These results indicate that
TGF-β-induced miR10a/10b expression inhibits PTEN expression, which
results in brain tumor cell migration.
miR10a/10b expression positively
correlates with PTEN expression in clinical human glioma
patients
As TGF-β-induced miR10a and miR10b expression
promotes cell migration via targeting PTEN, it was further
investigated whether PTEN expression was associated with the
miR10a/10b expression detected in the cells of human clinical
glioma specimens. As shown in Fig. 5B
and C, PTEN and miR10a/10b expression is correlated in brain
tumor patients.
Discussion
Metastatic brain tumors are malignant neoplasms that
spread to the brain from elsewhere in the body and represent the
most common neurologic manifestation of cancer, occurring in up to
15% of cancer patients. Brain metastases are the most common type
of intracranial tumor in adults, accounting for ~40% of
intracranial neoplasms (25). With
the improving survival rates of cancer patients, the incidence of
brain metastases has been rising (2).
TGF-β elicits tumor-promoting effects through its
ability to induce EMT, which enhances invasiveness and metastasis
(26). TGF-β induces EMT through
the activation of downstream signaling pathways, including Smad and
non-Smad signaling pathways. TGF-β induces Akt activation through
PI3K during EMT in various cell types. Once activated, Akt
initiates the mTOR signaling pathway which is involved in cell
survival, growth, migration and invasion (5).
It has been reported that TGF-β promotes the
expression of miR181b, miR192 and miR21 (27–29).
In addition, Takahashi et al reported that TGF-β induced the
expression of miR10a in Treg cells (22). The present study demonstrated that
TGF-β promotes the expression of miR10a and miR10b.
The microRNAs miR10a and mi10b enhance cell
migration. The homolog of miR10a, miR10b, has been suggested to
enhance tumor cell migration and the invasion of metastatic breast
cancer cells by repressing the translation of HoxD10 (16). In addition, miR10a is believed to
regulate the metastatic properties of HCC by directly targeting
EphA4 (13). miR-10b inhibits
translation of the mRNA of HOXD10, a transcription factor known for
its roles in cell motility, resulting in the increased expression
of a pro-metastatic gene, RHOC. It also promotes the cell migration
and invasion of human esophageal squamous cell carcinoma cells by
the direct regulation of KLF4 (30). In addition, miR-10b targets
neurofibromin 1 mRNA, leading to the activation of RAS signaling in
neurofibromatosis type I (31).
The overexpression of miR10b in non-metastatic cell lines has been
demonstrated to promote tumor invasion and metastasis in a
xenograft mouse model (21).
TGF-β-induced miR10a/10b targets PTEN. It has been
reported that PTEN, an important tumor suppressor, is regulated by
multiple miRNAs (32). It has also
been demonstrated that miR21 increases tumor cell proliferation,
migration and invasion through targeting PTEN (33). The present study indicated that
miR10a and miR10b target PTEN.
In conclusion, the present study demonstrated that
miR10a/10b expression is associated with TGF-β expression in human
glioma tissues and it further affirmed that TGF-β induces the
expression of miR10a/10b in human glioma cells. Furthermore, it
demonstrated that TGF-β-induced miR10a/10b expression promotes the
migration of human glioma cells through targeting the tumor
suppressor, PTEN. This study may provide a number of suggestions
for human glioma clinical treatment.
References
|
1
|
Chan XH, Nama S, Gopal F, Rizk P, Ramasamy
S, Sundaram G, et al: Targeting glioma stem cells by functional
inhibition of a prosurvival oncomiR-138 in malignant gliomas. Cell
Rep. 2:591–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X, Yang H, Gong B, Jiang C and Yang
L: Combined gene expression and protein interaction analysis of
dynamic modularity in glioma prognosis. J Neurooncol. 107:281–288.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathupala SP, Mittal S, Guthikonda M and
Sloan AE: MicroRNA and brain tumors: a cause and a cure? DNA Cell
Biol. 26:301–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu H, Hu ZL, Wen JF, Wang KS and Liu Y:
TGF-β promotes invasion and metastasis of gastric cancer cells by
increasing fascin1 expression via ERK and JNK signal pathways. Acta
Biochim Biophys Sin (Shanghai). 41:648–656. 2009.
|
|
5
|
Kaminska B, Kocyk M and Kijewska M: TGF
beta signaling and its role in glioma pathogenesis. Adv Exp Med
Biol. 986:171–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connolly EC, Freimuth J and Akhurst RJ:
Complexities of TGF-β targeted cancer therapy. Int J Biol Sci.
8:964–978. 2012.
|
|
7
|
Nicoloso MS and Calin GA: MicroRNA
involvement in brain tumors: from bench to bedside. Brain Pathol.
18:122–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaur A, Jewell DA, Liang Y, Ridzon D,
Moore JH, Chen C, Ambros VR and Israel MA: Characterization of
microRNA expression levels and their biological correlates in human
cancer cell lines. Cancer Res. 67:2456–2468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pang JC, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mansfield JH, Harfe BD, Nissen R, Obenauer
J, Srineel J, Chaudhuri A, et al: MicroRNA-responsive ‘sensor’
transgenes uncover Hox-like and other developmentally regulated
patterns of vertebrate microRNA expression. Nat Genet.
36:1079–1083. 2004.
|
|
12
|
Garzon R, Pichiorri F, Palumbo T, Iuliano
R, Cimmino A, Aqeilan R, et al: MicroRNA fingerprints during human
megakaryocytopoiesis. Proc Natl Acad Sci USA. 103:5078–5083. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan Y, Luo YC, Wan HY, et al: MicroRNA-10a
is involved in the metastatic process by regulating Eph tyrosine
kinase receptor A4-mediated epithelial-mesenchymal transition and
adhesion in hepatoma cells. Hepatology. 57:667–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss FU, Marques IJ, Woltering JM,
Vlecken DH, Aghdassi A, Partecke LI, et al: Retinoic acid receptor
antagonists inhibit miR-10a expression and block metastatic
behaviour of pancreatic cancer. Gastroenterology. 137:2136–2145.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ørom UA, Nielsen FC and Lund AH:
MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and
enhances their translation. Mol Cell. 30:460–471. 2008.PubMed/NCBI
|
|
16
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–60.
2012.PubMed/NCBI
|
|
18
|
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H
and Liu Z: MicroRNA-10b promotes migration and invasion through
KLF4 in human esophageal cancer cell lines. J Biol Chem.
285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garzon R, Garofalo M, Martelli MP,
Briesewitz R, Wang L, Fernandez-Cymering C, et al: Distinctive
microRNA signature of acute myeloid leukemia bearing cytoplasmic
mutated nucleophosmin. Proc Natl Acad Sci USA. 105:3945–3950. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lund AH: miR-10 in development and cancer.
Cell Death Differ. 17:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi H, Kanno T, Nakayamada S,
Hirahara K, Sciumè G, Muljo SA, et al: TGF-β and retinoic acid
induce the microRNA miR-10a, which targets Bcl-6 and constrains the
plasticity of helper T cells. Nat Immunol. 13:587–595. 2012.
|
|
23
|
Luo H, Yang Y, Duan J, Wu P, Jiang Q and
Xu C: PTEN-regulated AKT/FoxO3a/Bim signaling contributes to
reactive oxygen species-mediated apoptosis in selenite-treated
colorectal cancer cells. Cell Death Dis. 4:e4812013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wakefield LM, Letterio JJ, Chen T,
Danielpour D, Allison RS, Pai LH, Denicoff AM, Noone MH, Cowan KH,
O’Shaughnessy JA, et al: Transforming growth factor-beta1
circulates in normal human plasma and is unchanged in advanced
metastatic breast cancer. Clin Cancer Res. 1:129–136.
1995.PubMed/NCBI
|
|
25
|
Patel RR and Mehta MP: Targeted therapy
for brain metastases: improving the therapeutic ratio. Clin Cancer
Res. 13:1675–1683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGF-β in cancer. FEBS Letters.
586:1959–1970. 2012.
|
|
27
|
Wang B, Hsu SH, Majumder S, Kutay H, Huang
W, Jacob ST and Ghosal K: TGFbeta-mediated upregulation of hepatic
miR-181b promotes hepatocarcinogenesis by targeting TIMP3.
Oncogene. 29:1787–1797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung AC, Huang XR, Meng X and Lan HY:
miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc
Nephrol. 21:1317–1325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dey N, Ghosh-Choudhury N, Kasinath BS and
Choudhury GG: TGFβ-stimulated microRNA-21 utilizes PTEN to
orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and
matrix expansion. PLoS One. 7:e423162012.
|
|
30
|
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H
and Liu Z: MicroRNA-10b promotes migration and invasion through
KLF4 in human esophageal cancer cell lines. J Biol Chem.
285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chai G, Liu N, Ma J, Li H, Oblinger JL,
Prahalad AK, et al: MicroRNA-10b regulates tumorigenesis in
neurofibromatosis type 1. Cancer Sci. 101:1997–2004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI
|
|
33
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|