Introduction
Chronic kidney disease (CKD) is currently considered
to be a predominant public health burden and a major cause of
morbidity and mortality for diabetic and hypertensive patients
worldwide. Recent clinical studies have revealed that persistent
proteinuria and serum FGF-23 levels are powerful risk factors for
the progression of end-stage renal disease (1–4).
Fibroblast growth factor-23 (FGF-23) is a newly
identified member of the FGF family. FGF-23 is secreted from the
bone and acts on the kidney to promote urinary inorganic phosphate
(Pi) excretion and to suppress vitamin D synthesis, thereby
maintaining Pi and calcium homeostasis (5,6). An
increasing number of studies have confirmed that the circulatory
levels of FGF-23, PTH and Pi are independent risk factors for CKD
progression, and are closely correlated with vascular calcification
and secondary hyperparathyroidism (7–9).
Proteinuria is a powerful and independent risk
factor for kidney disease, cardiovascular disease and all-cause
mortality (10). Thus, the
reduction in proteinuria invariably translates into protection from
a decline in renal function in order to improve the clinical
outcome. All studies are committed to reduce proteinuria, improve
renal function and extenuate complications in CKD patients
(8–10).
Thiazolidinediones (TZDs) are ligands known to bind
and activate the nuclear peroxisome proliferator-activated
receptor-γ (PPAR-γ). TZDs are used to treat diabetes mellitus, and
are known to exert their glucose-lowering effects by reducing
insulin resistance through the stimulation of PPAR-γ nuclear
receptor. In addition to improving glycemic control, TZDs have been
demonstrated to exert beneficial effects on renal and vascular
protection (11–15). In the present study, the effect of
rosiglitazone (ROG) on proteinuria and renal function in CKD models
induced by adenine was analyzed, and the contribution of the
Pi-PTH-FGF-23 system was investigated.
Materials and methods
Animal study protocols
This study was conducted according to the guidelines
of the National Institute of Health on the care and use of
laboratory animals. The project was approved by the Institutional
Animal Care and Use Committee of Sun Yat-Sen University. All rats
were housed individually and were provided with normal laboratory
chow and tap water ad libitum throughout the study. The
animals were handled daily to minimize handling stress. Male SD
rats (weight, 250–280 g) were obtained from the Animal Center of
Sun Yat-Sen University (Guangzhou, China). The SD rats were divided
randomly into four groups (n=6 per group): i) Normal group,
intragastric administration of saline consistently for four weeks;
ii) ROG controls, intragastric administration of rosiglitazone
(GlaxoSmithKline plc., Tianjin, China) 10 mg/kg/day consistently
for four weeks; iii) CKD model group, intragastric administration
of 200 mg/kg/day adenine (Sigma-Aldrich, St. Louis, MO, USA)
consistently for four weeks; and iv) ROG treatment group,
intragastric administration of ROG (10 mg/kg/day) and adenine (200
mg/kg/day) consistently for four weeks. All rats were sacrificed,
and the kidney tissues and blood samples were collected at the end
of four weeks. Urine samples of 24-h were collected on the day
prior to the rats being sacrificed.
Biochemistry assay
The blood and urine samples from the rats were
centrifuged at 1,120 × g for 10 min. The levels of serum creatine
(Cr), blood urea nitrogen (BUN), uric acid (UA), calcium,
phosphorus, intact parathyroid hormone (iPTH), alkaline phosphatase
(ALP) and the 24-h protein/Cr ratio of the urine were tested by an
autoanalyzer (Abbott, Chicago, IL, USA) in duplicate.
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of FGF-23 were measured by ELISA
(FGF-23 ELISA kit; Immutopics, San Clemente, CA, USA) according the
manufacturer’s instructions.
Hematoxylin and eosin (HE) staining and
pathological analysis
The kidneys were fixed in 4% paraformaldehyde
overnight and embedded with paraffin for HE staining.
Semi-quantitative histological scoring was performed by a
pathologist. The scoring of the tubular interstitial damage index
(TIDI) used the following criteria: The tubular atrophy and
interstitial infiltrates were scored as absent, 0; involving
<25% of the biopsy area, 1; involving 26 to 50% of the biopsy
area, 2; and involving >50% of the biopsy area, 3. The presence
of proteinaceous casts was scored as absent, 0; isolated, 1;
present in ≤10% of tubules, 2; and present in >10% of tubules,
3. Tubular dilation was scored as absent, 0; mild, 1; moderate, 2,
when associated with flattening of the tubular epithelium; or
severe, 3. Tubular dilation was scored as severe, 3, when the
diameter exceeded that of the glomeruli; these tubules were often
filled with proteinaceous casts.
Statistical analysis
The statistical analysis was conducted using
analysis of variance (SPSS 13.0 software: SPSS, Inc., Chicago, IL,
USA). P<0.05 was used to indicate a statistically significant
difference. The results are expressed as the mean ± SE.
Results
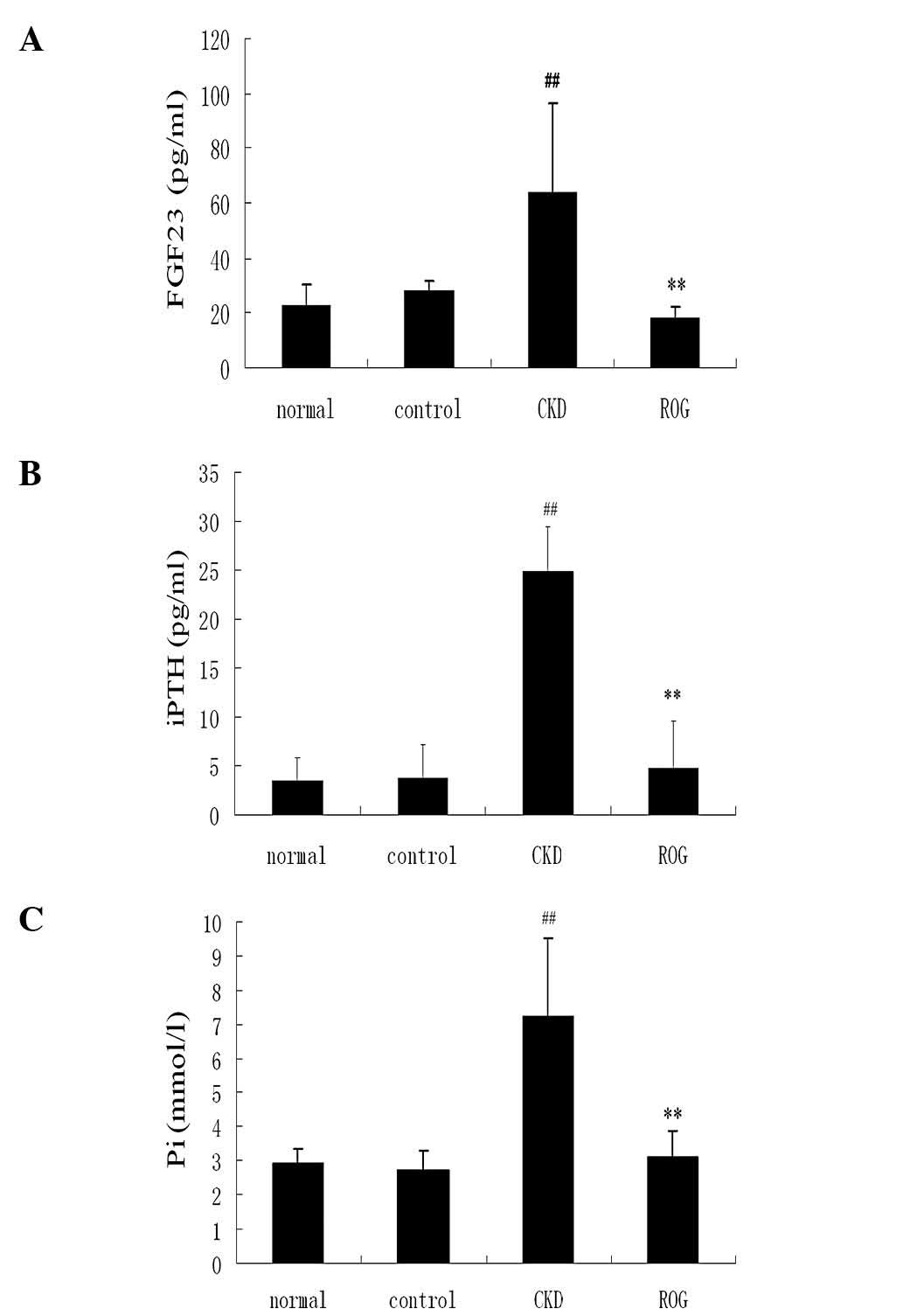
Effect of ROG on the Pi-PTH-FGF-23
system
The serum levels of FGF-23 were significantly higher
in the CKD model group compared with those of the normal group
(64.3±13.2 versus 23.2±7.2 pg/ml; P<0.01). The value of FGF-23
was significantly decreased in the ROG treatment group compared
with the CKD model group (17.9±4.5 versus 64.3±13.2 pg/ml;
P<0.01). The serum levels of iPTH and Pi were also significantly
higher in the CKD model compared with the normal group (P<0.01),
but significantly reduced following treatment with ROG in
comparison with the CKD model (P<0.01; Fig. 1A–C). However, there were no
significant differences in serum calcium and ALP levels among the
four groups.
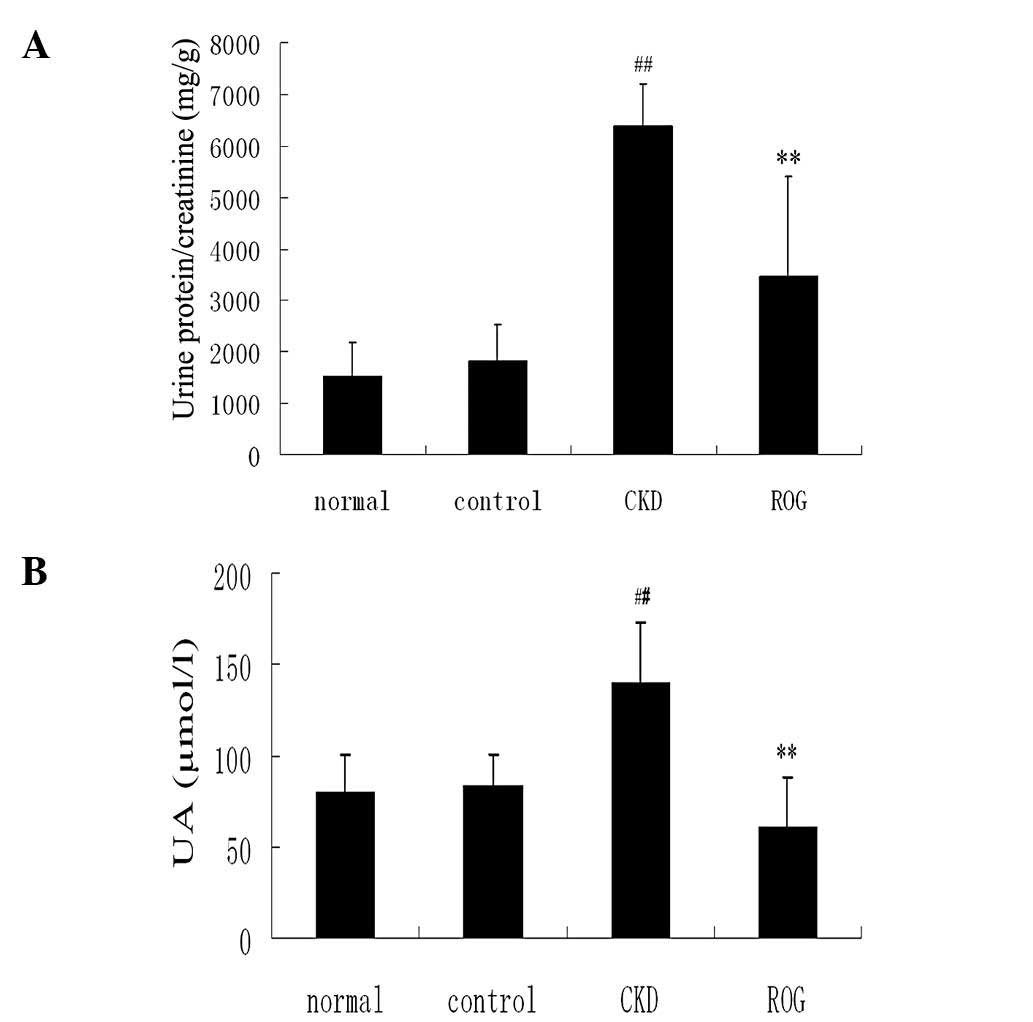
Effect of ROG on proteinuria and renal
function
The ratio of protein/Cr of the urine was
significantly increased in the CKD models compared with that in the
normal group (6,391.4±1,923.0 versus 1,526.1±719.5 mg/g;
P<0.01). However, ROG significantly reduced the proteinuria
content compared with the CKD models (3,469.75±887.55 versus
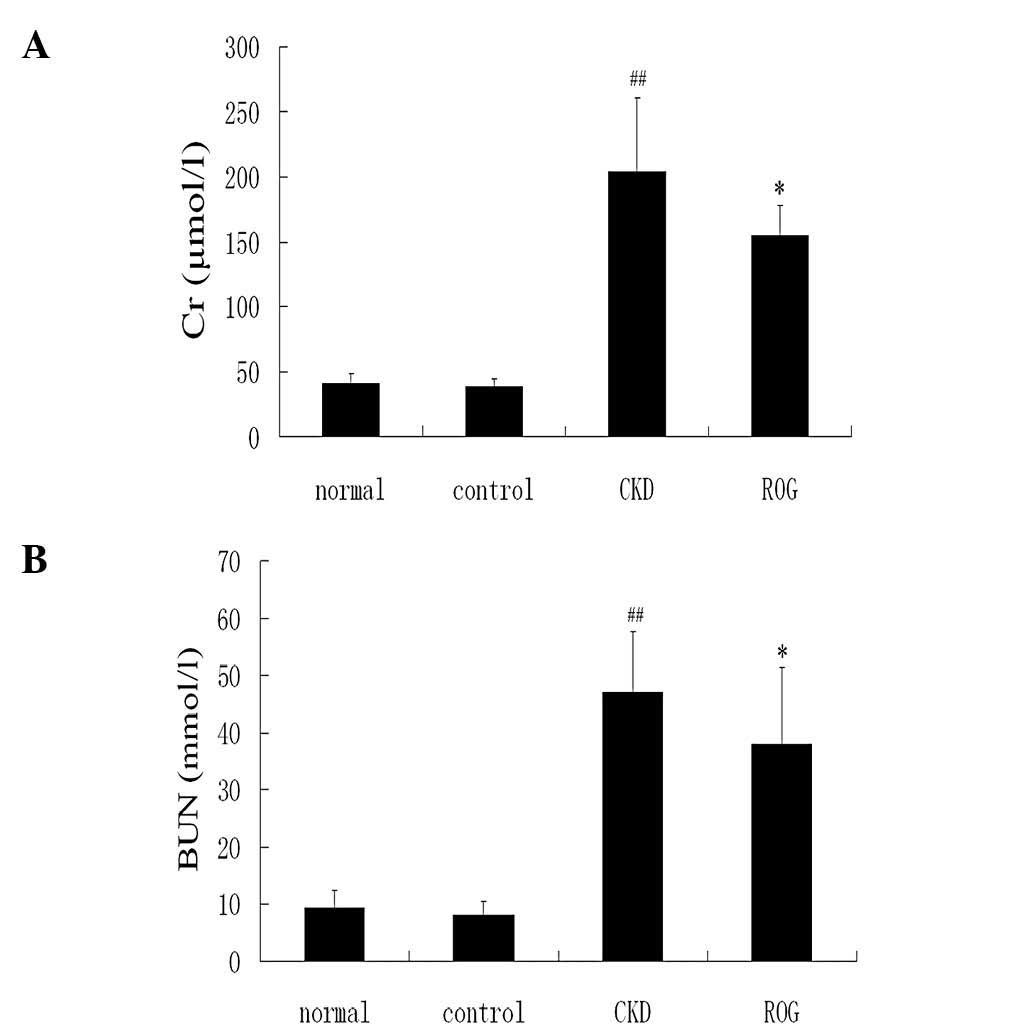
6,391.41±1,923.02 mg/g; P<0.01)(Fig. 2A–C). Serum Cr, BUN and UA levels
were also significantly increased in the CKD models group compared
with the normal group. It is noteworthy that the levels of renal
function were significantly improved following treatment with ROG
(Fig. 3A–C).
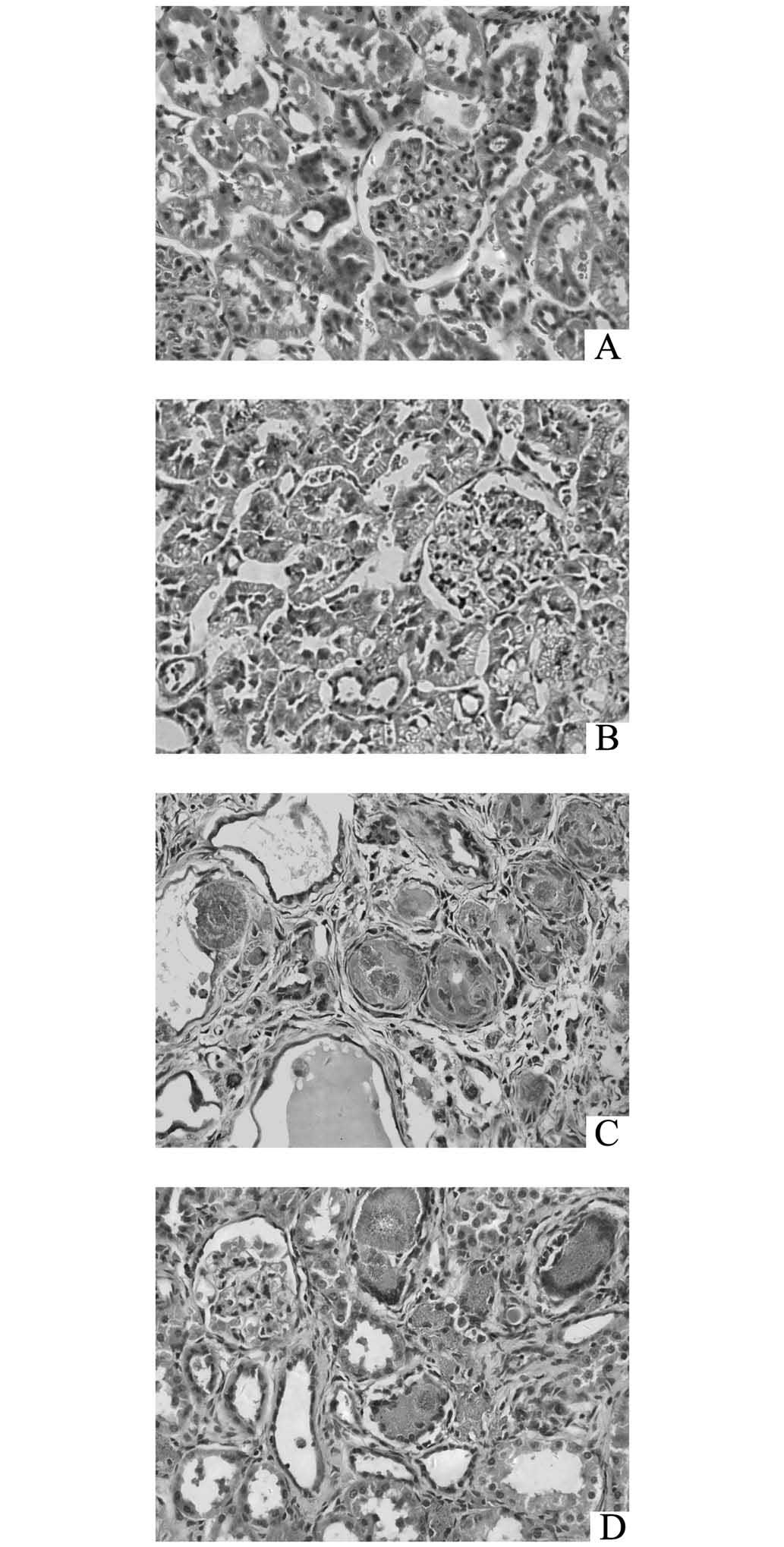
Renal pathological variation
HE staining demonstrated that the proximal
convoluted tubules were swollen, while the distal convoluted
tubules and the collecting ducts were expanded with considerable
protein casts and 2,8-dihydroxyadenine crystals in the lumina
(Fig. 4C). Monocytes proliferated
around the crystals and created nodules, and inflammatory cells
infiltrated into the interstitial tissue (Fig. 4C). However, ROG markedly improved
the aforementioned pathological lesions, as shown in Fig. 4D. The TIDI values of the CKD models
were significantly increased compared with those of the controls
(P<0.01), while ROG also significantly improved the TIDI
(P<0.05, Table I).
 | Table IComparison of the TIDI in the various
groups of the experiment. |
Table I
Comparison of the TIDI in the various
groups of the experiment.
| Parameter | Normal controls | ROG controls | CKD models | ROG treatment
group |
|---|
| TIDI | 0.53±0.19 | 0.61±0.22 | 9.33±1.74a | 5.95±0.89a,b |
Discussion
In the present study, the CKD rat models were
successfully induced with an intragastric administration of
adenine, and the serum levels of FGF23, PTH and Pi were confirmed
to be consistent with the levels in the lesions of the kidney in
this model. Furthermore, this is the first study to demonstrate
that ROG was able to attenuate renal damage and metabolic disorders
in adenine-induced CKD models. ROG, a PPAR-γ agonist, has been
indicated to exert cardiovascular and renal protection through the
improvement of anti-inflammation, antiproliferation and
antioxidative stress (13–18). These data demonstrated that ROG
exerts a polyphenic profit on the cardiovascular and renal system.
However, the effect of PPAR-γ on renal proteinuria and renal
function are not yet fully understood.
The present study confirmed the effect of ROG on
FGF-23 levels and proteinuria, each a risk factor of CKD, thus
providing a novel insight into the renoprotective properties of the
drug. The results of this study demonstrated that the serum FGF-23,
PTH and Pi levels were markedly increased in adenine-induced CKD
rats, but that ROG was able to effectively attenuate the
augmentation. FGF-23 is part of a previously unrecognized hormonal
bone-PTH-kidney axis. Mineral and bone metabolism may be viewed as
a complex system in which three hormones (PTH, calcitriol and
FGF-23) act on three key organs (the kidneys, intestines and bones)
to adjust plasma, calcium and phosphorus concentrations (19). Mineral disorders, including an
increase in FGF-23 and PTH, and hyperphosphatemia, are independent
risk factors in CKD progression and mortality (3,20,21).
The present data revealed that ROG reduced the serum levels of
FGF-23, PTH and phosphorus in the CKD rats, indicating that ROG may
have a beneficial effect on the kidney through the attenuation of
mineral disorders in adenine-induced CKD models.
It was also observed that the intervention with ROG
decreased the proteinuria and protected the renal function in the
adenine-induced CKD models. The present results were consistent
with other experimental studies that demonstrated that TZDs are
beneficial for renal and cardiovascular protection (13,18,22,23).
ROG intervention attenuated hyperuricemia, which is generally
acknowledged as an independent risk factor of CKD. Thus, the
current study revealed that ROG, a PPAR-γ agonist may decrease
proteinuria and attenuate metabolic disorders, including mineral
disorders and hyperuricemia, in adenine-induced CKD models.
Therefore, a novel renoprotective effect of TZDs may be indicated
based on the present study. Further studies are required to confirm
these findings.
Acknowledgements
The authors would like to thank Professor Wenfang
Chen from the Department of Pathology, The First Affiliated
Hospital, Sun Yat-Sen University. This study was financially
supported by the Natural Science Foundation of Guangdong Province,
China (grant no. 2012B031800448) and the Foundation of The
Educational Ministry (grant no. 2013BSD).
References
|
1
|
Bello AK, Hemmelgarn B, Lloyd A, James MT,
Manns BJ, Klarenbach S, et al: Associations among estimated
glomerular filtration rate, proteinuria, and adverse cardiovascular
outcomes. Clin J Am Soc Nephrol. 6:1418–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarnak MJ and Astor BC: Implications of
proteinuria: CKD progression and cardiovascular outcomes. Adv
Chronic Kidney Dis. 18:258–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faul C: Fibroblast growth factor 23 and
the heart. Curr Opin Nephrol Hypertens. 21:369–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakano C, Hamano T, Fujii N, Obi Y, Matsui
I, Tomida K, et al: Intact fibroblast growth factor 23 levels
predict incident cardiovascular event before but not after the
start of dialysis. Bone. 50:1266–1274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Razzaque MS and Lanske B: The emerging
role of the fibroblast growth factor-23-klotho axis in renal
regulation of phosphate homeostasis. J Endocrinol. 194:1–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimada T, Kakitani M, Yamazaki Y,
Hasegawa H, Takeuchi Y, Fujita T, et al: Targeted ablation of Fgf23
demonstrates an essential physiological role of FGF23 in phosphate
and vitamin D metabolism. J Clin Invest. 113:561–568. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seiler S, Heine GH and Fliser D: Clinical
relevance of FGF-23 in chronic kidney disease. Kidney Int Suppl.
76:S34–S42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Abbadi MM, Pai AS, Leaf EM, Yang HY,
Bartley BA, Quan KK, et al: Phosphate feeding induces arterial
medial calcification in uremic mice: role of serum phosphorus,
fibroblast growth factor-23, and osteopontin. Kidney Int.
75:1297–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutiérrez OM, Mannstadt M, Isakova T,
Rauh-Hain JA, Tamez H, Shah A, et al: Fibroblast growth factor 23
and mortality among patients undergoing hemodialysis. N Engl J Med.
359:584–592. 2008.PubMed/NCBI
|
|
10
|
Sharma SK, Zou H, Togtokh A, Ene-Iordache
B, Carminati S, Remuzzi A, et al: Burden of CKD, proteinuria, and
cardiovascular risk among Chinese, Mongolian, and Nepalese
participants in the International Society of Nephrology screening
programs. Am J Kidney Dis. 56:915–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reel B, Guzeloglu M, Bagriyanik A, Atmaca
S, Aykut K, Albayrak G and Hazan E: The effects of PPAR-γ agonist
pioglitazone on renal ischemia/reperfusion injury in rats. J Surg
Res. 182:176–184. 2013.
|
|
12
|
Basturk T, Unsal A, Ulas T, Koc Y, Sakaci
T, Ahbap E and Borlu F: Effects of rosiglitazone treatment on
insulin resistance and TNF-alpha levels in patients with chronic
kidney disease: a prospective study. Eur Rev Med Pharmacol Sci.
16:1519–1524. 2012.PubMed/NCBI
|
|
13
|
Setti G, Hayward A, Dessapt C, Barone F,
Buckingham R, White K, et al: Peroxisome proliferator-activated
receptor-γ agonist rosiglitazone prevents albuminuria but not
glomerulosclerosis in experimental diabetes. Am J Nephrol.
32:393–402. 2010.
|
|
14
|
Ren L, Liu N, Zhi H, Li Y, Li Y, Tang R,
et al: Vasculoprotective effects of rosiglitazone through
modulating renin-angiotensin system in vivo and vitro. Cardiovasc
Diabetol. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alessi A, França Neto OR, Prim C, Silva
RF, Noronha Ld, Brofman PR, et al: Rosiglitazone and vascular
injury in hypercholesterolemic rabbits: neointimal formation
assessment. Arq Bras Cardiol. 95:283–288. 2010.PubMed/NCBI
|
|
16
|
Nakaya H: Roles of PPARgamma in preventing
the development of atherosclerosis in LDL receptor null mice. Nihon
Rinsho. 68:229–234. 2010.(In Japanese).
|
|
17
|
Schiffrin EL: Peroxisome
proliferator-activated receptors and cardiovascular remodeling. Am
J Physiol Heart Circ Physiol. 288:H1037–H1043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu W, Yu Q, Zhang J and Liu D:
Rosiglitazone ameliorates diabetic nephropathy by reducing the
expression of Chemerin and ChemR23 in the kidney of
streptozotocin-induced diabetic rats. Inflammation. 35:1287–1293.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergwitz C and Jüppner H: Regulation of
phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med.
61:91–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pontoriero G, Cozzolino M, Locatelli F and
Brancaccio D: CKD patients: the dilemma of serum PTH levels.
Nephron Clin Pract. 116:c263–c268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levin A, Djurdjev O, Beaulieu M and Er L:
Variability and risk factors for kidney disease progression and
death following attainment of stage 4 CKD in a referred cohort. Am
J Kidney Dis. 52:661–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kincaid-Smith P, Fairley KF, Farish S,
Best JD and Proietto J: Reduction of proteinuria by rosiglitazone
in non-diabetic renal disease. Nephrology (Carlton). 13:58–62.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Betz B, Schneider R, Kress T, Schick MA,
Wanner C and Sauvant C: Rosiglitazone affects nitric oxide
synthases and improves renal outcome in a rat model of severe
ischemia/reperfusion injury. PPAR Res. 2012:2193192012. View Article : Google Scholar : PubMed/NCBI
|