Introduction
Alzheimer disease (AD) is a progressive
neurodegenerative disease. The pathological features of AD include
senile plaque deposits and neurofibrillary tangles. The major
constituent of senile plaques is amyloid-β (Aβ) peptides, which are
generated as cleavage fragments by the action of γ and β secretase
on the amyloid precursor protein (APP) metabolism. Due to their
inherently disordered and ‘sticky’ nature, the resulting Aβ
peptides easily aggregate into oligomers, then fibrils and mature
plaques in the brain (1). A
previous study suggested that Aβ oligomers are the most toxic form
of Aβ peptide and are critical in inducing cognitive impairment and
synaptic dysfunction (2), however,
the cell mechanisms of Aβ oligomer-mediated neurotoxicity are
poorly defined. Experimental evidence supports the hypothesis that
the disease process starts with the binding of oligomeric
assemblies of Aβ to proteins on the surface of nerve cells
(3). The identity of the molecules
to which oligomers of Aβ bind to remains largely enigmatic. A
number of binding proteins of Aβ have been identified on the neuron
cell surface, including the N-methyl-D-aspartate receptor (4), integrins and the α7 nicotinic
acetylcholine receptor (5).
ATP synthase, traditionally studied in the
mitochondria, where it generates the majority of cellular ATP, has
also been detected on the plasma membranes of several cell types
(6,7). The ecto-ATP synthase serves as a
multi-ligand receptor to regulate specific biological effects
(8–10). Therefore, it was valuable to study
the properties of ATP synthase on the neuronal surface, which have
yet to be clearly elucidated. In a previous study, it was found
that the presence of ATP synthase on the neuronal surface is
responsible for the production of extracellular ATP (11) and that the cell surface ATP
synthase α is a binding protein for Aβ on neural cells. Previous
observations have demonstrated a novel function for Aβ in
regulating ATP synthase activity through interaction with the α
subunit of ATP synthase (12).
In the present study, the effect of the exogenous
addition of oligomeric Aβ on neuronal cells and its effect on the
APP/Fe65 signaling pathway was observed. ATP synthase was confirmed
to be located on the surface of neuron cells and oligomeric Aβ was
observed to induce neuron damage by LDH release, which originated
from the breakdown in the integrity of the plasma membrane. From
these observations, the expression of APP and Fe65 following
oligomeric Aβ treatment was studied. Results showed that inhibition
of the surface ATP synthase may reduce the neuronal damage and
decrease APP and Fe65 expression. These results confirmed that the
cell surface ATP synthase has a pathophysiological role in
Aβ-induced neuronal toxicity.
Materials and methods
Cell culture
Cortices of embryos were obtained from pregnant
Sprague-Dawley E16–E17 rats (Experimental Animal Center of Shanghai
University of Traditional Chinese Medicine) and dissociated with
0.125% trypsin for 10 min at 37°C, then fetal bovine serum (FBS)
was added to halt trypsinization. Cell suspensions were filtered
through stainless steel mesh filter and centrifuged for 6 min at
555 × g. The cells were plated onto poly-L-lysine-coated plates or
dishes according to the manufacturer’s instructions with Dulbecco’s
modified Eagle’s medium (Gibco-BRL, Carlsbad, CA, USA) and
supplemented with 10% FBS. Following 24 h, the medium was replaced
with Neurobasal medium containing B27 supplement. Cultures were
maintained for 8–10 days. Experiments were performed according to
the Guide for the Care and Use of Medical Laboratory Animals
(Ministry of Health, China, 1998) and the guidelines of the
Shanghai University of Traditional Chinese Medical Laboratory
Animal Care and Use Committee.
Preparation of aggregated
Aβ1–40 and electron microcopy
Aggregates of Aβ1–40 were produced by
dissolving a peptide in ddH2O to 700 μM, immediately
diluting in PBS to 350 μM and incubating at 37°C for 7 days. The
aggregated states were checked by electron microscopy. Briefly,
Aβ1–40 solution was absorbed onto a carbon-coated copper
grid and stained negatively with 1% phosphotungstic acid. Following
drying, images were captured by electron microscopy (JEM-2100,
JEOL, Tokyo, Japan) operating at 120 kV.
LDH release assay
Neurotoxicity of Aβ was evaluated by analyzing LDH
content in culture medium. Neuronal cells were seeded in 96-well
plates and 1% Triton X-100 was used as a positive control.
Supernatant (50 μl) was transferred to a fresh 96-well plate and an
equal volume of freshly prepared reaction mixture was added at 37°C
for 10 min and 0.1 M citric acid 20 μl was added to end the
reaction. The absorbance was measured at 570 nm.
Immunofluorescence staining
The mitochondria of primary cultured neurons on
coverslips were first labeled by incubating with a mitochondrial
dye, the Mitotracker (GenMed Scientifics Inc., Shanghai, China),
then the cells were fixed with 4% paraformaldehyde for 30 min at
room temperature and blocked in 5% BSA for 30 min with or without
0.2% Triton X-100. The cells were incubated with the primary
antibodies with or without 0.2% Triton X-100, including anti-ATP
synthase α, anti-APP-C (both 1:200; BD Biosciences, San Jose, CA,
USA) and anti-Fe65 (1:200; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), overnight at 4°C. After being washed three times with
0.01 M PBS, the cells were incubated with FITC or Texas
Red-conjugated secondary antibodies (1:200; Santa Cruz
Biotechnology, Inc.) at 37°C for 1 h. For nuclear staining, the
cells were incubated with 2 μg/ml DAPI for 5 min at 37°C, washed
three times and fluorescence signals were detected by confocal
laser scanning microscopy (TCS-SP2; Leica, Wetzlar, Germany) or
observed under a fluorescence microscope (BX51; Olympus, Tokyo,
Japan).
Western blot analysis
Total protein was extracted and the concentration in
samples was determined by Micro-BCA protein assay (Pierce
Biotechnology Inc., Rockford, IL, USA). Samples were resolved by
SDS-PAGE and transferred onto polyvinylidene fluoride membranes.
The membranes were incubated with primary antibodies anti-APP-C
(1:1,000), anti-Fe65 (1:1,000) and anti-GADPH (1:5,000; all Abcam,
Cambridge, MA, USA) at 4°C overnight. Following washing, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies for 1 h at room temperature. Blots were
visualized by enhanced chemiluminescence.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5 (GrahPad Software, San Diego, CA, USA). Measurement
data are expressed as mean ± SD. Differences were assessed using
the Student’s t-test for comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of Aβ on the viability of primary
cortical neuron by ATP synthase
To confirm the oligomer state of Aβ aggregates,
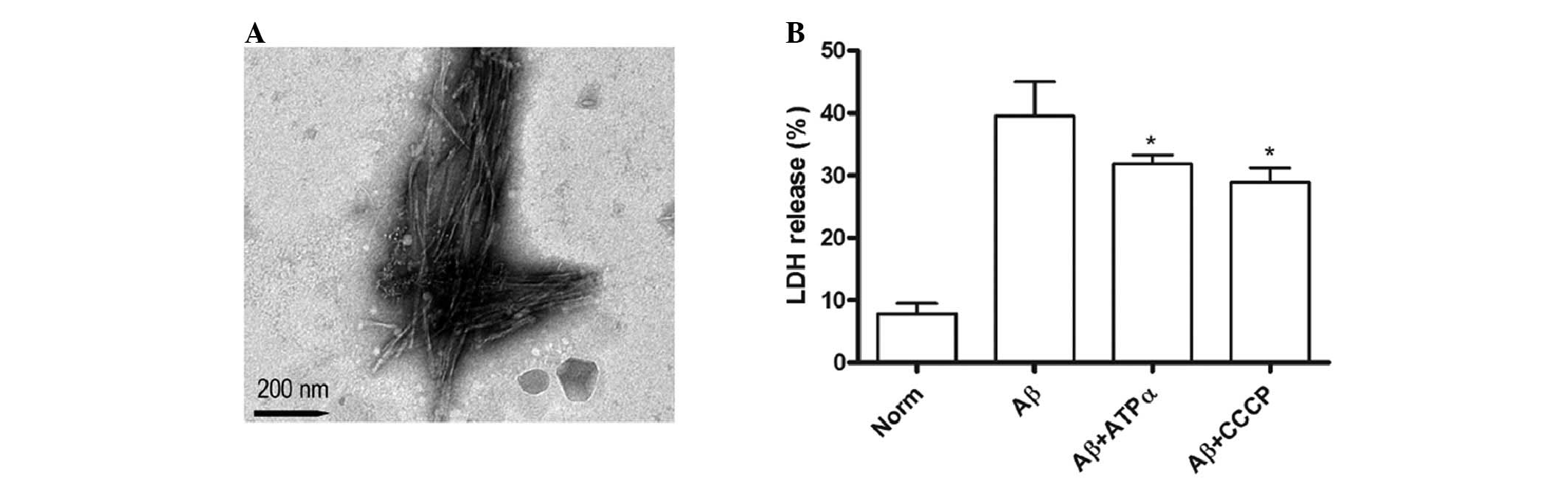
electron microscopy was used to probe the aggregation. Robust
fibrils were primarily observed (Fig.
1A). Increasing activity of LDH released from cells into
culture medium also indicated the damage to neurons. LDH activity
was assayed in the supernatant of the control, exposed to
Aβ1–40 (5 μmol/l) and treated with anti-ATP synthase α
antibody (0.25 μg/ml) or CCCP (1 μg/ml) in the presence of Aβ
groups. The results showed that Aβ treatment promoted more LDH
release compared with the control group. Furthermore, the data
suggested that groups exposed to anti-ATP synthase α antibody or
CCCP in the presence of Aβ had significantly lower leakage of LDH,
indicating that the cytotoxicity of Aβ on the neuron may be
occurring through the neuronal surface ATP synthase pathway
(Fig. 1B).
ATP synthase is located on the cell
surface of primary cultured neurons
In a previous study (11) it was suggested that the expression
of the ATP synthase complex is located on the neuronal surface. In
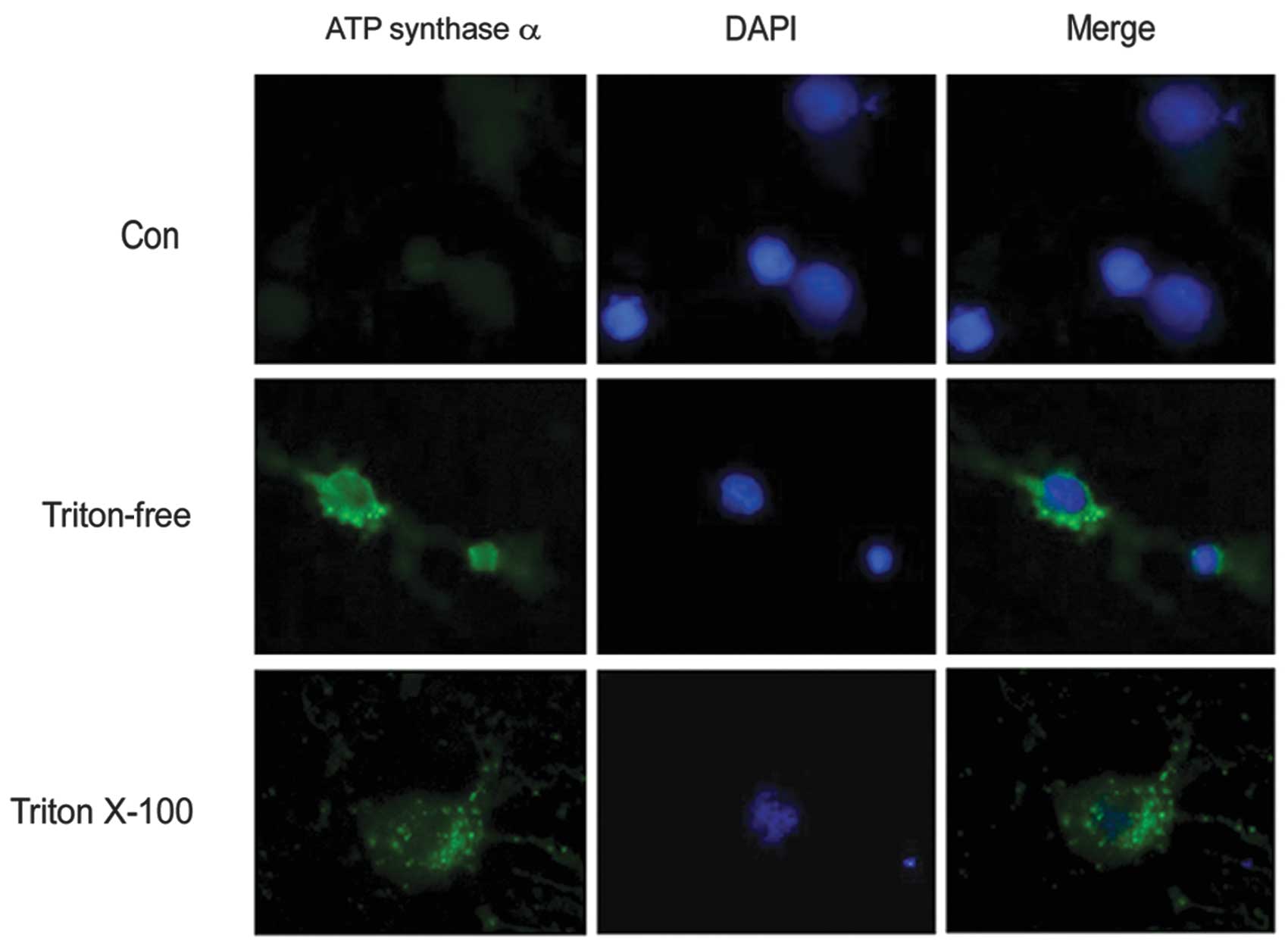
the present study, the primary cultured neurons were immunostained
with anti-ATP synthase α antibody with or without Triton X-100. The
results demonstrated that subunits of ATP synthase were distributed
on the neuronal cell body as well as on the surface (Fig. 2). To confirm that the expression of
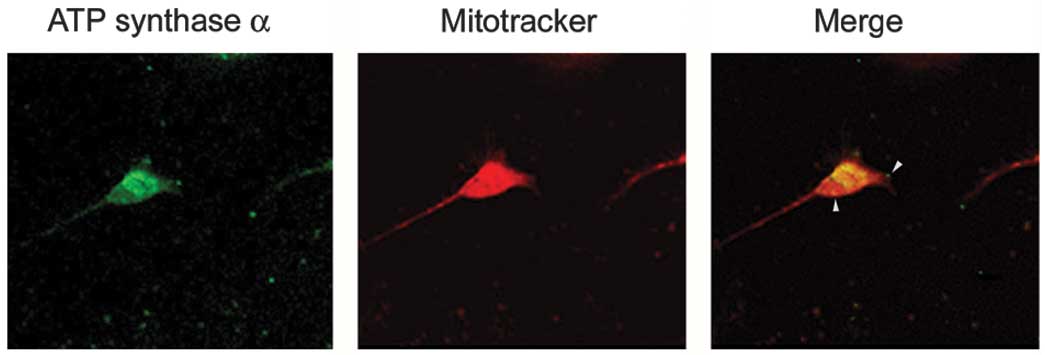
ATP synthase complex is on the neuronal surface, live neurons
prelabeled with Mitotracker were immunostained with an anti-ATP
synthase α antibody. Triton X-100, which is commonly used to
permeabilize cells, was omitted to decrease the antibody in the
neuron. The results showed that the α subunits of ATP synthase
appeared as a punctate distribution on the neuronal surface
(Fig. 3).
Aβ inhibits the APP expression levels
through neuronal surface ATP synthase
In the current study, primary neuronal cultures
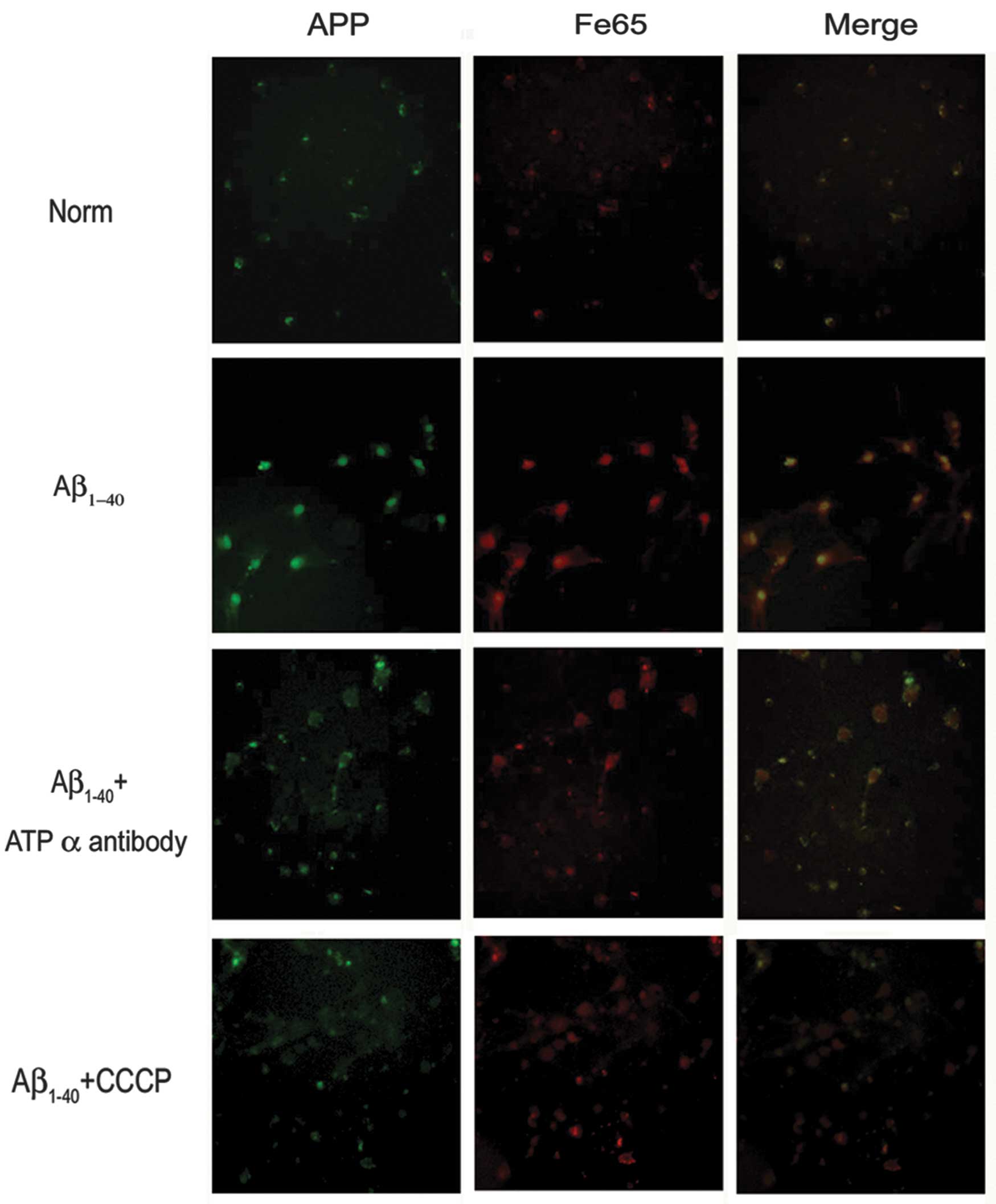
exposed to Aβ led to an increase in APP expression levels. The
adaptor protein Fe65 has been shown to mediate APP metabolism and
increase Aβ production, thus the present study showed that exposure
to Aβ, led to an increase in Fe65 levels. However,
immunofluorescence studies showed that groups exposed to the
anti-ATP synthase α antibody or CCCP in the presence of Aβ had
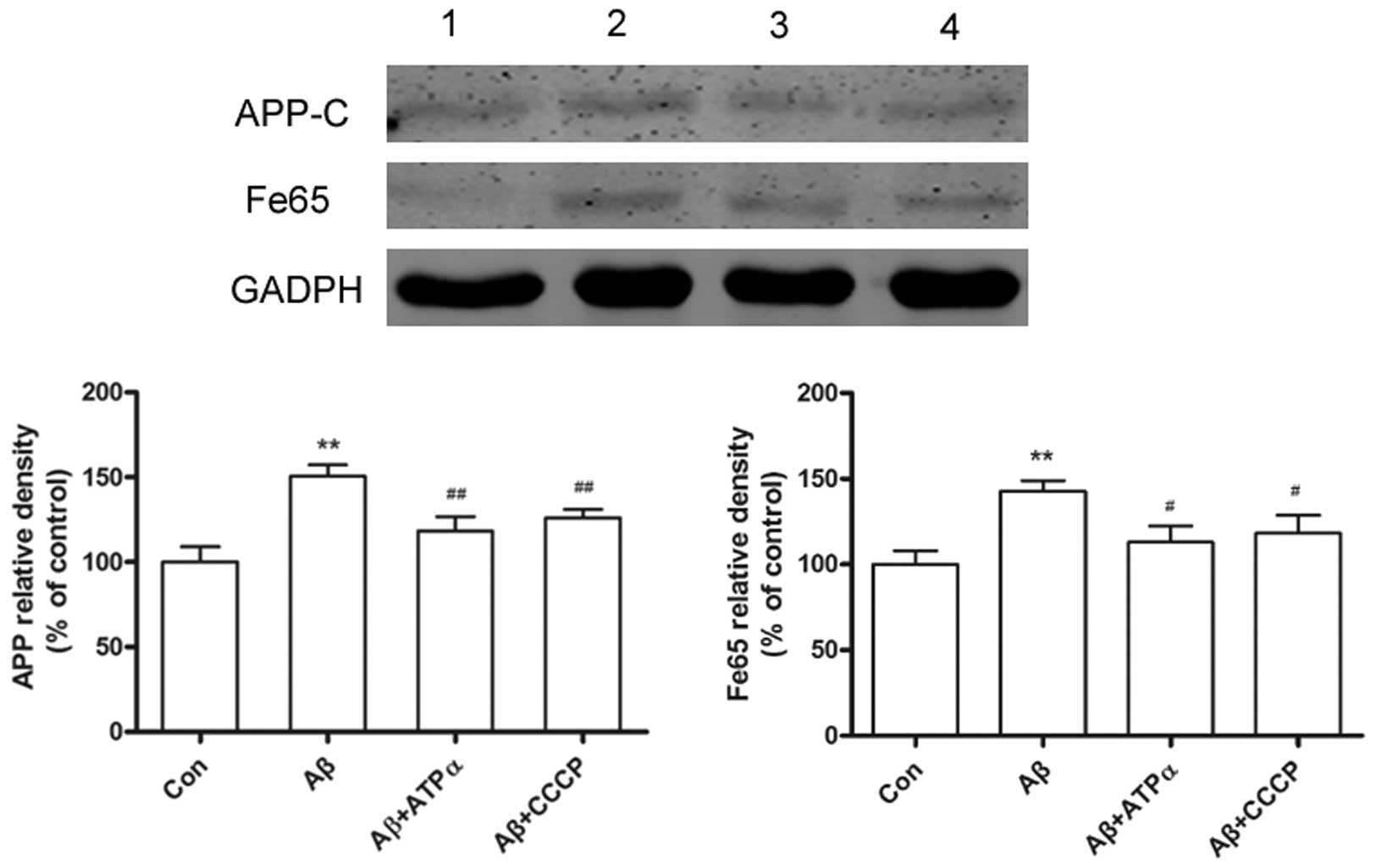
significantly decreased APP and Fe65 levels (Fig. 4). To determine the inhibition of
neuronal surface ATP synthase α, the APP and Fe65 levels were
analyzed following pretreatment with the ATP synthase α antibody or
CCCP in the presence of Aβ on cultured neurons by western blot
analysis. Results showed that the cytotoxicity of Aβ on the primary
cultured neuron was regulated by neuronal surface ATP synthase α
(Fig. 5).
Discussion
Aβ is the primary component of amyloid deposits in
AD, which is capable of interacting with a number of molecular
partners and regulating its activity (13,14).
The cell surface is the first site of interaction between
extracellular Aβ and neurons. Our previous study (11) showed that ATP synthase was present
on the surface of cultured neural cells and demonstrated that the
α-subunit of ATP synthase was located in the senile plaques of
APP/PS1 transgenic mice and co-labeled with Aβ. Cell surface ATP
synthase acts to bind a number of ligands and to trigger hydrolysis
or synthesis of ATP, lipid metabolism and cell death. In contrast
to the studies of surface ATP synthase on hepatocytes and
endothelial cells (15,16), ATP synthase on neurons has been
less studied. For instance, the α-subunit of ATP synthase not only
co-deposits with neurofibrillary tangles in Alzheimer’s disease,
but also binds with amyloid-β peptide and the amyloid precursor
protein and it appears that ecto-ATP synthase may be involved in
neurodegeneration (17,18).
APP is an integral membrane protein from which the
β-amyloid peptide is generated. APP was hypothesized to be involved
in the signal transduction processes, due to its transmembrane
structure (19). The Fe65 gene is
primarily expressed in the neurons of specific regions of the
mammalian nervous system and encodes a protein containing two types
of protein-protein interaction domains: the WW domain and the
phosphotyrosine interaction/phosphotyrosine binding (PID/PTB)
domain. The PID/PTB domains were demonstrated to interact with the
intracellular domain of APP (20).
By overexpression or knockdown, Fe65 has been shown to modulate APP
metabolism, increase APP translocation to the plasma membrane,
which was accompanied by an increase in Aβ production and sAPP
secretion (21,22). Thus, it was hypothesized that the
APP changes may affect the interaction with Fe65.
The current observations suggest that Aβ affects an
increase in APP production and Fe65 by binding to the surface ATP
synthase. Schmidt et al indicated that APP binds surface ATP
synthase and that the binding sequence of APP is similar to the ATP
synthase binding sequence of the inhibitor of F1, a naturally
occurring inhibitor of the ATP synthase complex in mitochondria
(18). A physiologically relevant
negative feedback mechanism is hypothesized to be operating,
closely coordinating the levels of APP expression, surface ATP
synthase and Aβ production. Fe65 was first shown to interact with
APP in a yeast two-hybrid system (23). A previous study showed that Fe65
binds directly to the cytoplasmic domain of APP through its
carboxyl-terminal-phosphotyrosine interaction domains subsequently
(20). The current observations
are in agreement with an important role of surface ATP synthase in
Aβ-mediated neurotoxicity and hypothesize that one of the
mechanisms that play a role in the neurodegenerative processes are
observed in AD. However, the surface ATP synthase trigger neuronal
stress mechanisms in the presence of Aβ require further
investigation.
Acknowledgements
The study was supported by grants from the Shanghai
Health Bureau Youth Fund (no. 20114Y104) and the Innovation Program
of Shanghai Municipal Education Commission (no: 09YZ118).
References
|
1
|
Kayed R, Head E, Thompson JL, et al:
Common structure of soluble amyloid oligomers implies common
mechanism of pathogenesis. Science. 300:486–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fifre A, Sponne I, Koziel V, et al:
Microtubule-associated protein MAP1A, MAP1B, and MAP2 proteolysis
during soluble amyloid beta-peptide-induced neuronal apoptosis.
Synergistic involvement of calpain and caspase-3. J Biol Chem.
281:229–240. 2006. View Article : Google Scholar
|
|
3
|
Selkoe DJ: Soluble oligomers of the
amyloid beta-protein impair synaptic plasticity and behavior. Behav
Brain Res. 192:106–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You H, Tsutsui S, Hameed S, et al: Aβ
neurotoxicity depends on interactions between copper ions, prion
protein, and N-methyl-D-aspartate receptors. Proc Natl Acad Sci
USA. 109:1737–1742. 2012.
|
|
5
|
Verdier Y, Zarándi M and Penke B: Amyloid
beta-peptide interactions with neuronal and glial cell plasma
membrane: binding sites and implications for Alzheimer’s disease. J
Pept Sci. 10:229–248. 2004.PubMed/NCBI
|
|
6
|
Kim BW, Choo HJ, Lee JW, Kim JH and Ko YG:
Extracellular ATP is generated by ATP synthase complex in adipocyte
lipid rafts. Exp Mol Med. 36:476–485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burrell HE, Wlodarski B, Foster BJ, et al:
Human keratinocytes release ATP and utilize three mechanisms for
nucleotide interconversion at the cell surface. J Biol Chem.
280:29667–29676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Champagne E, Martinez LO, Collet X and
Barbaras R: Ecto-F1Fo ATP synthase/F1 ATPase: metabolic and
immunological functions. Curr Opin Lipidol. 17:279–284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mowery YM and Pizzo SV: Targeting cell
surface F1F0 ATP synthase in cancer therapy. Cancer Biol Ther.
7:1836–1838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vantourout P, Radojkovic C, Lichtenstein
L, Pons V, Champagne E and Martinez LO: Ecto-F1-ATPase:
a moonlighting protein complex and an unexpected apoA–I receptor.
World J Gastroenterol. 16:5925–5935. 2010.
|
|
11
|
Xing SL, Yan J, Yu ZH and Zhu CQ: Neuronal
cell surface ATP synthase mediates synthesis of extracellular ATP
and regulation of intracellular pH. Cell Biol Int. 35:81–86.
2011.PubMed/NCBI
|
|
12
|
Xing SL, Chen B, Shen DZ and Zhu CQ:
β-amyloid peptide binds and regulates ectopic ATP synthase α-chain
on neural surface. Int J Neurosci. 122:290–297. 2012.
|
|
13
|
Magdesian MH, Carvalho MM, Mendes FA, et
al: Amyloid-beta binds to the extracellular cysteine-rich domain of
Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem.
283:9359–9368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaked GM, Kummer MP, Lu DC, Galvan V,
Bredesen DE and Koo EH: Abeta induces cell death by direct
interaction with its cognate extracellular domain on APP (APP
597–624). FASEB J. 20:1254–1256. 2006.PubMed/NCBI
|
|
15
|
Fabre AC, Vantourout P, Champagne E, et
al: Cell surface adenylate kinase activity regulates the
F(1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human
hepatocytes. Cell Mol Life Sci. 63:2829–2837. 2006. View Article : Google Scholar
|
|
16
|
Martinez LO, Jacquet S, Esteve JP, et al:
Ectopic beta-chain of ATP synthase is an apolipoprotein A–I
receptor in hepatic HDL endocytosis. Nature. 421:75–79. 2003.
|
|
17
|
Sergeant N, Wattez A, Galván-valencia M,
et al: Association of ATP synthase alpha-chain with neurofibrillary
degeneration in Alzheimer’s disease. Neuroscience. 117:293–303.
2003.PubMed/NCBI
|
|
18
|
Schmidt C, Lepsverdize E, Chi SL, et al:
Amyloid precursor protein and amyloid beta-peptide bind to ATP
synthase and regulate its activity at the surface of neural cells.
Mol Psychiatry. 13:953–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guénette SY, Chen J, Jondro PD and Tanzi
RE: Association of a novel human FE65-like protein with the
cytoplasmic domain of the beta-amyloid precursor protein. Proc Natl
Acad Sci USA. 93:10832–10837. 1996.PubMed/NCBI
|
|
20
|
Zambrano N, Buxbaum JD, Minopoli G, et al:
Interaction of the phosphotyrosine interaction/phosphotyrosine
binding-related domains of Fe65 with wild-type and mutant
Alzheimer’s beta-amyloid precursor proteins. J Biol Chem.
272:6399–6405. 1997.PubMed/NCBI
|
|
21
|
Ando K, Iijima KI, Elliott JI, Kirino Y
and Suzuki T: Phosphorylation-dependent regulation of the
interaction of amyloid precursor protein with Fe65 affects the
production of beta-amyloid. J Biol Chem. 276:40353–40361. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Z, Dong Y, Maeda U, Xia W and Tanzi
RE: RNA interference silencing of the adaptor molecules ShcC and
Fe65 differentially affect amyloid precursor protein processing and
Abeta generation. J Biol Chem. 282:4318–4325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fiore F, Zambrano N, Minopoli G, Donini V,
Duilio A and Russo T: The regions of the Fe65 protein homologous to
the phosphotyrosine interaction/phosphotyrosine binding domain of
Shc bind the intracellular domain of the Alzheimer’s amyloid
precursor protein. J Biol Chem. 270:30853–30856. 1995.PubMed/NCBI
|