Introduction
Following liver injury, the repair process comprises
activation and proliferation of hepatic stellate cells (HSCs),
which produce extracellular matrix (ECM) proteins (1–3).
Once activated, HSCs upregulate gene expression of ECM components,
matrix-degrading enzymes and their respective inhibitors, which in
turn results in matrix remodeling. The balance between ECM
deposition and remodeling determines whether fibrosis progresses or
regresses (4,5). Antifibrogenic therapies aim to
inhibit the activation of profibrogenic cells to prevent fibrillar
collagen-I deposition, degrade the excessive ECM to recover the
normal liver architecture and restore functional liver mass. Matrix
metalloproteinases (MMPs) are important regulators of the ECM as
they regulate cellular inflammation, ECM deposition and tissue
reorganization. MMP-13, also called collagenase-3, is a member of
the large family of MMPs and is important in the degradation of
components of the ECM, particularly collagens. It degrades collagen
type II efficiently and also collagen type I, III and X (6–7).
Mitogen-activated protein kinase (MAPK) cascades are
signaling systems that transmit stimuli from outside the cell to
the nucleus. Recent studies have demonstrated that MAPK signaling,
converging on c-Jun NH2-terminal kinase (JNK) and p38 MAPK, plays a
central role in liver injury and compensatory HSC proliferation,
thus it has attracted considerable attention as a therapeutic
target (8,9). JNKs and p38 MAPKs, also called
stress-activated MAPKs, are preferentially activated by
proinflammatory cytokines, including lipopolysaccharide endotoxin
(LPS), tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1).
Following activation, stress-activated MAPKs phosphorylate specific
serine/threonine residues of target substrates and exert a variety
of cellular functions, including cell death, survival,
proliferation, migration and inflammation (10–13).
IL-1 is a pro-inflammatory cytokine and has long
been implicated in promoting tissue fibrosis (14,15,16).
Our previous studies have demonstrated that IL-1β is able to
upregulate the gene expression of tissue inhibitors of matrix
metalloproteinases (TIMPs) in rat HSCs via c-Jun N-terminal kinase
and p38 MAPK pathways (17–20).
In the present study, we demonstrate the association between the
effects of IL-1β upregulating MMP-13 mRNA expression and JNK-p38
MAPK pathways in rat HSCs.
Materials and methods
Cell culture and treatments
The HSC cell line (characteristics of the
fat-storing cell line, CFSC) was provided by Professor Greenwell,
Marion Bessin Liver Research Center, Albert Einstein College of
Medicine (Bronx, NY, USA). The phenotype of the CFSC cell line is
activated HSCs. Following spontaneous immortalization in culture,
cells were seeded and grown in RPMI-1640 (Gibco-BRL, Grand Island,
NY, USA) containing 10% fetal calf serum (FCS; Sijiqing Biological
Products Company, Hangzhou, Zhejiang, China), 4 mmol/l glutamine, 1
mmol/l HEPES and 100 U/ml penicillin/streptomycin, at 37°C and 5%
CO2. When the cells were 80–90% confluent, cell culture
was continued in a non-serum medium for 12 h to synchronize the
cells at G0 phase. HSC proliferation was detected 24 h
following the addition of IL-1β (10 μg/l; PeproTech Inc., Rocky
Hill, NJ, USA). The JNK inhibitor SP600125 and p38 MAPK inhibitor
SB203580 were purchased from Sigma Chemicals International (St.
Louis, MO, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
RT-PCR was performed in order to measure the
expression levels of MMP-13 mRNA in rat HSCs. The sequences of the
primers for MMP-13 sense and antisense were 5′-GCG GGA ATC CTG AAG
AAG TCT AC-3′ (sense) and 5′-TTG GTC CAG GAG GAA AAG CG-3′
(antisense), a 424 bp long fragment was amplified. The sequences of
the primers for GAPDH sense and antisense were 5′-GGC CCC TCT GGA
AAG CTG TG-3′ (sense) and 5′-CCG CCT GCT TCA CCA CCT TCT-3′
(antisense), a 239 bp long fragment was amplified. Total RNA was
extracted from cultured HSCs using TRIzol reagent according to the
manufacturer’s instructions. Next, 2 μg RNA of each sample was
reverse transcribed using a random primer and reverse transcriptase
in 25 μl of volume. Subsequently, the PCR was performed in 25 μl of
reaction mixture containing 5 μl cDNA templates, 2.5 μl 10X
PCR-buffer, 1 μl 10 mmol/l dNTPs, 1.5 μl 15 pmol/l MMP-13 or GAPDH
primers and 2.5 U Taq DNA polymerase. The cycle number was 35
cycles for MMP-13 and 30 cycles for GAPDH. PCR was conducted for 5
min at 94°C for initial DNA denaturation, followed by individual
cycles of denaturation (at 94°C for 45 sec), annealing (at 56°C for
35 sec), polymerization (at 72°C for 45 sec) and then a final
extension of 5 min at 72°C. PCR products were electrophoresed on 2%
agarose gel, stained with ethidium bromide (EB) and quantitated
using Gel-Pro Analyzer Version 3.0. The intensity of the MMP-13
band was compared with the intensity of the GAPDH band, and the
amount of MMP-13 mRNA was estimated.
Western blot analysis
The HSCs were lysed on ice by lysis buffer (RIPA, 50
mol/l Tris-HCl (pH 7.5), 150 mol/l NaCl, 10% glycerol, 1% Nonidet
P-40, 1% SDS, 0.5% deoxycholate, 1.0 mol/l PMSF and 1 mol/l sodium
orthovanadate). Protein samples (80 μg) were subjected to 10%
SDS-PAGE gel electrophoresis and then transferred onto a
nitrocellulose membrane by electroblotting. The membrane was
incubated at 4°C overnight in Tris-buffered saline/Tween-20 (20
mol/l Tris-HCl, pH 7.4, 150 mol/l NaCl, 0.05% Tween-20) with 5%
nonfat milk. Following blocking, the membranes were incubated for 5
h at room temperature in Tris-buffered saline (TBS) buffer (50
mol/l Tris-HCl and 150 mol/l NaCl) containing a 1:100 dilution of
mouse anti-phospho-JNK monoclonal antibody, anti-phospho-p38
monoclonal antibody (Santa Cruz Biotechnology, Inc., CA, USA) or
rabbit anti-β-actin polyclonal antibody (Zhongshanjinqiao, Beijing,
China). Next, membranes were incubated for 120 min at room
temperature in TBS containing a 1:3,000 dilution of anti-mouse IgG
(H+L)/HRP, 1:1,000 dilution of anti-mouse IgM (H+L)/HRP (Zhongshan
Biotechnology, Beijing, China) or 1:3,000 dilution of goat
anti-rabbit IgG antibody (Zhongshan Biotechnology). The level of
phospho-JNK protein and phospho-p38 protein was normalized to the
level of β-actin protein.
Statistical analysis
The data are presented as the mean ± SD of each
group. The statistical software SPSS 11.0 (Bizinsight Information
Technology Co., Ltd., Beijing, China) was used. Intergroup
differences between two groups were analyzed by an independent
t-test. The differences among multiple groups were analyzed by
analysis of variance (ANOVA). P<0.05 was considered to indicate
a statistically significant difference.
Results
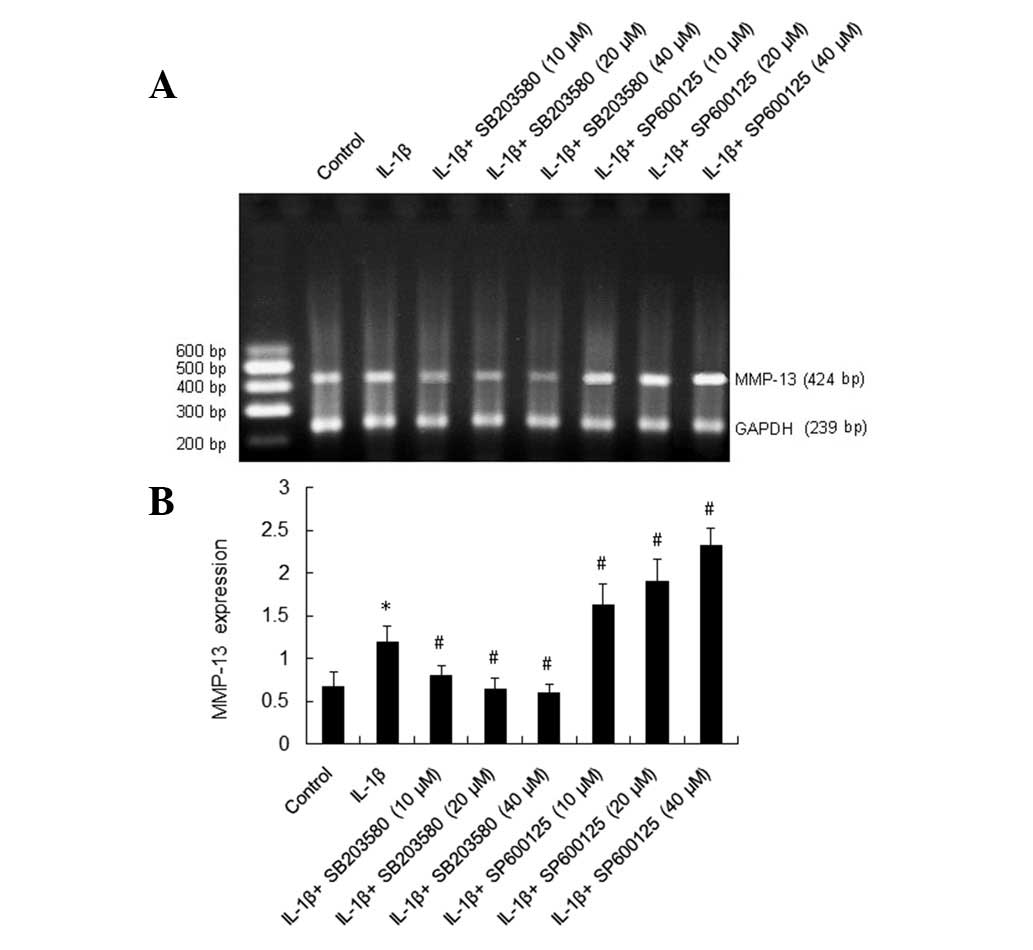
IL-1β upregulates mRNA expression of
MMP-13
We examined the mRNA expression of MMP-13 in rat
HSCs with RT-PCR. The ratio of MMP-13/GAPDH represents the
expression of MMP-13 mRNA. The data demonstrate that MMP-13 mRNA
expression (1.20±0.18) in the group treated with IL-1β (10 μg/l)
for 24 h was significantly higher than that in the control group
(0.68±0.17). There was statistical significance between the two
groups (P<0.01; Fig. 1).
IL-1β activates JNK and p38 in a
time-dependent manner
JNK activity was represented by the ratio of the
mean value of JNK1 and JNK2/β-actin, and p38 activity was
represented by the ratio of p38/β-actin. JNK activity following
IL-1β treatment for 0, 5, 15, 30, 60, and 120 min was 0.982
(0.299), 1.501 (0.720), 2.133 (0.882), 3.360 (0.452), 2.181 (0.789)
and 1.385 (0.368), respectively. We observed that JNK activity
slightly increased following IL-1β treatment for 5 min,
significantly increased following IL-1β treatment for 15 min and
reached its peak following IL-1β treatment for 30 min. The activity
returned to the initial level following IL-1β treatment for 120
min. The differences between JNK activity following treatment for
15, 30 and 60 min were significantly higher than the corresponding
values in the control group (P<0.01, P<0.01, P<0.01). The
p38 activity following IL-1β treatment for 0, 5, 15, 30, 60 and 120
min was 1.061 (0.310), 2.020 (0.863), 2.380 (0.573), 2.973 (0.953),
2.421 (0.793) and 1.755 (0.433), respectively. The p38 activity
slightly increased following treatment for 5 min and reached its
peak after 30 min. The activity returned to the initial level
following treatment for 120 min. The differences between p38
activity following treatment for 5, 15, 30 or 60 min were
significantly higher than the corresponding values in the control
(P<0.01, P<0.01, P<0.01, P<0.01, respectively; Fig. 2).
 | Figure 2JNK and p38 activity following IL-1β
stimulation in rat HSCs. (A) Respresentative Western blot analysis
results of JNK and p38; (B) densitometry of Western blotting
analyzed by Gel-Pro software. Control group was treated with
culture media only, IL-1β group was treated with IL-1β (10 μg/l)
for 0, 5, 15, 30, 60 and 120 min, then MAPK activity, including JNK
and p38 in rat HSCs was evaluated in all groups by Western
blotting. Data are expressed as the mean ± SD (n=6 per group).
*P<0.01 vs. 0 of JNK; #P<0.05 vs. 0 of
p38; ##P<0.01 vs. 0 of p38. IL-1β, interleukin-1β;
JNK, c-Jun N-terminal kinase; HSCs, hepatic stellate cells; MAPK,
mitogen-activated protein kinase. |
Effect of SP600125 and SB203580 on
IL-1β-induced expression of MMP-13 mRNA in rat HSCs
Following treatment with the inhibitor of the p38
MAPK pathway, SB203580, at 10, 20 and 40 μmol/l, the expression of
MMP-13 mRNA was 0.81 (0.11), 0.64 (0.14) and 0.60 (0.10),
respectively, compared with 1.20 (0.18) in the control group,
thereby suggesting decreased MMP-13 mRNA expression. The inhibitory
effect of SB203580 was concentration dependent [0.81 (0.11) vs.
1.20 (0.18), P<0.01; 0.64 (0.14) vs. 1.20 (0.18), P<0.01; and
0.60 (0.10) vs. 1.20 (0.18), P<0.01]. By contrast, following
treatment with the inhibitor of the JNK pathway, SP600125, at 10,
20 and 40 μmol/l, the expression of MMP-13 mRNA was 1.63 (0.24),
1.91 (0.26), 2.33 (0.20), respectively, compared with 1.20 (0.18)
in the control group, thereby suggesting increased MMP-13 mRNA
expression (P<0.01, P<0.01, P<0.01, respectively; Fig. 1).
Discussion
At present, liver diseases constitute a major
medical problem worldwide. Liver damage mainly occurs due to viral
infections, excessive alcohol consumption, autoimmune reactions or
as a consequence of adverse drug effects. Following liver tissue
damage, HSCs undergo a transition from a quiescent to an activated
phenotype and increase proliferation and synthesis of the ECM
(21–23). Activated HSCs express MMPs, the key
enzyme in the degradation of ECM; however, they also express TIMPs.
Numerous cytokines may affect the activation of HSCs and regulate
the secretion of MMPs and TIMPs.
There is increasing evidence supporting an important
role for cytokines in various aspects of inflammatory liver
diseases, including IL-1, IL-6 and TNF-α (24–26).
These cytokines are produced in the liver by Kupffer cells and
hepatocytes, playing roles in lipid metabolism and hepatic
inflammation (27–29). We have recently demonstrated that
IL-1β is able to promote the gene expression of TIMPs,
proliferation and type I collagen synthesis in rat HSCs (17–20).
In the present study, MMP-13 mRNA expression following treatment
with IL-1β for 24 h was significantly higher than that in the
control group. This suggests that IL-1β is also able to upregulate
MMP-13 gene expression in rat HSCs. Therefore, our study revealed
that IL-1β played a pivotal role in fibrosis regression, in part
through the expression of MMP-13.
MAPKs, including extracellular signal-regulated
kinases, JNK and p38 MAPK, belong to a family of Ser/Thr protein
kinases that mediate numerous complex cellular programs, including
gene expression, mitosis, differentiation, proliferation and cell
survival in response to different stimuli (12–14,30).
IL-1 is able to activate JNK and p38 MAPK in various types of
cells. It has been demonstrated previously that IL-1β is capable of
promoting proliferation, type I collagen synthesis and TIMP-1 gene
expression via c-Jun N-terminal kinase and p38 MAPK pathways in rat
HSCs. Our present data demonstrated that following stimulation with
IL-1β, the two MAPK pathways, JNK and p38 MAPK, were both
activated. Following stimulation with IL-1β for 15 min, the level
of phosphorylated JNK significantly increased compared with that in
the control and reached the peak 30 min later. The level returned
to the initial level 120 min later. By contrast, following
stimulation with IL-1β for 5 min, the level of activated p38
significantly increased compared with that in the control and
reached the peak 30 min later. The level returned to the initial
level 120 min later. This finding suggested that IL-1β is able to
activate JNK and p38 in a time-dependent manner and further
confirmed that IL-1β is able to upregulate MMP-13 gene expression
through JNK and p38 pathways in HSCs. To investigate the
association between MAPK phosphorylation and MMP-13 gene expression
induced by IL-1β, HSCs were pretreated with SB203580 and SP600125.
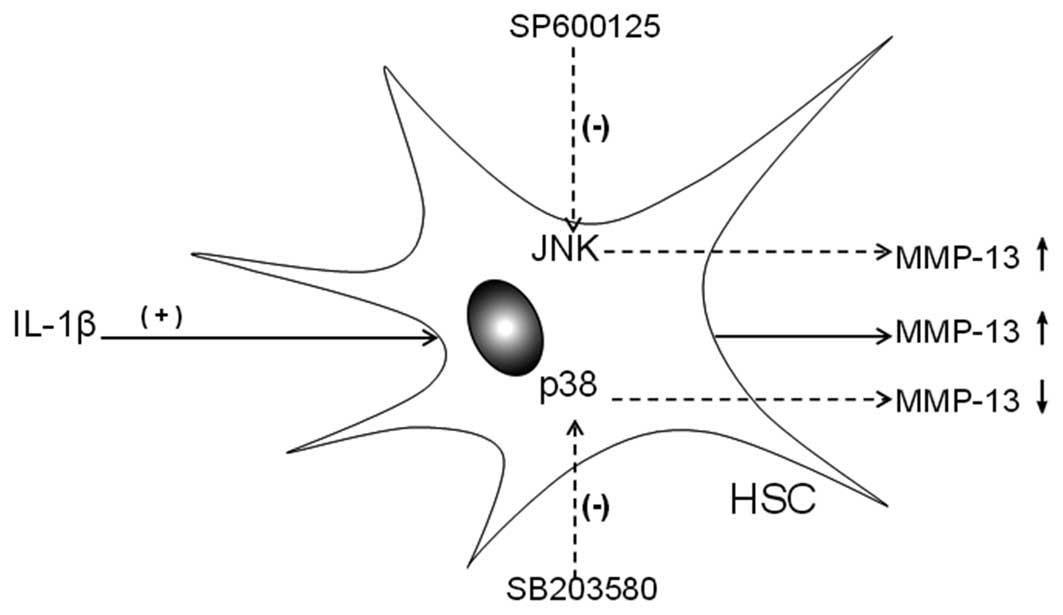
Our study demonstrated that inhibition of the JNK pathway is able
to increase MMP-13 gene expression; however, inhibition of the p38
pathway is able to inhibit MMP-13 gene expression. Taken together
with the results of the present study, these data suggest that
upregulation of JNK appears to be deleterious, whereas p38 MAPK may
protect IL-1β-mediated MMP-13 gene expression in HSCs. This finding
further suggested that JNK and p38 played important roles in
IL-1β-induced MMP-13 gene expression in HSCs. Although these two
pathways have opposite functions, their co-regulation may increase
MMP-13 gene expression (Fig. 3).
MMP-13 gene expression is usually controlled by the co-effects of
different signaling pathways rather than any single pathway. JNK
and p38 constitute a small part of the complex intracellular
signaling pathway through which IL-1β promotes HSC MMP-13 gene
expression. Further studies are needed in order to elucidate other
pathways, which may also be involved.
In conclusion, we provided evidence that IL-1β is
capable of promoting MMP-13 mRNA expression in rat HSCs and the JNK
and p38 MAPK pathways were involved in this process. In summary,
IL-1β-induced MMP-13 mRNA expression is possibly mediated by
cytoplasmic JNK and p38 MAPK pathways and they play a distinct role
in this process. Thus, JNK and p38 MAPK pathways co-operatively
mediate MMP-13 mRNA expression in rat HSCs. A better understanding
of these pathways may be useful in order to identify targets for
hepatic fibrosis therapy.
References
|
1
|
Rashid ST, Humphries JD, Byron A, et al:
Proteomic analysis of extracellular matrix from the hepatic
stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel
constituents of fibrotic liver. J Proteome Res. 11:4052–4064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwiecinski M, Noetel A, Elfimova N, et al:
Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis
in hepatic stellate cells by miRNA-29 induction. PLoS One.
6:e245682011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atorrasagasti C, Aquino JB, Hofman L, et
al: SPARC downregulation attenuates the profibrogenic response of
hepatic stellate cells induced by TGF-β1 and PDGF. Am J Physiol
Gastrointest Liver Physiol. 300:G739–G748. 2011.PubMed/NCBI
|
|
4
|
Szuster-Ciesielska A, Mizerska-Dudka M,
Daniluk J and Kandefer-Szerszeń M: Butein inhibits ethanol-induced
activation of liver stellate cells through TGF-β, NFκB, p38, and
JNK signaling pathways and inhibition of oxidative stress. J
Gastroenterol. 48:222–237. 2013.PubMed/NCBI
|
|
5
|
Dechêne A, Sowa JP, Gieseler RK, et al:
Acute liver failure is associated with elevated liver stiffness and
hepatic stellate cell activation. Hepatology. 52:1008–1016.
2010.PubMed/NCBI
|
|
6
|
Veidal SS, Nielsen MJ, Leeming DJ and
Karsdal MA: Phosphodiesterase inhibition mediates matrix
metalloproteinase activity and the level of collagen degradation
fragments in a liver fibrosis ex vivo rat model. BMC Res Notes.
5:6862012. View Article : Google Scholar
|
|
7
|
Hironaka K, Sakaida I, Matsumura Y, et al:
Enhanced interstitial collagenase (matrix metalloproteinase-13)
production of Kupffer cell by gadolinium chloride prevents pig
serum-induced rat liver fibrosis. Biochem Biophys Res Commun.
267:290–295. 2000. View Article : Google Scholar
|
|
8
|
Nakagawa H and Maeda S: Molecular
mechanisms of liver injury and hepatocarcinogenesis: focusing on
the role of stress-activated MAPK. Patholog Res Int.
2012:1728942012.PubMed/NCBI
|
|
9
|
Hwang IS, Kim JE, Lee YJ, et al:
Protective effects of gomisin A isolated from Schisandra chinensis
against CCl(4)-induced hepatic and renal injury. Int J Mol Med.
31:888–898. 2013.PubMed/NCBI
|
|
10
|
Sato H, Tanaka T and Tanaka N: The effect
of p38 mitogen-activated protein kinase activation on inflammatory
liver damage following hemorrhagic shock in rats. PLoS One.
7:e301242012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv Z and Xu L: Salvianolic acid B inhibits
ERK and p38 MAPK signaling in TGF-β1-stimulated human hepatic
stellate cell line (LX-2) via distinct pathways. Evid Based
Complement Alternat Med. 2012:9601282012.
|
|
12
|
Zhou P, Gross S, Liu JH, et al:
Flavokawain B, the hepatotoxic constituent from kava root, induces
GSH-sensitive oxidative stress through modulation of IKK/NF-kappaB
and MAPK signaling pathways. FASEB J. 24:4722–4732. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brugger J, Schick MA, Brock RW, et al:
Carbon monoxide has antioxidative properties in the liver involving
p38 MAP kinase pathway in a murine model of systemic inflammation.
Microcirculation. 17:504–513. 2010.PubMed/NCBI
|
|
14
|
Aden N, Nuttall A, Shiwen X, et al:
Epithelial cells promote fibroblast activation via IL-1alpha in
systemic sclerosis. J Invest Dermatol. 130:2191–2200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gieling RG, Wallace K and Han YP:
Interleukin-1 participates in the progression from liver injury to
fibrosis. Am J Physiol Gastrointest Liver Physiol. 296:G1324–G1331.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He X, Mekasha S, Mavrogiorgos N, et al:
Inflammation and fibrosis during chlamydia pneumoniae infection is
regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol.
184:5743–5754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Ai X, Zhang J and Yao X:
Differential role of YiGanKang decoction in IL-1β induction of
IL-1RI and AP-1 in rat hepatic stellate cell. J Ethnopharmacol.
141:599–602. 2012.PubMed/NCBI
|
|
18
|
Zhang Y and Yao X: Suppressive effects of
YiGanKang, a combination of Chinese herbs, on collagen synthesis in
hepatic stellate cell. J Ethnopharmacol. 134:949–952. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y and Yao X: Role of c-Jun
N-terminal kinase and p38/activation protein-1 in
interleukin-1β-mediated type I collagen synthesis in rat hepatic
stellate cells. APMIS. 120:101–107. 2012.PubMed/NCBI
|
|
20
|
Zhang YP, Yao XX and Zhao X:
Interleukin-1β up-regulates tissue inhibitor of matrix
metalloproteinase-1 mRNA and phosphorylation of c-jun N-terminal
kinase and p38 in hepatic stellate cells. World J Gastroenterol.
12:1392–1396. 2006.
|
|
21
|
Karimian G, Buist-Homan M, Mikus B, et al:
Angiotensin II protects primary rat hepatocytes against bile
salt-induced apoptosis. PLoS One. 7:e526472012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kocabayoglu P and Friedman SL: Cellular
basis of hepatic fibrosis and its role in inflammation and cancer.
Front Biosci (Schol Ed). 5:217–230. 2013.PubMed/NCBI
|
|
23
|
Li S, Wang L, Yan X, et al: Salvianolic
acid B attenuates rat hepatic fibrosis via downregulating
angiotensin II signaling. Evid Based Complement Alternat Med.
2012:1607262012.PubMed/NCBI
|
|
24
|
Hozawa S, Nakamura T, Nakano M, et al:
Induction of matrix metalloproteinase-1 gene transcription by
tumour necrosis factor alpha via the p50/p50 homodimer of nuclear
factor-kappa B in activated human hepatic stellate cells. Liver
Int. 28:1418–1425. 2008. View Article : Google Scholar
|
|
25
|
Sclavons C, Burtea C, Boutry S, Laurent S,
Vander Elst L and Muller RN: Phage display screening for tumor
necrosis factor- α-binding peptides: detection of inflammation in a
mouse model of hepatitis. Int J Pept. 2013:3484092013.
|
|
26
|
Kumar R, Prakash S, Chhabra S, et al:
Association of pro-inflammatory cytokines, adipokines &
oxidative stress with insulin resistance & non-alcoholic fatty
liver disease. Indian J Med Res. 136:229–236. 2012.
|
|
27
|
Seki S, Nakashima H, Nakashima M and
Kinoshita M: Antitumor immunity produced by the liver Kupffer
cells, NK cells, NKT cells, and CD8 CD122 T cells. Clin Dev
Immunol. 2011:8683452011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jacob A, Zhou M, Wu R, Halpern VJ,
Ravikumar TS and Wang P: Pro-inflammatory cytokines from Kupffer
cells downregulate hepatocyte expression of adrenomedullin binding
protein-1. Biochim Biophys Acta. 1772:766–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gandhi CR, Murase N and Starzl TE: Cholera
toxin-sensitive GTP-binding protein-coupled activation of augmenter
of liver regeneration (ALR) receptor and its function in rat
kupffer cells. J Cell Physiol. 222:365–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacob A, Rajan D, Pathickal B, et al: The
inhibitory effect of ghrelin on sepsis-induced inflammation is
mediated by the MAPK phosphatase-1. Int J Mol Med. 25:159–64.
2010.PubMed/NCBI
|