Introduction
Airway inflammation is an essential characteristic
of allergic asthma (1). Findings
of previous studies have demonstrated that a number of cell types
and cytokines contribute to this inflammation and increase
bronchial sensitivity to various stimuli. Nerve growth factor (NGF)
is a well-studied mediator in allergic asthma (2–5). NGF
plays a critical role in neuro-immune mechanisms of asthma, which
induce airway inflammation by causing neurogenic inflammation and
enhancing the effects of immune cells. NGF may originate from the
infiltrating inflammatory cells and it has been reported that NGF
formation has been found in various immune organs, including the
spleen, lymph nodes, thymus and immune cells, such as mast cells,
eosinophils, and B and T cells (6). The NGF-mediated tyrosine kinase
receptor A (TrkA) pathway has been reported to participate in the
pathogenesis of asthma. Interleukin (IL)-1β is important in airway
responsiveness and inflammation in bronchial asthma (7,8).
Moreover, IL-4 is a T-helper (Th)2 cytokine that has a crucial role
in allergic inflammation and airway remodeling (9,10).
The ankyrin-rich membrane spanning protein (ARMS),
originally identified as a substrate for protein kinase D
(Kidins220), serves as a novel downstream target of Trk receptor
tyrosine kinases. A previous study showed that TrkA and ARMS
association persisted for a number of hours (2). NGF-induced neuronal differentiation
is mediated by TrkA receptor and ARMS association directly, which
leads to sustained mitogen-activated protein kinase activation,
resulting in neurite outgrowth (11,12).
Previous studies have shown that expression of ARMS in the lung
increased in asthmatic mice, compared with controls (13,14).
Taken together, results of those studies suggest that ARMS plays a
fundamental role as a signaling molecule in the NGF pathway. NGF
and TrkA participate in the pathogenesis of asthma. However, the
effect of Kidins220/ARMS in the immuno-inflammation of asthma has
not been adequately addressed.
The aim of the current study was to investigate the
role of the NGF-mediated Kidins220/ARMS pathway in
immuno-inflammation of asthma. Results indicate that Kidins220/ARMS
was overexpressed in the spleen and peripheral blood of asthmatic
BALB/c mice and may participate in immuno-inflammation of asthma
through the NGF-mediated signaling pathway.
Materials and methods
Animals
Female BALB/c mice (age, 6–8 weeks; weight, 18–22 g)
were purchased from Beijing Laboratory Animal Research Center
(Beijing, China) and maintained under specific pathogen-free
conditions. All animal experiments were approved by the Ethics
Committee for Animal Use and Care at the Institute of Education of
China Medical University (Shenyang, China).
Sensitization and challenge of animal
model
In total, 42 BALB/c mice were randomly divided into
four groups: Control, ovalbumin (OVA), anti-NGF and anti-ARMS
groups. Groups contained 10 mice, with the exception of the OVA
group which had 12 mice. An animal model of asthma was established
according to previous studies (15,16)
with specific modifications. In the OVA group, mice were sensitized
to 20 μg OVA absorbed in 2.0 mg aluminium hydroxide, per injection,
administered intraperitoneally on day 1. On days 8, 15 and 22, mice
were sensitized again to 10 μg OVA absorbed in 1.0 mg aluminium
hydroxide, per injection. On day 23, mice were administered inhaled
aerosols of 4% OVA in phosphate-buffered saline (PBS) for 25–30 min
(until the onset of bronchial obstruction) daily for 7 consecutive
days. Mice in the anti-NGF group were also subjected to OVA
sensitization and asthma induction in the same manner. Intranasal
application of 50 μl (1:50 dilution) polyclonal goat antimouse NGF
antibody (1:4,000 dilution blocks bioactivity of 5 ng/ml NGF; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) dissolved in sterile
PBS was performed 3 h prior to each airway allergen challenge in
the anti-NGF group. Intranasal treatment with anti-NGF antibody was
performed according to the methods of Braun et al (17), Choi
et al (18) and Nagai et al (19), with minor
modifications. Mice in the anti-ARMS group underwent intranasal
application of goat polyclonal anti-ARMS antibody that had been
diluted 1:25 in PBS (Santa Cruz Biotechnology, Inc.) (14). Mice in the control group were
administered PBS alone by injection and were challenged with PBS.
All the animals were humanely sacrificed within 24 h following the
last OVA or PBS exposure.
Immunohistochemical detection of ARMS in
the spleen
Excised spleen tissue was infused with 4%
paraformaldehyde and postfixed in sucrose for 24 h. The spleens
were then immersed in 30% sucrose in PBS for an additional 24 h,
frozen in liquid nitrogen and stored at −80°C. Frozen sections were
blocked with rabbit serum for 30 min at room temperature and then
incubated with goat polyclonal anti-ARMS antibody (1:50 dilution;
0.01 mol/l PBS; pH 7.4) at 4°C overnight. After being washed twice
with PBS, the sections were incubated with 1:200 antigoat IgG
conjugated to fluorescein isothyanate (Santa Cruz Biotechnology,
Inc.) for 30 min at room temperature. For the negative control, PBS
was used instead of primary antibodies. The slides were then
observed and images were captured using a microscope (Olympus,
Tokyo, Japan).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR) for ARMS mRNA in
the spleen and peripheral blood
RT-PCR was performed using the Takara RNA PCR kit
(AMV) 3.0 (Takara Bio, Inc., Dalian, China). Total RNA was
extracted from the spleen tissue and white blood cells (WBCs) of
peripheral blood and purified using RNAout (Takara Bio, Inc.),
according to the manufacturer’s instructions. The primers for ARMS
were 5′-AGG AGG ATG GGC GGA AGT C-3′ (forward) and 5′-CAT AAA CAG
GCT ACG GGA GG-3′ (reverse) and primers for GAPDH were 5′-CAG TGG
CAA AGT GGA GAT TGT TG-3′ (forward) and 5′-CAG TCT TCT GGG TGG CAG
TGA T-3′ (reverse). The reaction was carried out for 30 cycles
using a 94°C, 30 sec denaturing step, a 54°C, 40 sec annealing step
and a 72°C, 1 min extension step. The procedures conformed to a
standard protocol. For the semi-quantitative measurement of ARMS
density, the involved slides were photographed and assessed using
computer-aided image analysis (Motic Images Advanced 3.2; Motic,
Xiamen, China).
Western blot analysis for ARMS, IL-1β and
IL-4 in the spleen and peripheral blood
Blood was collected from the eyeball using heparin,
then diluted 2-fold with lymphocyte separation medium (ICN-Flow
Laboratories, Costa Mesa, CA, USA) and centrifuged for 20 min at
9,690 × g. WBCs were collected and stored at −70°C. Protein
homogenates from the samples of WBCs and spleen tissues were
prepared by rapid homogenization in 10 volumes of ice-cold RIPA
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). Protein concentrations of the cell and tissue lysates were
determined using the Enhanced BCA Protein assay kit (Beyotime
Institute of Biotechnology) and the supernatants were boiled in
sodium dodecyl sulfate sample buffer for 5 min. Equal amounts of
lysate proteins were separated using 7.5 or 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto a polyvinylidene difluoride membrane (Amersham Pharmacia
Biotech, Uppsala, Sweden). Following blocking, the blots were
incubated with specific primary antibody overnight at 4°C and then
incubated for 1 h with horseradish peroxidase-conjugated secondary
antibody. Bound antibodies were detected using an enhanced
chemiluminescence kit with a Lumino-Image analyzer (Taitec, Tokyo,
Japan). Integrated density values were analyzed using a
computerized image analysis system (Fluor Chen 2.0; Bio-Rad,
Hercules, CA, USA) and normalized to those of β-actin.
Statistical analysis
Data were analyzed using statistical software (SPSS
15.0; SPSS Inc., Chicago, IL, USA). Data are expressed as mean ±
SEM. Differences between groups were analyzed, as appropriate,
using t-tests and one-way analysis of variance followed by the
Fisher’s least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
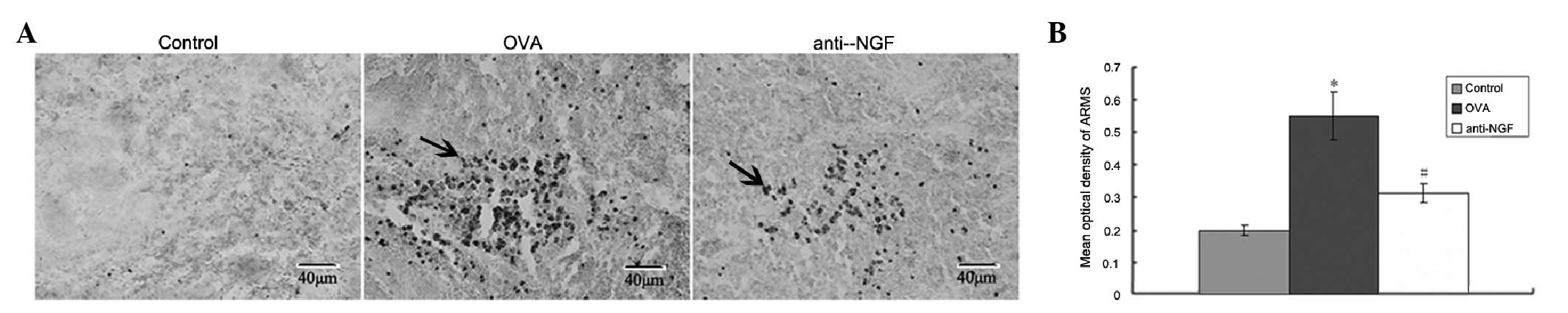
Immunohistochemical detection of ARMS in
the spleen
Expression of ARMS, mainly in the cytoplasm, was
visible as brownish yellow particles (Fig. 1A). Optical density analysis
revealed that the expression of ARMS in the OVA group increased
when compared with the control group. Expression of ARMS in
anti-NGF group was lower than that in the asthmatic model group
(P<0.05; Fig. 1B).
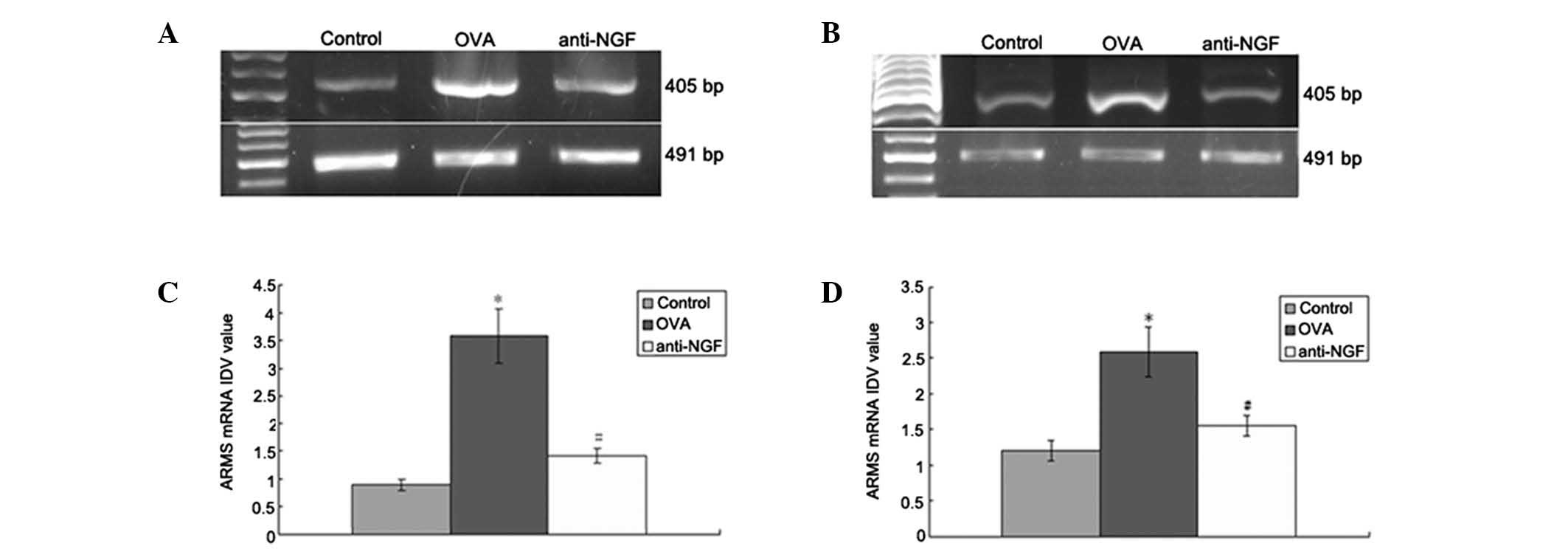
Expression of ARMS mRNA in the spleen and
peripheral blood
RT-PCR was used to detect the mRNA expression levels
of ARMS in the spleen (Fig. 2A)
and WBCs of the peripheral blood (Fig.
2B). In the control group ARMS mRNA expression levels were low,
but were markedly increased in the OVA group (P<0.01). However,
anti-NGF treatment significantly decreased ARMS mRNA upregulation
caused by OVA (P<0.01; Fig. 2C and
D).
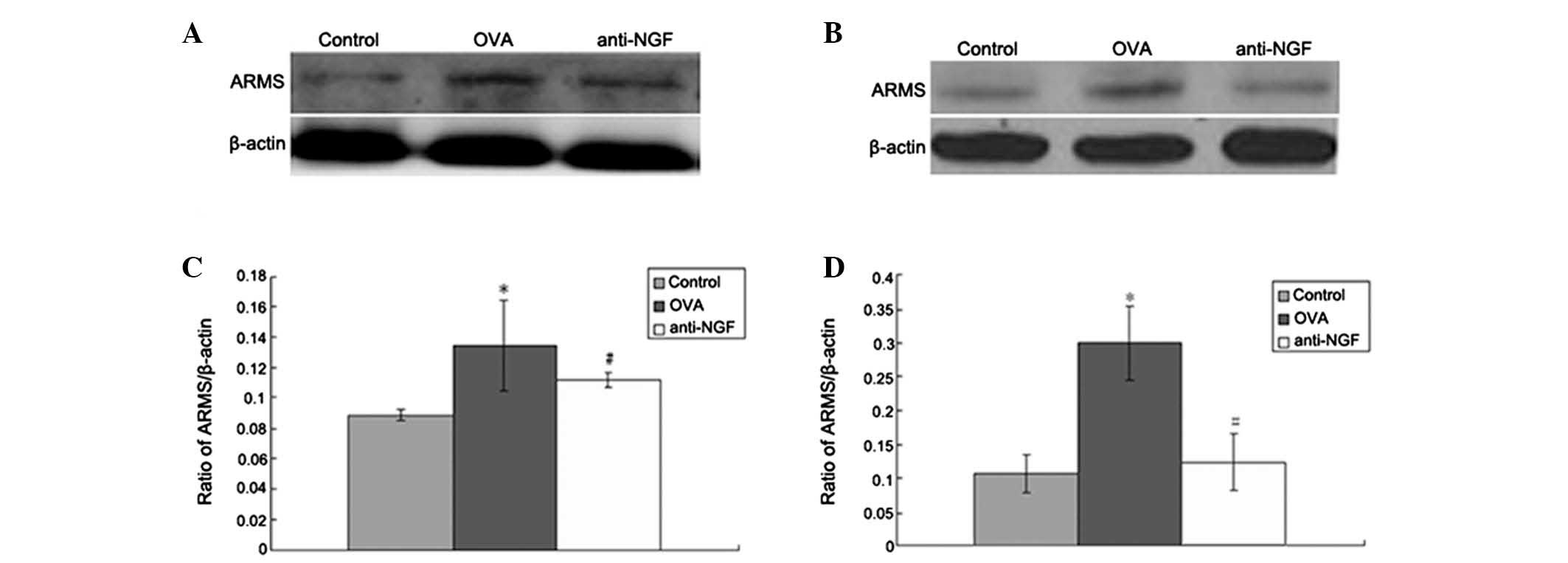
Expression of ARMS protein in the spleen
and peripheral blood
Protein levels of ARMS in the spleen (Fig. 3A) and WBCs of peripheral blood
(Fig. 3B) were detected by western
blot analysis. In the control group ARMS protein was expressed at
low levels, but these levels were markedly increased in the OVA
group (P<0.01 or P<0.05). However, anti-NGF or anti-ARMS
treatment significantly decreased ARMS upregulation caused by OVA
(P<0.01 or P<0.05; Fig. 3C and
D).
Expression of IL-1β and IL-4 protein in
the spleen and peripheral blood
Expression of IL-1β (Fig. 4A) and IL-4 (Fig. 4B) in the spleen was detected. In
the control group IL-1β and IL-4 proteins were expressed at low
levels, but these levels were markedly increased in the OVA group
(P<0.01 or P<0.05). However, anti-NGF or anti-ARMS treatment
significantly decreased IL-1β and IL-4 upregulation caused by OVA
(P<0.01 or P<0.05; Fig. 4C and
D).
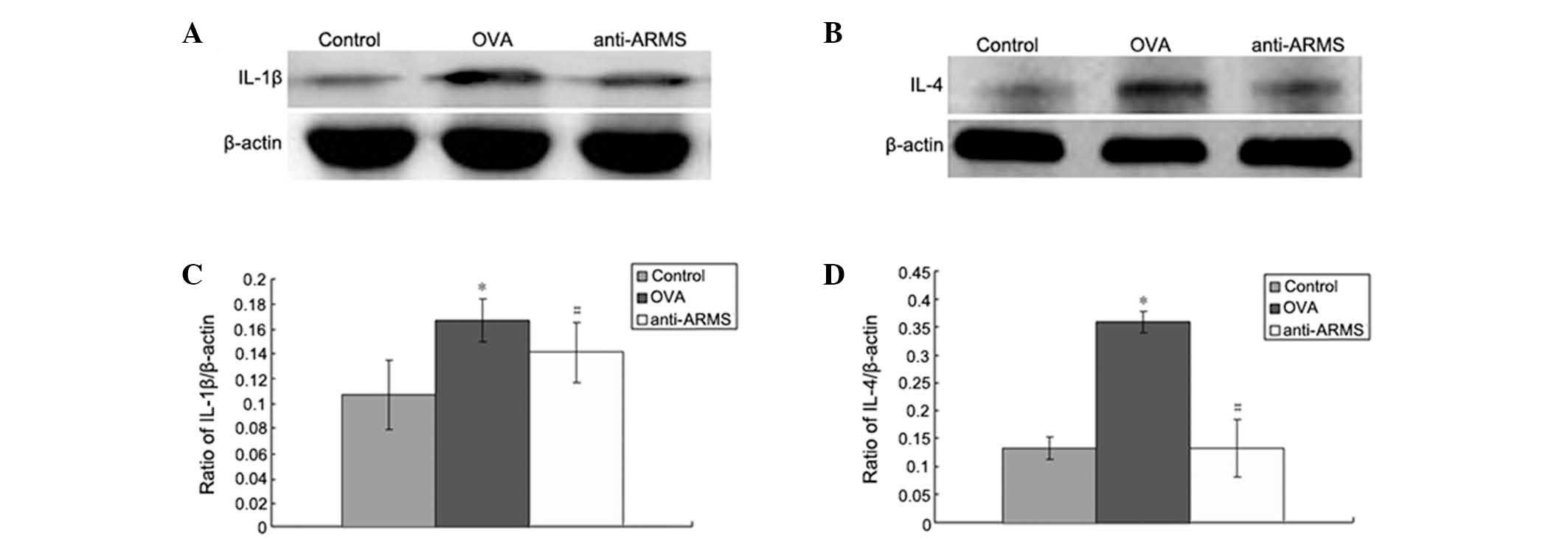 | Figure 4Expression of IL-1β and IL-4 protein
in the spleen by western blot analysis. Representative image of (A)
IL-1β and (B) IL-4. Optical density values for (C) IL-1β, relative
to β-actin, *P<0.01, vs. control and
#P<0.05, vs. OVA and for (D) IL-4, relative to
β-actin, *P<0.01, vs. control and
#P<0.01 vs. OVA. Data are expressed as mean ± SEM
(n=6). ARMS, ankyrin-rich membrane spanning; OVA, ovalbumin; IL,
interleukin. |
Discussion
Kidins220/ARMS is a multifunctional scaffold protein
that interacts with a number of transmembrane receptors, including
Trks and p75NTR (11,20,21).
Intracellular scaffolding proteins have a key role in the
association between various receptors, which induce cross-talk of
downstream signaling (22).
Kidins220/ARMS functions as a unique downstream adaptor protein of
Trk receptor tyrosine kinases (23), which activates downstream enzymes
by binding with NGF-TrkA (24).
Furthermore, NGF-TrkA is important in bronchial
hyper-responsiveness and inflammation (25,26)
in asthma. Although it has been previously demonstrated that the
expression of ARMS in the lung increased in asthmatic mice compared
with that in control mice (8,9),
little was known about the involvement of Kidins220/ARMS in the
spleen and blood.
In the present study, the expression of ARMS in the
spleen and WBCs of peripheral blood, as measured by RT-PCR, western
blot analyses and immunohistochemistry, increased in the OVA group
compared with the control group. Intervention with anti-NGF
antibody resulted in a significant reduction in ARMS expression in
the spleen and WBCs of peripheral blood in the OVA group. ARMS
blockade was also associated with a reduction of inflammatory
cytokines, including IL-1β and IL-4, in the spleen of
OVA-sensitized mice. Previously, studies indicated that Th2 cells
over produced cytokines (e.g., IL-4, 5 and 13) and histamines in
allergic asthma (27,28). Th1-associated IL-1β and TNF-α, as
well as Th2-associated IL-4, are considered to be the key cytokines
that induce airway hyper-responsiveness, infiltration of
eosinophils into the lungs and mucus secretion in allergic asthma
(29,30). The immunopathological hallmark of
allergic asthma is the infiltration of eosinophils and Th2 cells in
affected tissue. Th2 cells are prominent for immune deviation in
allergic asthma (31,32), but the immunological mechanism
requires further investigation.
Allergic asthma is a Th2-dominant disease
characterized by increased airway inflammation and IgE production,
as well as airway hyper-reactivity. It has been reported that NGF
may affect the viability of peripheral blood eosinophils from
healthy donors (33,34). Moreover, previous studies have
revealed elevated serum levels of NGF in subject responses to
allergic asthma, particularly in patients with severe allergic
asthma (35). There was a regular
expression of ARMS in the spleen and WBCs of peripheral blood,
while a higher expression was shown in the asthmatic group as
compared to the control group. Th1/Th2 cytokine immune imbalance
may indirectly induce airway neurogenic inflammation by regulating
NGF expression. Furthermore, NGF may promote and magnify
immunological inflammation. IL-1, a lymphocyte-activating factor,
enhances adhesion and gasification of inflammatory cells to the
airway inflamed regions (36),
inducing the endosomatic immunological process. This finding
indicates that NGF-mediated ARMS signaling may regulate
immune-activation by upregulating IL-1β and IL-4 in the spleen and
WBCs of peripheral blood in asthma. Thus, the present study on
Kidins220/ARMS in the spleen and peripheral blood may clarify the
mechanism involved in asthma. In addition, targeting Kidins220/ARMS
on immune cells may be a new promising therapeutic option for the
treatment of allergic asthma.
Acknowledgements
The study was supported by grants from the Education
Department Project of Heilongjiang Province (no. 12511218).
References
|
1
|
Sanico AM, Stanisz AM, Gleeson TD, Bora S,
Proud D, Bienenstock J, Koliatsos VE and Togias A: Nerve growth
factor expression and release in allergic inflammatory disease of
the upper airways. Am J Respir Crit Care Med. 161:1631–1635. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoyle GW: Neurotrophins and lung disease.
Cytokine Growth Factor Rev. 14:551–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frossard N, Freund V and Advenier C: Nerve
growth factor and its receptors in asthma and inflammation. Eur J
Pharmacol. 500:453–465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lommatzsch M, Braun A and Renz H:
Neurotrophins in allergic airway dysfunction: what the mouse model
is teaching us. Ann NY Acad Sci. 992:241–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aloe L, Bracci-Laudiero L, Bonini S and
Manni L: The expanding role of nerve growth factor: from
neurotrophic activity to immunologic diseases. Allergy. 52:883–894.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gosset P, Tsicopoulos A, Wallaert B,
Vannimenus C, Joseph M, Tonnel AB and Capron A: Increased secretion
of tumor necrosis factor alpha and interleukin-6 by alveolar
macrophages consecutive to the development of the late asthmatic
reaction. J Allergy Clin Immunol. 88:561–571. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wills-Karp M, Uchida Y, Lee JY, Jinot J,
Hirata A and Hirata F: Organ culture with proinflammatory cytokines
reproduces impairment of the beta-adrenoceptor-mediated relaxation
in tracheas of a guinea pig antigen model. Am J Respir Cell Mol
Biol. 8:153–159. 1993. View Article : Google Scholar
|
|
8
|
Jie Z, Jin M, Cai Y, Bai C, Shen Y, Yuan
Z, Hu Y and Holgate S: The effects of Th2 cytokines on the
expression of ADAM33 in allergen-induced chronic airway
inflammation. Respir Physiol Neurobiol. 168:289–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshinaka T, Nishii K, Yamada K, Sawada H,
Nishiwaki E, Smith K, Yoshino K, Ishiguro H and Higashiyama S:
Identification and characterization of novel mouse and human
ADAM33s with potential metalloprotease activity. Gene. 282:227–236.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Averill S, Delcroix JD, Michael GJ,
Tomlinson DR, Fernyhough P and Priestley JV: Nerve growth factor
modulates the activation status and fast axonal transport of ERK
1/2 in adult nociceptive neurones. Mol Cell Neurosci. 18:183–196.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arevalo JC, Yano H, Teng KK and Chao MV: A
unique pathway for sustained neurotrophin signaling through an
ankyrin-rich membrane-spanning protein. EMBO J. 23:2358–2368. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neubrand VE, Cesca F, Benfenati F and
Schiavo G: Kidins220/ARMS as a functional mediator of multiple
receptor signalling pathways. J Cell Sci. 125:1845–1854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni X, Li X, Fang X, Li N, Cui W and Zhang
B: NGF/TrkA-mediated Kidins220/ARMS signaling activated in the
allergic airway challenge in mice. Ann Allergy Asthma Immunol.
105:299–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni X, Li X, Fang X, Li N, Cui W, Zhang B
and Liu Y: Kidins220/ARMS contributes to airway inflammation and
hyper-responsiveness in OVA-sensitized mice. Respir Physiol
Neurobiol. 175:97–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanacker NJ, Palmans E, Kips JC and
Pauwels RA: Fluticasone inhibits but does not reverse
allergen-induced structural airway changes. Am J Respir Crit Care
Med. 163:674–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen HH, Xu F, Zhang GS, Wang SB and Xu
WH: CCR3 monoclonal antibody inhibits airway eosinophilic
inflammation and mucus overproduction in mouse model of asthma.
Acta Pharmacol Sin. 27:1594–1599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Braun A, Appel E, Baruch R, Herz U,
Botchkarev V, Paus R, Brodie C and Renz H: Role of nerve growth
factor in a mouse model of allergic airway inflammation and asthma.
Eur J Immunol. 28:3240–3251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi JR, Lee CM, Jung ID, Lee JS, Jeong
YI, Chang JH, Park HJ, Choi IW, Kim JS, Shin YK, et al: Apigenin
protects ovalbumin-induced asthma through the regulation of GATA-3
gene. Int Immunopharmacol. 9:918–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagai T, Arai Y, Emori M, Nunome SY, Yabe
T, Takeda T and Yamada H: Anti-allergic activity of a Kampo
(Japanese herbal) medicine ‘Sho-seiryu-to (Xiao-Qing-Long-Tang)’ on
airway inflammation in a mouse model. Int Immunopharmacol.
4:1353–1365. 2004.
|
|
20
|
Kong H, Boulter J, Weber JL, Lai C and
Chao MV: An evolutionarily conserved transmembrane protein that is
a novel downstream target of neurotrophin and ephrin receptors. J
Neurosci. 21:176–185. 2001.PubMed/NCBI
|
|
21
|
Chang MS, Arevalo JC and Chao MV: Ternary
complex with Trk, p75, and an ankyrin-rich membrane spanning
protein. J Neurosci Res. 78:186–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cesca F, Yabe A, Spencer-Dene B, Arrigoni
A, Al-Qatari M, Henderson D, Phillips H, Koltzenburg M, Benfenati F
and Schiavo G: Kidins220/ARMS is an essential modulator of
cardiovascular and nervous system development. Cell Death Dis.
2:e2262011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HJ, Park HW, Lee SJ, Arevalo JC, Park
YS, Lee SP, Paik KS, Chao MV and Chang MS: Ankyrin repeat-rich
membrane spanning/Kidins220 protein interacts with mammalian Septin
5. Mol Cells. 30:143–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frossard N, Naline E, Olgart Hoglund C,
Georges O and Advenier C: Nerve growth factor is released by
IL-1beta and induces hyperresponsiveness of the human isolated
bronchus. Eur Respir J. 26:15–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nockher WA and Renz H: Neurotrophins in
allergic diseases: from neuronal growth factors to intercellular
signaling molecules. J Allergy Clin Immunol. 117:583–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Humbert M, Menz G, Ying S, Corrigan CJ,
Robinson DS, Durham SR and Kay AB: The immunopathology of extrinsic
(atopic) and intrinsic (non-atopic) asthma: more similarities than
differences. Immunol Today. 20:528–533. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elenkov IJ and Chrousos GP: Stress
hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and
susceptibility to disease. Trends Endocrinol Metab. 10:359–368.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaiko GE and Foster PS: New insights into
the generation of Th2 immunity and potential therapeutic targets
for the treatment of asthma. Curr Opin Allergy Clin Immunol.
11:39–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wills-Karp M: IL-12/IL-13 axis in allergic
asthma. J Allergy Clin Immunol. 107:9–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kay AB: Allergy and allergic diseases.
First of two parts. N Engl J Med. 344:30–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Umetsu DT, McIntire JJ, Akbari O, Macaubas
C and DeKruyff RH: Asthma: an epidemic of dysregulated immunity.
Nat Immunol. 3:715–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noga O, Englmann C, Hanf G, Grutzkau A,
Seybold J and Kunkel G: The production, storage and release of the
neurotrophins nerve growth factor, brain-derived neurotrophic
factor and neurotrophin-3 by human peripheral eosinophils in
allergics and non-allergics. Clin Exp Allergy. 33:649–654. 2003.
View Article : Google Scholar
|
|
34
|
Solomon A, Aloe L, Pe’er J, Frucht-Pery J,
Bonini S, Bonini S and Levi-Schaffer F: Nerve growth factor is
preformed in and activates human peripheral blood eosinophils. J
Allergy Clin Immunol. 102:454–460. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonini S, Lambiase A, Lapucci G, Properzi
F, Bresciani M, Bracci Laudiero ML, Mancini MJ, Procoli A, Micera
A, Sacerdoti G, et al: Nerve growth factor and asthma. Allergy.
57(Suppl 72): 13–15. 2002. View Article : Google Scholar
|
|
36
|
Nambu A and Nakae S: IL-1 and allergy.
Allergol Int. 59:125–135. 2010. View Article : Google Scholar
|