Introduction
Apoptosis is the active process of programmed cell
death, which occurs during a number of important physiological
conditions and has become an area of extensive investigation in
cancer research to eliminate precancerous and/or cancerous cells
(1,2). Apoptosis is a tightly regulated
process characterized by cell shrinkage, plasma membrane blebbing
and chromatin condensation that is consistent with DNA cleavage in
ladders (1,3). Caspase (a family of cysteine
proteases) activation is often regulated by specific cellular
factors, including members of the Bcl-2 family and/or the death
receptor-related gene products (4). The inhibitor of the apoptosis protein
(IAP) family proteins, X-linked IAP (XIAP), cellular inhibitor of
apoptosis protein (cIAP)-1, cIAP-2 and survivin, by contrast, are
endogenous inhibitors of apoptosis. However, the majority of cancer
cells block apoptosis, which allows them to survive regardless of
genetic and morphological transformations. Therefore, the induction
of apoptotic cell death is an important mechanism underlying the
effect of numerous anti-cancer drugs.
Reactive oxygen species (ROS) are important
regulators of apoptosis. ROS production has been shown to be part
of the signaling process, whereby a number of bioactive agents
activate a cascade that functions to eliminate cancer cells
(5,6). Accordingly, excessive generation of
ROS has been shown to result in cell damage and initiate certain
effects (7). Although ROS are
important in cell signaling, extended elevated levels of ROS lead
to severe damage to DNA, RNA and proteins, which eventually results
in cell death via apoptotic or necrotic mechanisms (8). In addition, ROS may act as
intracellular messengers that are induced by a diverse range of
stimuli and trigger apoptosis (9).
With the growth of ecological movements, natural
products have become more popular for the prevention or treatment
of cancer. This has been paralleled by an increase in research
focused on natural products. The biological activities of the
polysaccharides isolated from mushrooms, fungi, yeasts, algae,
lichens and plants have attracted increased attention in the
biochemical and medical areas due to their immunomodulatory,
anticancer and antitumor effects (10). A previous study indicated cytotoxic
activity of the polysaccharide against human cancer cell lines and
demonstrated that the polysaccharide derived from herbal medicine
may induce cell apoptosis (11). A
number of bioactive polysaccharides have been isolated from
botanical sources, the majority of which are relatively nontoxic
with no significant side effects (12).
Soy sauce is currently used as a liquid seasoning in
cooking worldwide (13) and its
various biological activities have been reported, including
antioxidative (14), antiplatelet
(15) activities and
immunomodulatory effect (16) and
the inhibition of angiotensin I-converting enzyme (17). Soy soluble polysaccharides (SPS)
have been reported for hypoallergenicity and antiallergic activity,
and be effective for allergic disorders. Therefore, soy sauce is
considered to be not only a liquid seasoning but also a functional
anti-cancer food (13,18). In the present study, SSPS was
observed to induce a number of characteristic apoptotic symptoms in
HCT-116 human colon cancer cells, including chromatin condensation,
activation of caspases, cleavage of poly ADP-ribose polymerase
(PARP) and mitochondrial depolarization. ROS were shown to be
potential causes of the caspase activation that leads to
SSPS-induced apoptosis.
Materials and methods
Reagents
4,6-diamidino-2-phenylindole (DAPI),
N-acetyl-L-cysteine (NAC),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyl-tetrazolium
bromide (MTT) and propidium iodide (PI) were purchased from the
Sigma-Aldrich (St. Louis, MO, USA). The caspase inhibitors
z-DEVD-fmk (caspase-3 inhibitor), z-IETD-fmk (caspase-8 inhibitor),
z-LEHD-fmk (caspase-9 inhibitor) and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) were purchased from Calbiochem (San Diego, CA, USA). Mouse
monoclonal antibodies Bcl-2, Bad, caspase-3, actin and rabbit
polyclonal antibodies against Bcl-xL, Bax, Bid, cytochrome c,
caspase-8, caspase-9, XIAP, cIAP-1, cIAP-2 and PARP were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
peroxidase-labeled donkey anti-rabbit immunoglobulin and
peroxidase-labeled sheep anti-mouse immunoglobulin were purchased
from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
Preparation of soy sauce
Fermented soybean, a seed for soy sauce, was
prepared by multi-step fermentation (19). Soy sauce was fermented and aged
using fermented soybean. The prepared Meju was mixed with a
brine solution (20%) at a ratio of 1:2 (w/v) and was placed in
individual cylindrical mash tanks. Throughout the aging process,
the temperature was maintained at 15°C. The total aging period was
three months.
Isolation and preparation of SSPS
SSPS were prepared according to the method described
previously by Kikuchi et al(20). Soy sauce (10 ml) was dialyzed
overnight using seamless cellulose tubing [width, 43 mm; diameter
27 mm; 12,000 MW; dialysis membrane (Viskase Companies, Inc.,
Darien, IL, USA)] in water at 4°C and freeze-dried.
Cell line, cell culture and MTT
assay
HCT-116 human colon cancer cells were obtained from
the American Type Culture Collection (Rockville, MD, USA) and
cultured in RPMI-1640 supplemented with 10% fetal bovine serum and
1% penicillin-streptomycin at 37°C in a humidified chamber
containing 95% air and 5% CO2. For the cell viability
assay, cells were cultured in the absence and presence of various
concentrations of SSPS for 24 h. Measurement of cell viability was
determined using the MTT assay, which is based on the conversion of
MTT to MTT-formazan by mitochondrial enzymes.
Flow cytometry analysis
Following treatment with SSPS, the cells were
collected, washed with cold phosphate-buffered saline (PBS) and
fixed in 75% ethanol at 4°C for 30 min. Prior to analysis, the
cells were washed once with PBS, suspended in a cold PI solution
containing 100 μg/ml RNase A, 50 μg/ml PI, 0.1% (w/v) sodium
citrate and 0.1% (v/v) NP-40 and incubated on ice for 30 min in the
dark. Flow cytometry analyses were conducted using a flow cytometer
(FACSCalibur; BD Biosciences, San Jose, CA, USA). Cell-Quest
software was used to determine the relative DNA content based on
the presence of red fluorescence. The sub-G1 population was
calculated to estimate the apoptotic cell population (21).
DAPI staining
Cells were washed with ice-cold PBS and fixed with
4% paraformaldehyde (Sigma-Aldrich) in PBS for 10 min at room
temperature. Fixed cells were washed with PBS and stained with DAPI
solution for 10 min at room temperature. The cells were washed
twice with PBS and analyzed by fluorescence microscopy (EM-900;
Carl Zeiss AG, Oberkochen, Germany).
Determination of caspase activity
The enzymatic activity of the caspases induced by
SSPS was assayed using a colorimetric assay kit (R&D Systems,
Minneapolis, MN, USA) according to manufacturer’s instructions.
Briefly, cells were lysed in the supplied lysis buffer. The lysed
cells were centrifuged at 20,000 × g for 20 min, and equal amounts
of protein (100 μg per 50 μl) were incubated with 50 μl of a
reaction buffer and 5 μl of the colorimetric tetrapeptides,
Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) for caspase-3,
Ile-Glu-Thr-Asp (IETD)-pNA for caspase-8 and Leu-Glu-His-Asp
(LEHD)-pNA for caspase-9, respectively. Caspase activity was
determined by measuring changes in absorbance at 405 nm using the
enzyme-linked immunosorbent assay reader.
Protein extraction and western blot
analysis
Cells were harvested and lysed with lysis buffer (20
mM sucrose, 1 mM EDTA, 20 μM Tris-HCl, pH 7.2, 1 mM DTT, 10 mM KCl,
1.5 mM MgCl2 and 5 μg/ml aprotinin) for 30 min. The
protein concentration was measured using a Bio-Rad protein assay
(Bio-Rad, Hercules, CA, USA). For western blot analysis, an equal
quantity of protein was subjected to electrophoresis on sodium
dodecyl sulfate (SDS)-polyacrylamide gel and transferred to a
nitrocellulose membrane (Schleicher & Schuell, Inc., Keene, NH,
USA). Blots were probed with the desired antibodies for 1 h,
incubated with the diluted enzyme-linked secondary antibody and
visualized by enhanced chemiluminescence according to the
manufacturer’s instructions (Amersham Pharmacia Biotech).
Mitochondrial membrane potential (MMP,
Δψm) assay
The MMP was determined using the lipophilic cationic
probe JC-1. JC-1 is a radiometric, dual-emission fluorescent dye
that is absorbed and concentrated by respiring mitochondria and can
reflect changes in MMP in living cells. There are two excitation
wavelengths, 527 nm (green) for the monomer form and 590 nm (red)
for the JC-1-aggregate form. With normal mitochondrial function,
MMP is high and red fluorescence is predominant. However, when
there is mitochondrial injury, the MMP is reduced, leading to an
increase in green fluorescence. The relative intensity of red and
green fluorescent signals indicate whether mitochondria are
damaged. In the current study, cells were trypsinized and cell
pellets were resuspended in PBS and incubated with 10 μM JC-1 for
20 min at 37°C. Cells were analyzed using flow cytometry.
Preparation of mitochondrial
proteins
Cells were treated with SSPS and harvested with
ice-cold PBS. The mitochondrial and cytosolic fractions were
isolated using a mitochondrial fractionation kit according to the
manufacturer’s instructions (Active Motif, Carlsbad, CA, USA). Cell
lysates (30 μg protein/lane) were fractionated in
SDS-polyacrylamide gels prior to transfer to the nitrocellulose
membranes (Schleicher & Schuell) using standard
instructions.
Measurement of intracellular ROS
generation
Generation of ROS was determined in cells treated
with SSPS in the presence and absence of NAC and was evaluated with
20,70-dichlorofluorescein diacetate (DCF-DA; Molecular Probes,
Leiden, The Netherlands) as described previously (22). Cells were incubated with 10 μM
DCF-DA at 37°C for 30 min, washed with PBS and FL-1 fluorescence
was measured by flow cytometery.
Statistical analysis
The data are expressed as the mean ± SD. A
statistical comparison was performed using one-way analysis of
variance followed by Fisher’s exact test. The significant
differences between the groups were determined using unpaired
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of cell growth and induction
of apoptosis by SSPS in HCT-116 cells
To investigate the effect of SSPS on the cell growth
of HCT-116 cells, cells were exposed to specific concentrations (0,
0.3, 0.6, 0.9 and 1.2 mg/ml) of SSPS. Following 24 h incubation,
the cell viability was measured using an MTT assay. As shown in
Fig. 1A, treatment with SSPS
significantly inhibited the viability of cells and these effects
occurred in a concentration-dependent manner.
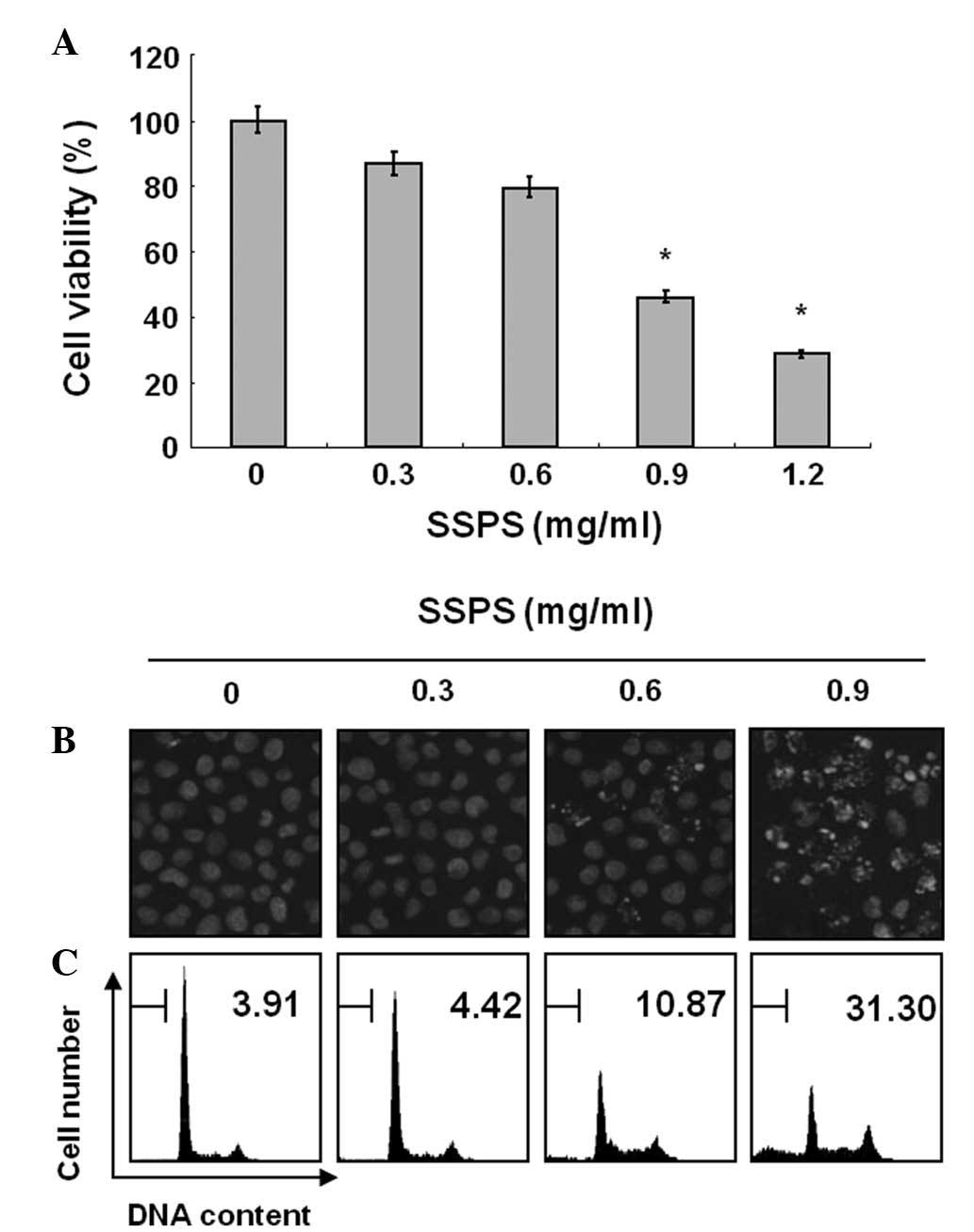 | Figure 1Inhibition of cell growth and
induction of apoptosis by SSPS in HCT-116 human colon cancer cells.
(A) Cells were plated at a volume of 4×104 cells per
60-mm plate and incubated for 24 h. Cells were treated with varying
concentrations of SSPS for 24 h and cell viability was measured by
an MTT assay. Data are expressed as the mean ± SD of three
independent experiments. For statistical analysis, Student’s t-test
was performed. *P<0.05 vs. contol. (B) Following cell
treatment with SSPS for 24 h, cells were stained with DAPI for 10
min and imaged by fluorescence microscopy using a blue filter
(magnification, ×400). (C) Effects of SSPS on the cell cycle
distribution in HCT-116 cells. Cells were incubated with specific
concentrations of SSPS for 24 h. To quantify the degree of
apoptosis induced by SSPS, cells were evaluated for the sub-G1 DNA
content using a flow cytometer. Data represent the means of two
independent experiments. SPSS, soy soluble polysaccharide; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyl-tetrazolium
bromide; DAPI, 4,6-diamidino-2-phenylindole. |
To elucidate whether SSPS inhibits the viability of
HCT-116 cells by inducing apoptosis, cells treated with SSPS were
examined following DAPI staining. The control cells exhibited an
intact nuclear structure, whereas, cells treated with SSPS had
chromosomal condensation and formation of apoptotic bodies
(Fig. 1B). Morphological analysis
of HCT-116 cells treated with SSPS indicated that the cells had
undergone gross morphological changes indicative of apoptosis,
including chromatin condensation and formation of apoptotic
bodies.
Thus, to assess how SSPS affected cell growth, the
degree of apoptosis was determined by analyzing the quantity of
sub-G1 DNA in HCT-116 cells that were treated with SSPS using flow
cytometry. As shown in Fig. 1C, a
significant accumulation of cells with sub-G1 DNA content was noted
in a dose-dependent manner, but no other markedly detectable cell
cycle changes were observed in HCT-116 cells treated with the
indicated concentration of SSPS for 24 h. These results
demonstrated that the cytotoxic effects observed in SSPS-treated
HCT-116 cells are associated with the induction of apoptosis.
Caspase involvement in SSPS-induced
apoptosis
Caspases are known to serve as important mediators
of apoptosis and to contribute to the general apoptotic morphology
through the cleavage of various cellular substrates, including
PARP, an endogenous substrate of caspase-3 (23). Therefore, the expression levels and
activities of caspase-3, -8 and -9 during SSPS-induced apoptosis
were investigated. As shown in Fig.
2A, western blot analyses showed that SSPS concentration
dependently induced a marked change in the cleavage of caspase-3,
-8 and -9. Subsequent western blot analyses showed the progressive
proteolytic cleavage of PARP in HCT-116 cells following SSPS
treatment, indicating that the activation of caspase is involved in
the SSPS-induced apoptotic pathway. To further quantify the
proteolytic activation of caspases in HCT-116 cells, the lysates
normalized for protein from the cells treated with SSPS were
assayed for their activities using colorimetric assay kits. As
shown in Fig. 2B, treatment with
SSPS significantly increased the activities of caspase-3, -8 and 9
compared with control cells.
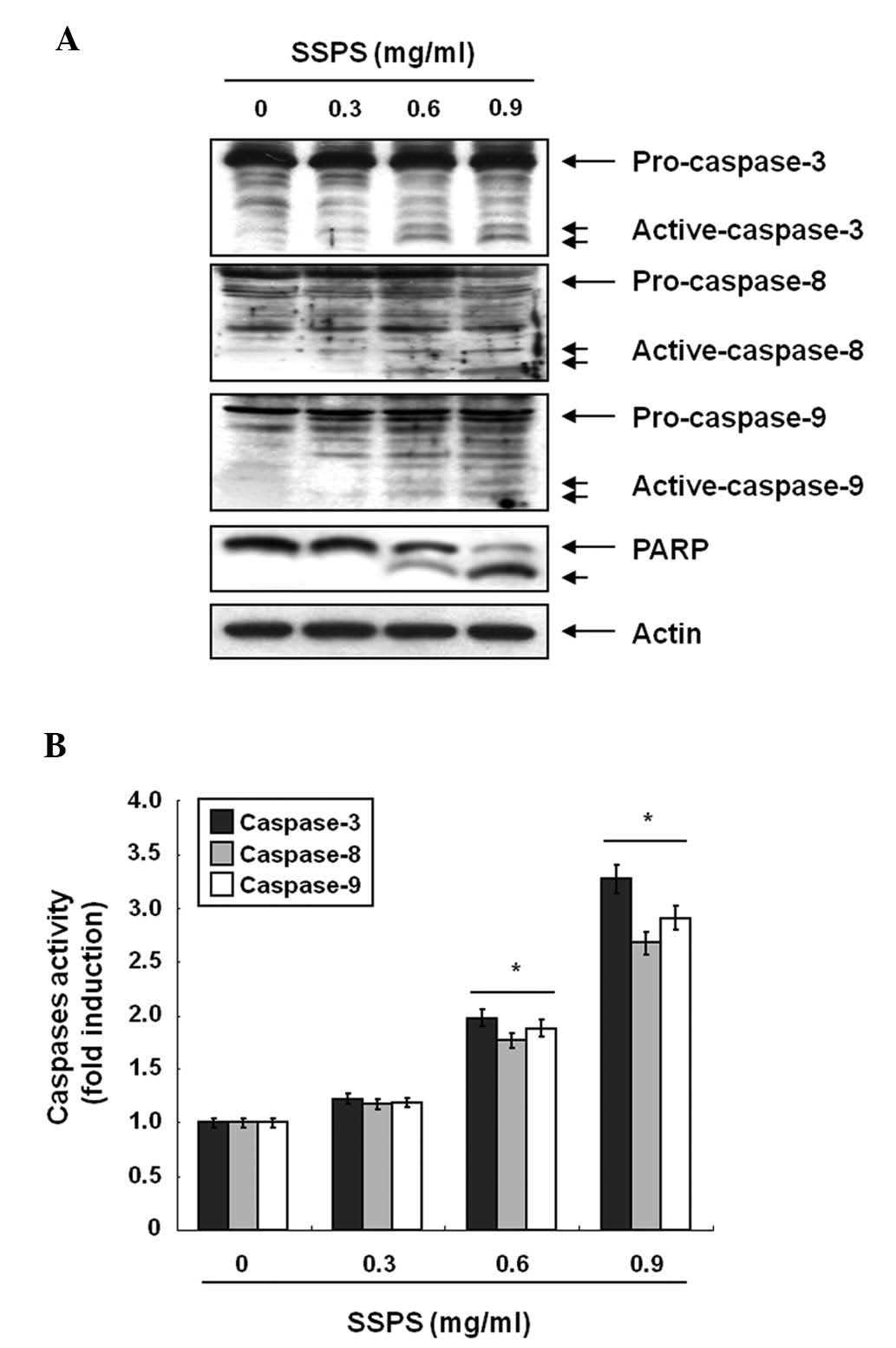 | Figure 2SSPS-induced caspase activation in
HCT-116 cells. (A) Cells were treated with the indicated
concentrations of SSPS for 24 h. Equal quantities of cell lysates
(30 μg) were resolved by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis, transferred to nitrocellulose membranes and probed
with antibodies against caspase-3, -8, -9 and PARP. Actin was used
as an internal control. (B) Cell lysates obtained from cells grown
under the same conditions as (A) were assayed for in vitro
caspase-3, -8 and -9 activity using DEVD-pNA, IETD-pNA and
LEHD-pNA, respectively, as substrates. The relative fluorescent
products were measured. Data are presented as the mean ± SD of
representative experiments performed a minimum of three times.
Significance was determined by Student’s t-test.
*P<0.05, vs. control. SPSS, soy soluble
polysaccharide; pNA, p-nitroaniline; PARP, poly (ADP-ribose)
polymerase. |
Effects of SSPS on the expression of
Bcl-2 and IAP family proteins and a loss of MMP
(Δψm) in HCT-116 cells
The involvement of Bcl-2 and IAP family proteins in
SSPS-mediated apoptosis was determined by western blot analysis. As
shown in Fig. 3A, when HCT-116
cells were treated with SSPS, a marked decrease in Bcl-2 and Bcl-xL
antiapoptotic protein expression was observed in a
concentration-dependent manner. In the case of the proapoptotic
proteins Bax and Bad, there was a marginal concentration-dependent
upregulation observed in HCT-116 cells treated with SSPS. In
addition, western blot analyses revealed that treatment with SSPS
induced the cleavage of Bid, generating a truncated form, tBid, a
Bcl-2 homology domain (BH)-3-only proapoptotic member of the Bcl-2
family. The levels of XIAP, cIAP-1 and cIAP-2 were markedly
inhibited by SSPS treatment, in a concentration dependent
manner.
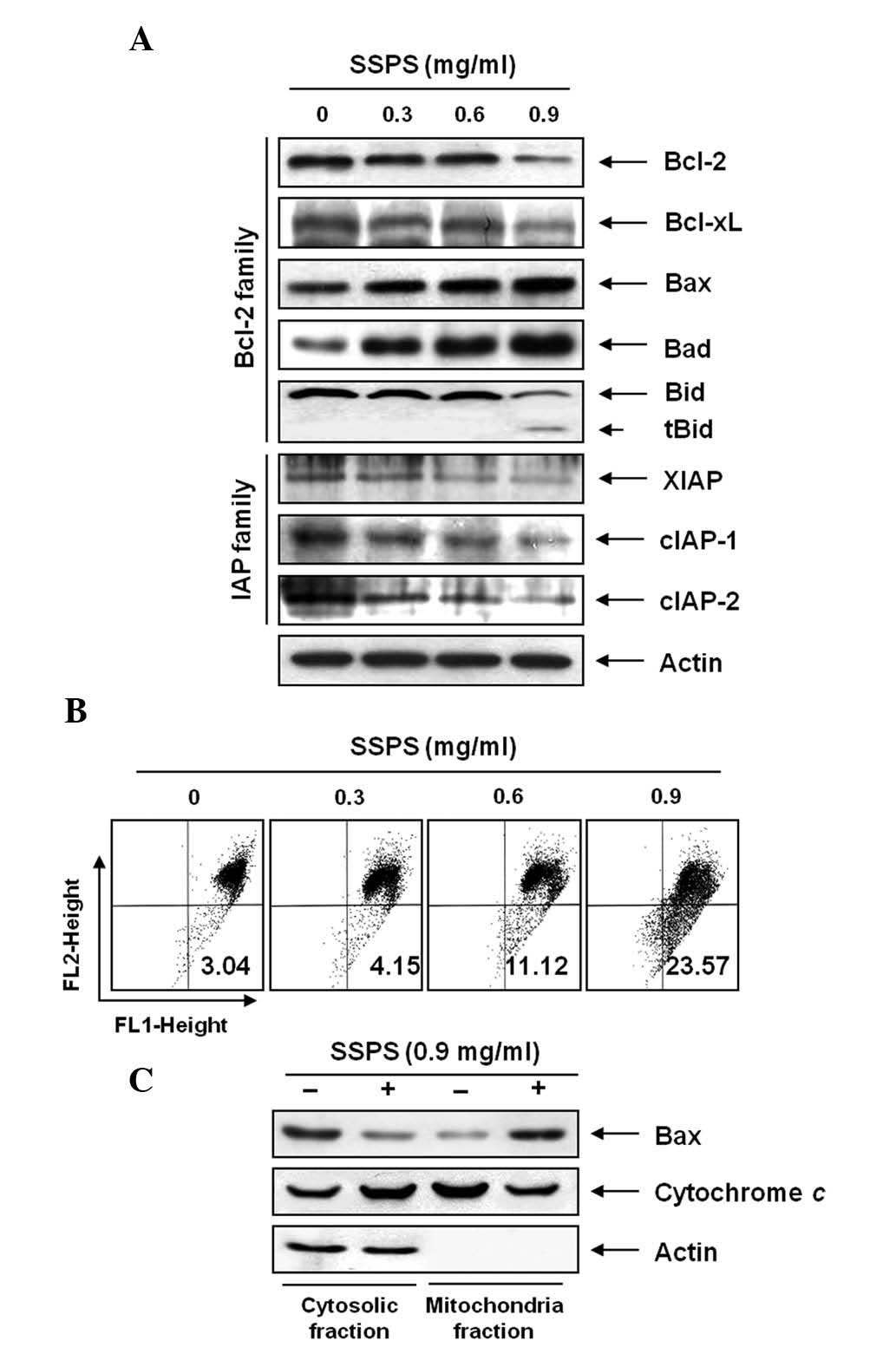 | Figure 3Effects of SSPS treatment on the
levels of Bcl-2 and inhibitor of apoptosis protein family proteins,
cytochrome c and mitochondrial membrane hyperpolarization in
HCT-116 cells. (A) Cells were treated with the indicated
concentrations of SSPS for 24 h. Proteins were detected using
western blot analysis with the indicated antibodies and enhanced
chemiluminescence detection. Actin was used as an internal control.
(B) Cells were stained with JC-1 for 20 min at 37°C. The mean JC-1
fluorescence intensity was detected using flow cytometry. Data
represent the means of two independent experiments. (C) Cytosolic
and mitochondrial extracts were prepared from control and
SSPS-treated cells, resolved by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes and probed with the anti-Bax and
anti-cytochrome c antibodies. SPSS, soy soluble
polysaccharide; JC -1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide; X-linked inhibitor of the apoptosis protein family
proteins; cIAP, cellular inhibitor of apoptosis protein. |
Furthermore, the involvement of the mitochondria in
SSPS-induced apoptosis of HCT-116 cells was investigated by
examining the effect of SSPS on the MMP values as well as the
levels of cytosolic and mitochondrial Bax and cytochrome c.
As shown in Fig. 3B, exposure of
HCT-116 cells to various concentrations of SSPS led to a
significant reduction in the level of MMP and this reduction
occurred in a dose-dependent manner. In addition, exposure of
HCT-116 cells to SSPS led to a significant increase in the level of
cytosolic proapoptotic protein cytochrome c and to the
decrease of Bax (Fig. 3C). By
contrast, treatment with SSPS induced a significant downregulation
of cytochrome c and upregulation of Bax protein in the
mitochondria. These results indicate that SSPS induced
mitochondrial dysfunction in HCT-116 cells.
SSPS-induced apoptosis is associated with
the generation of ROS
SSPS was investigated to determine whether it may
stimulate ROS generation in HCT-116 cells. In HCT-116 cells exposed
to SSPS for the indicated time periods, generation of ROS was
observed at 0.5 h and the levels continued to increase at 1 h
(Fig. 4A). The ROS scavenger NAC
at 10 mM significantly blocked the levels of ROS from the
SSPS-treated HCT-116 cells. NAC scavenges ROS in cells by
interacting with OH− and H2O2 and
thus affect ROS-mediated signaling pathways (24). As shown in Fig. 4B and C, pretreatment of HCT-116
cells with NAC attenuated chromatin condensation and formation of
apoptotic bodies and restored the accumulation of the sub-G1 DNA
population. In addition, blocking of the generation of ROS by
pretreatment of the cells with NAC prevented the SSPS-induced
modulation of Bcl-2 family proteins and the cleavage of PARP
(Fig. 4D). Inhibiting the
generation of ROS by pretreatment of the cells with NAC
significantly prevented the SSPS-induced loss of cell viability
(Fig. 4E).
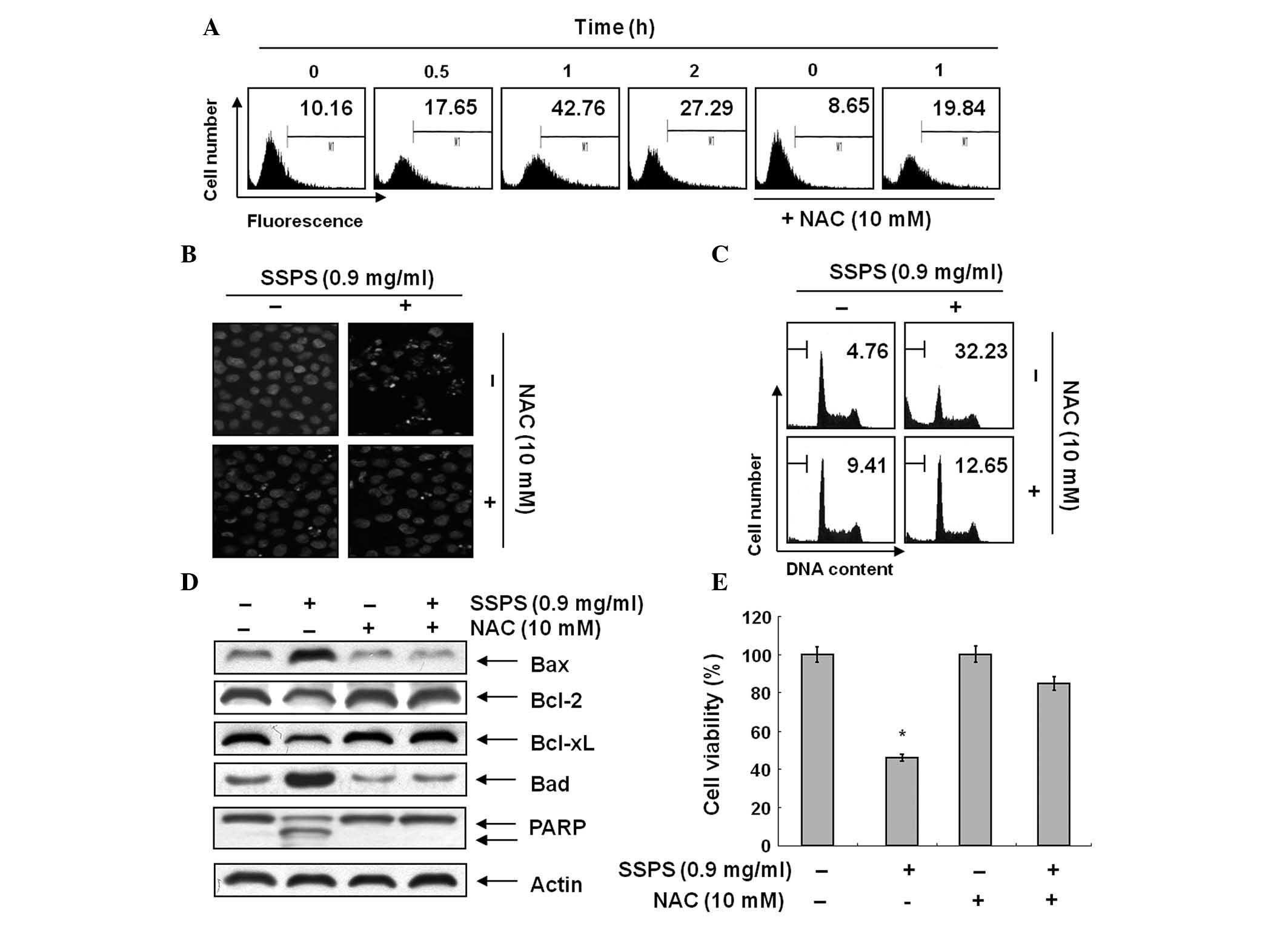 | Figure 4SSPS-induced apoptosis is associated
with ROS generation in HCT-116 cells. (A) Cells were treated with
or without NAC (10 mM) for 2 h prior to being challenged with 0.9
mg/ml SSPS for the indicated times. ROS generation was measured by
flow cytometry. (B) Cells were treated with or without NAC for 1 h
prior to being challenged with 0.9 mg/ml of SSPS for 1 h. Following
treatment with SSPS for 24 h, cells were stained with DAPI and
imaged by fluorescence microscopy using a blue filter
(magnification, ×400). (C) In a parallel experiment, cell cycle
distribution was analyzed by flow cytometry following 24 h
incubation. Experiments were performed three times independently,
with similar results obtained in each experiment. (D) Cellular
proteins extracted from cells grown under the same conditions as
(B) were separated by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis and transferred onto nitrocellulose membranes.
Membranes were probed with the indicated antibodies. Actin was used
as an internal control. (E) The cells under the same conditions as
(B) were evaluated for cell viability. *P<0.05, vs.
control. SPSS, soy sauce polysaccharide; ROS, reactive oxygen
species; NAC, N-acetyl-L-cysteine; DAPI,
4,6-diamidino-2-phenylindole; PARP, poly (ADP-ribose)
polymerase. |
Discussion
In 1972, Kikuchi and Yokotsuka (25) purified polysaccharides from soy
sauce and investigated their properties in detail. Polysaccharides
obtained from soybeans, one of the predominant raw materials of soy
sauce, contained a large quantitiy of galacturonic acid and were
marginally hydrolyzed by mold enzymes (20,25).
Matsushita et al(16) and
Kobayashi et al(26)
reported immunomodulatory and anti-allergic activities of
Shoyu polysaccharides from soy sauce; however, the cellular
and molecular mechanisms responsible for the apoptotic effects of
SSPS have not yet been determined in cancer cells. In the current
study, the inhibition of cell viability was investigated by SSPS,
and the concentration-dependent changes in the chromatin
condensation of nuclei were determined using HCT-116 cells.
Apoptosis is mediated via the activation of an
extrinsic (death receptor-mediated) or intrinsic
(mitochondrial-mediated) pathway, regulated by caspases, death
receptors and the Bcl-2 family (27). Mitochondrial dysfunction, including
the loss of MMP (Δψm),
which is accompanied by the alteration of Bcl-2 family proteins
(28) and release of cytochrome
c from the mitochondria into the cytosol is associated with
apoptosis (29). Bid is a member
of the BH-3-only family of proapoptotic proteins that initiate
apoptotic cell death. During death receptor-induced apoptosis, Bid
is activated through cleavage by caspase-8, generating a truncated
form, tBid. tBid and Bax cooperate to induce mitochondrial
dysfunction, including cytochrome c release (23). The IAP family proteins reportedly
block apoptosis due to their function as direct inhibitors that
bind to and inhibit a number of caspases (30). In the current study, SSPS-induced
apoptosis was shown to associate with a decrease in Bcl-2 and
cleavage of Bid. Furthermore, expression levels of the XIAP
proteins were decreased by SSPS (Fig.
2). Caspases are known to act as important mediators of
apoptosis and to contribute to the overall apoptotic morphology via
the cleavage of specific cellular substrates. In particular,
activation of caspase-3 is responsible for the proteolytic
degradation of a number of key proteins, including PARP, finally
leading to apoptosis (31). The
present results indicate that SSPS treatment induces activation of
caspase-3, -8 and -9 and concomitant proteolytic degradation and
downregulation of PARP proteins.
ROS have been shown to induce specific biological
processes, including apoptosis. Increased ROS induces the collapse
of MMP therefore triggering a series of mitochondria-associated
events, including apoptosis (8).
Overproduction of ROS was observed in SSPS-treated cells and the
SSPS-induced cell death was prevented by the antioxidant NAC,
indicating that ROS generation is involved in the SSPS-induced
apoptosis in HCT-116 cells.
In the present study, treatment with the natural
substance SSPS was shown to lead to increased apoptosis in the
human colon cancer cell line. The results demonstrate that SSPS may
significantly induce apoptosis in HCT-116 cells through a
mitochondria-mediated caspase pathway and ROS generation. The
results obtained in the present study show that SSPS may contribute
to the prevention or treatment of colorectal cancer.
References
|
1
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han SI, Kim YS and Kim TH: Role of
apoptotic and necrotic cell death under physiologic conditions. BMB
Rep. 41:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makin G and Dive C: Apoptosis and cancer
chemotherapy. Trends Cell Biol. 11:S22–S26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar
|
|
5
|
Ka H, Park HJ, Jung HJ, et al:
Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial
permeability transition in human promyelocytic leukemia HL-60
cells. Cancer Lett. 196:143–152. 2003. View Article : Google Scholar
|
|
6
|
Ueda S, Nakamura H, Masutani H, et al:
Baicalin induces apoptosis via mitochondrial pathway as prooxidant.
Mol Immunol. 38:781–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies KJ and Hochstein P: Ubisemiquinone
radicals in liver: implications for a mitochondrial Q cycle in
vivo. Biochem Biophys Res Commun. 107:1292–1299. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fiers W, Beyaert R, Declercq W and
Vandenabeele P: More than one way to die: apoptosis, necrosis and
reactive oxygen damage. Oncogene. 18:7719–7730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chakraborti T, Das S, Mondal M,
Roychoudhury S and Chakraborti S: Oxidant, mitochondria and
calcium: an overview. Cell Signal. 11:77–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Liu W, Wu J, et al: A polysaccharide
fraction of adlay seed (Coixlachryma-jobi L.) induces
apoptosis in human non-small cell lung cancer A549 cells. Biochem
Biophys Res Commun. 430:846–851. 2013.PubMed/NCBI
|
|
11
|
Chow LW, Lo CS, Loo WT, Hu XC and Sham JS:
Polysaccharide peptide mediates apoptosis by up-regulating p21 gene
and down-regulating cyclin D1 gene. Am J Chin Med. 31:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong SC, Yang BK, Ra KS, et al:
Characteristics of anti-complementary biopolymer extracted from
Coriolus versicolor. Carbohydr Polym. 55:255–263. 2004.
View Article : Google Scholar
|
|
13
|
Kataoka S: Functional effects of Japanese
style fermented soy sauce (shoyu) and its components. J Biosci
Bioeng. 100:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long LH, Kwee DC and Halliwell B: The
antioxidant activities of seasonings used in Asian cooking.
Powerful antioxidant activity of dark soy sauce revealed using the
ABTS assay. Free Radic Res. 32:181–186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuchiya H, Sato M and Watanabe I:
Antiplatelet activity of soy sauce as functional seasoning. J Agric
Food Chem. 47:4167–4174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsushita H, Kobayashi M, Tsukiyama R and
Yamamoto K: In vitro and in vivo immunomodulating activities of
Shoyu polysaccharides from soy sauce. Int J Mol Med. 17:905–909.
2006.PubMed/NCBI
|
|
17
|
Kinoshita E, Yamakoshi J and Kikuchi M:
Purification and identification of an angiotensin I-converting
enzyme inhibitor from soy sauce. Biosci Biotechnol Biochem.
57:1107–1110. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi M: Immunological functions of
soy sauce: hypoallergenicity and antiallergic activity of soy
sauce. J Biosci Bioeng. 100:144–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JO, Park MH, Choi YH, Ha YL and Ryu
CH: New fermentation technique for complete digestion of soybean
protein. J Microbiol Biotechnol. 17:1904–1907. 2007.PubMed/NCBI
|
|
20
|
Kikuchi T and Sugimoto H: Detailed
structure of an acidic polysaccharide in soy sauce, confirmed by
use of two kinds of purified pectinases. Agric Biol Chem. 40:87–92.
1975. View Article : Google Scholar
|
|
21
|
Jung EJ, Kim CW and Kim DR: Cytosolic
accumulation of gammaH2AX is associated with tropomyosin-related
kinase A-induced cell death in U2OS cells. Exp Mol Med. 40:276–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin DY, Kim GY, Li W, et al: Implication
of intracellular ROS formation, caspase-3 activation and Egr-1
induction in platycodon D-induced apoptosis of U937 human leukemia
cells. Biomed Pharmacother. 63:86–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong JW, Jin CY, Park C, et al: Induction
of apoptosis by cordycepin via reactive oxygen species generation
in human leukemia cells. Toxicol In Vitro. 25:817–824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon DO, Kim MO, Choi YH, Hyun JW, Chang
WY and Kim GY: Butein induces G(2)/M phase arrest and apoptosis in
human hepatoma cancer cells through ROS generation. Cancer Lett.
288:204–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kikuchi T and Yokotsuka T: Studies on the
polysaccharides from soy sauce Part I. Agric Biol Chem. 36:544–550.
1972. View Article : Google Scholar
|
|
26
|
Kobayashi M, Matsushita H, Yoshida K,
Tsukiyama R, Sugimura T and Yamamoto K: In vitro and in vivo
anti-allergic activity of soy sauce. Int J Mol Med. 14:879–884.
2004.PubMed/NCBI
|
|
27
|
Tsujimoto Y: Cell death regulation by the
Bcl-2 protein family in the mitochondria. J Cell Physiol.
195:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar
|
|
29
|
Green DR: Apoptotic pathways: ten minutes
to dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Z, Tian Y, Wang J, et al: A dimeric
Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP.
Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol
Chem. 282:30718–30727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|


















