Introduction
Diabetes is associated with a number of chronic
conditions, including inflammation, insulin resistance, hepatic
steatosis and cardiovascular disease. Recent studies have revealed
that gut microbes may play an important role in diabetes and
metabolic diseases. These microbes may affect homeostasis as
environmental factors. Over the past few years, new evidence has
demonstrated that the increasing prevalence of type 2 diabetes and
obesity may be partly attributed to disorders of the host gut
microbiota. Larsen et al(1)
suggested that type 2 diabetes in humans is associated with changes
in the composition of the intestinal microbiota. The authors of
that study found a reduction in the levels of Firmicutes and an
increase in the levels of Bacteroidetes in diabetic patients
compared with their non-diabetic counterparts.
Glucagon-like peptide 1 (GLP-1) is an incretin
hormone secreted by enteroendocrine L cells that are scattered
throughout the gastrointestinal mucosa. GLP-1 is crucial in the
treatment of diabetes as it stimulates insulin, suppresses the
secretion of glucagon, inhibits gastric emptying and reduces
appetite and food intake (2). The
secretion of GLP-1 has been shown to be markedly reduced in type 2
diabetes (3,4). However, the mechanism underlying this
change is poorly understood. We propose that damaged
enteroendocrine L cells are responsible for this incretin
defect.
Cani and Delzenne (5) found that the bacteria-related factor
lipopolysaccharide (LPS) may be responsible for the development of
diabetes. Subsequently, Creely et al(6) reinforced the hypothesis that LPS may
act as a gut microbiota-related factor in the development of type 2
diabetes in humans. LPS is a complex glycoprotein constituent of
the outer cell wall of gram-negative bacteria. It is continuously
released from the surface of replicating and dying gram-negative
bacteria, such as Bacteroidetes, into the gastrointestinal tract.
LPS levels increase significantly in the gut of diabetic patients,
which makes them interact constantly with the intestinal
epithelium. Enteroendocrine L cells, which are scattered among the
intestinal epithelium, are constantly exposed to high
concentrations of LPS in patients with type 2 diabetes. This
interaction may induce apoptosis in enteroendocrine L cells.
Increasing attention has been focused on how to
modulate the secretion of GLP-1 by enteroendocrine L cells.
However, few studies have focused on apoptosis of enteroendocrine L
cells potentially being a mechanism underlying the decreased
secretion of GLP-1 in patients with type 2 diabetes. In this study,
we investigated whether LPS was capable of inducing apoptosis in
the intestinal endocrine cell line STC-1. This cell line has been
established as a good cellular model for the study of GLP-1
secretion and transcription (7–9). The
results suggest that LPS induces apoptosis in STC-1 cells by
regulating the expression of three key proteins, caspase-3, Bax and
Bcl-2, which are critical for apoptosis.
Materials and methods
Cell cultures
STC-1 cells were obtained from the Guangzhou Jennio
Biotech Co., Ltd. (Guangzhou China). Cells were cultured in DMEM
(Hyclone, Logan, UT, USA) supplemented with 2.5% (vol/vol) fetal
bovine serum (Gibco, Carlsbad, CA, USA), 10% (vol/vol) horse serum
(Gibco), 100 U/ml penicillin (Life Technologies, Carlsbad, CA, USA)
and 100 U/ml streptomycin (Life Technologies), and incubated in a
humidified atmosphere containing 5% CO2 at 37°C. The
medium was changed every 2–3 days. When the cells reached 80–90%
confluence, they were trypsinized with 0.25% trypsin-EDTA
(Sigma-Aldrich, St. Louis, MO, USA) and subsequently replated into
culture flasks. Each experiment was repeated at least three times
(n≥3).
Cell viability assay
For the
3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT)
assay, 2×104 cells/well were seeded in 96-well plates
for 24 h at 37°C and starved for a further 24 h in serum-free DMEM.
Subsequently, the cells were exposed to LPS (Escherichia
coli 0111:B4; Sigma-Aldrich) at various concentrations (0, 0.1,
0.5, 1 and 10 μg/ml) at 100 μl/well. Following incubation for 24 h,
20 μl/well of MTT (Sigma-Aldrich) solution (5 mg/ml in PBS) was
added and the cells were cultured at 37°C for 4 h. Subsequently,
the supernatant was discarded carefully. The formazan crystals were
dissolved in 100 μl/well of DMSO. The plates were agitated for 5
min, and the absorbance of solubilized formazan was measured at 570
nm using an automatic microplate reader (Multiskan MK3, Thermo
Fisher Scientific Inc., Waltham, MA, USA). Cell survival was
expressed as the ratio of the absorbance of treated cells to the
untreated cells.
Hoechst 33258 staining
STC-1 cells (1×106 cells/well) were
exposed to various concentrations of LPS for 24 h. The cells were
washed in PBS and stained with Hoechst 33258 (5 μg/ml) for 5 min at
37°C. The stained cells were washed twice in PBS and then observed
immediately using a fluorescence microscope (Leica; Wetzlar,
Germany). An Ex/Em filter set of BP330–380/LP420 nm was used, and
the images were recorded using a color charge-coupled device
camera.
Analysis for annexin V
Cells (2×106) were seeded into six-well
plates for 24 h and starved for a further 12 h in serum-free DMEM.
Apoptotic cells were identified based on the translocation of
phosphatidylserine from the inner to the outer leaflet of the
plasma membrane, measured using an annexin V/propidium iodide (PI)
double staining apoptosis detection kit (Life Technologies).
Following treatment with or without various concentrations of LPS
for 24 or 6 h, the cells were washed three times with PBS and
immediately fixed in 4% paraformaldehyde for 15 min or harvested
and washed three times in cold PBS and resuspended in an
annexin-binding buffer. Subsequently, the fixed cells were added
with annexin V-fluorescein isothiocyanate (FITC) and PI buffer,
while the resuspended cells were added to annexin V-FITC and PI
directly. The reaction was incubated at room temperature for 15 min
in the dark. Stained cells were analyzed immediately using an
Accuri C6 flow cytometer (BD Biosciences; San Jose, California,
USA) and a fluorescence microscope (Leica). Fluorescence was
detected with an excitation wavelength of 488 nm.
For flow cytometry, the LPS exposure time was
reduced to 6 h. This change was due to the fact that flow cytometry
using annexin V staining is a more sensitive technique for
detecting apoptosis compared with the other techniques used in our
study.
Western blotting
In order to examine Bcl-2, Bax and caspase-3 protein
levels, cells were grown in culture flasks and treated with LPS as
described in a previous section. After washing in ice-cold PBS,
cells were lysed in a lysis buffer [50 mM Tris-HCl, pH 7.4; 150 mM
NaCl; 1 mM phenylmethanesulfonylfluoride (PMSF); 1 mM EDTA; 1%
Triton X-100; 1% sodium deoxycholate; and 0.1% sodium dodecyl
sulfate (SDS); Sigma-Aldrich] at 4°C for 30 min. Cell lysates were
centrifuged for 10 min at 15,400 rpm at 4°C, and the supernatants
were collected. The protein concentration of the samples was
estimated according to the BCA method (10). Subsequently, 50 μg of total protein
from each sample was separated via 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted
onto nitrocellulose membranes. The membranes were blocked with 2%
non-fat dry milk in PBS containing 0.25% Tween-20 overnight and
incubated with Bcl-2, Bax (Cell Signaling Technology, Beverly, MA,
USA) and caspase-3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA) antibodies for 2 h at room temperature. Following the three
washing steps, anti-horseradish peroxidase-conjugated secondary
antibodies (Beyotime, Haimen, China) were applied, and the
membranes were stained using enhanced chemiluminescence reagents
(Beyotime). The autoradiographs were scanned and semiquantitatively
quantified.
Statistical analyses
Data were presented as the means ± SD. Significant
differences between groups were evaluated using one-way analysis of
variance (ANOVA). P<0.05 was considered to indicate a
statistically significant difference.
Results
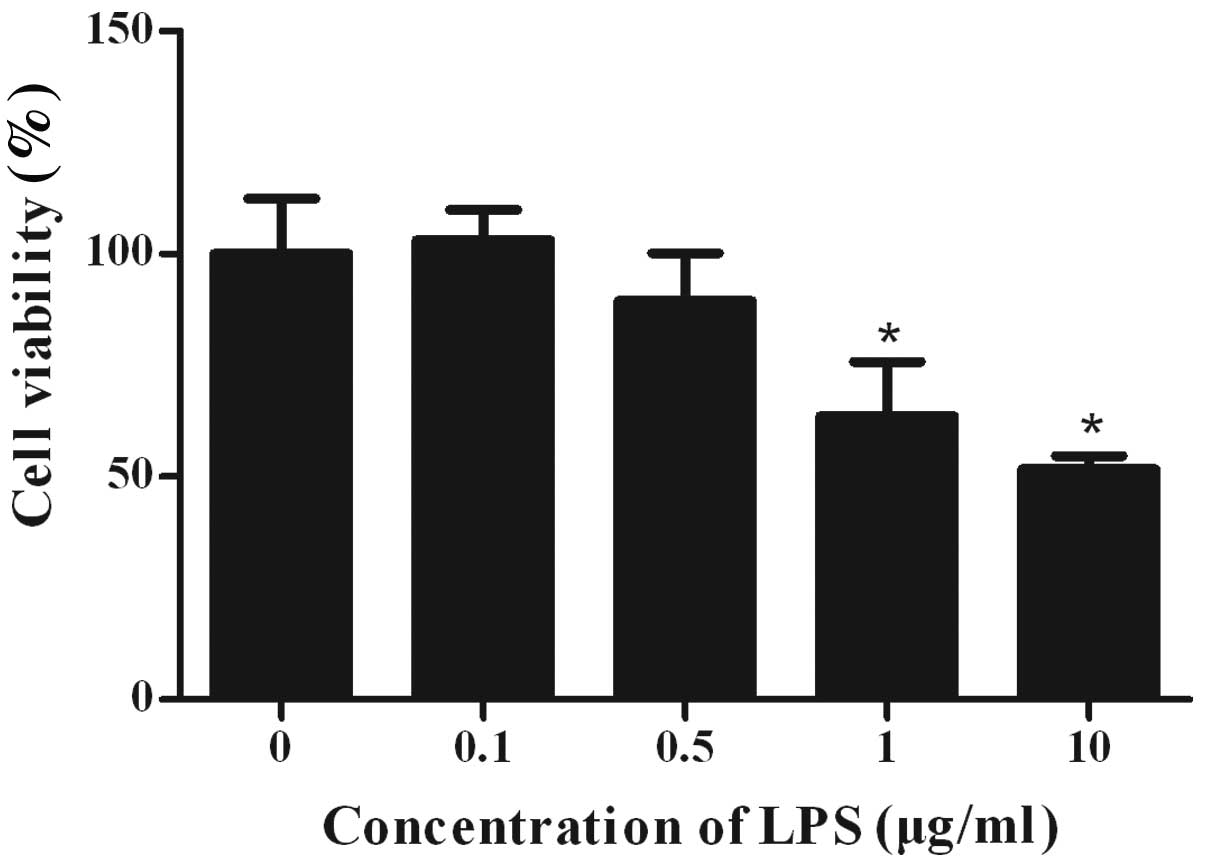
LPS affects the viability of STC-1
cells
STC-1 cells were cultured in the absence and
presence of varying concentrations of LPS for 24 h. The effects of
LPS on cell viability were assessed by MTT assay (Fig. 1). Exposure to increasing
concentrations of LPS induced a dose-dependent decrease in cell
survival. At the highest tested concentration of LPS (10 μg/ml),
the survival rate was 52±3% when compared with the control group.
At 1 μg/ml LPS, cell viability was reduced to 64±12%. However, no
significant growth inhibition was observed when LPS was added at a
concentration of 0.1 μg/ml and 0.5 μg/ml.
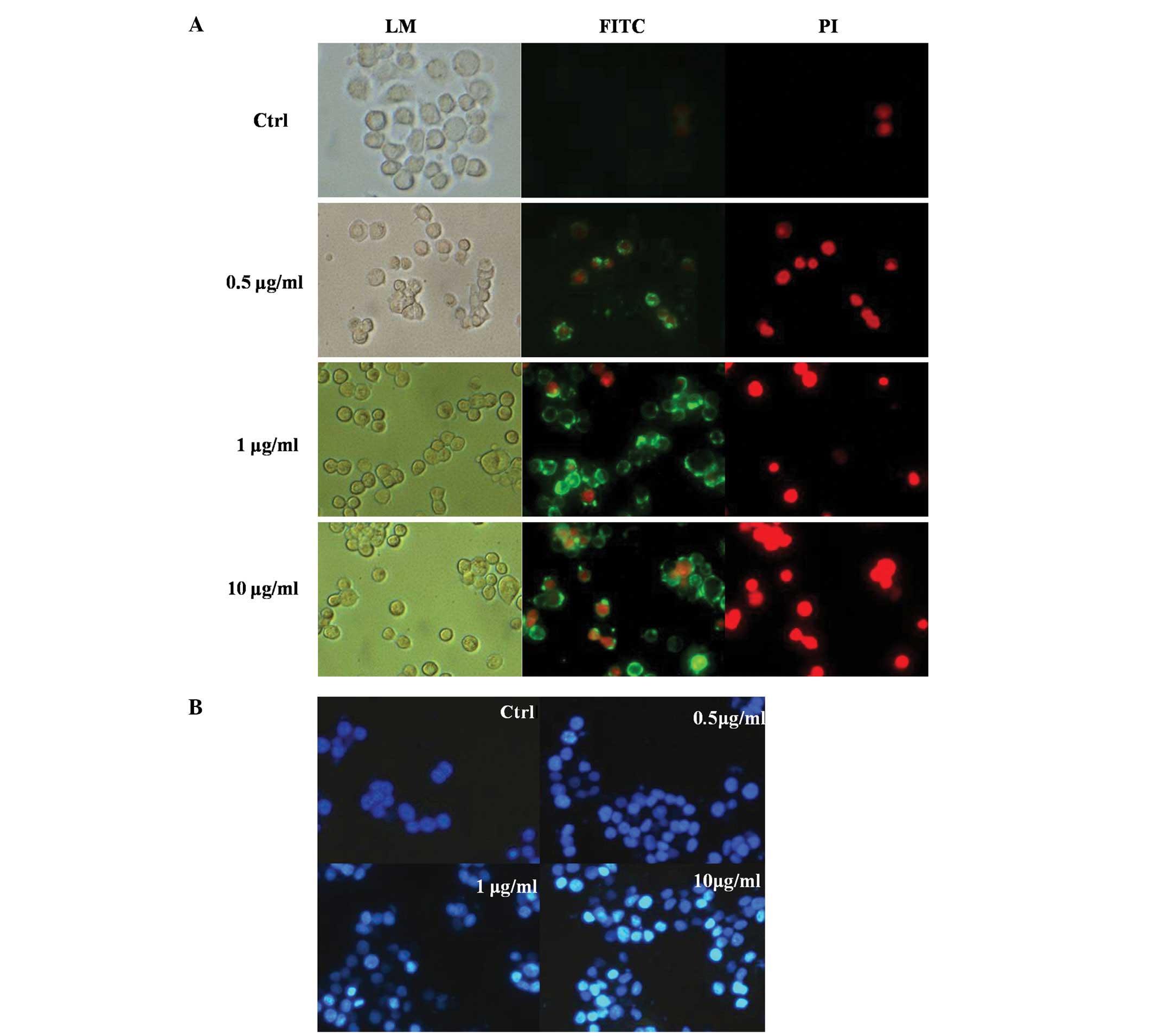
Cell morphology
Following staining with an annexin V/PI apoptosis
kit, living cells demonstrated little or no fluorescence, and
apoptotic cells exhibited green fluorescence. Necrotic cells
exhibited both red and green fluorescence. As shown in Fig. 2A, the cells show weak green
fluorescence, and no red fluorescence is found in the control
groups. The fluorescence intensity of the 0.5 μg/ml groups was
similar to that of the control group. However, the number of cells
with strongly green fluorescence increased with the increasing
concentrations of LPS (1–10 μg/ml). This indicates that apoptosis
was more prevalent at high concentrations of LPS.
The results of Hoechst 33258 staining were
consistent with the findings observed in a previous section. As
shown in Fig. 2B, normal STC-1
cells exhibited weak fluorescence. Following LPS treatment, the
cells demonstrated a brighter fluorescence, and typical apoptotic
characteristics, such as nuclear condensation, were observed in
cells treated with 1 or 10 μg/ml LPS.
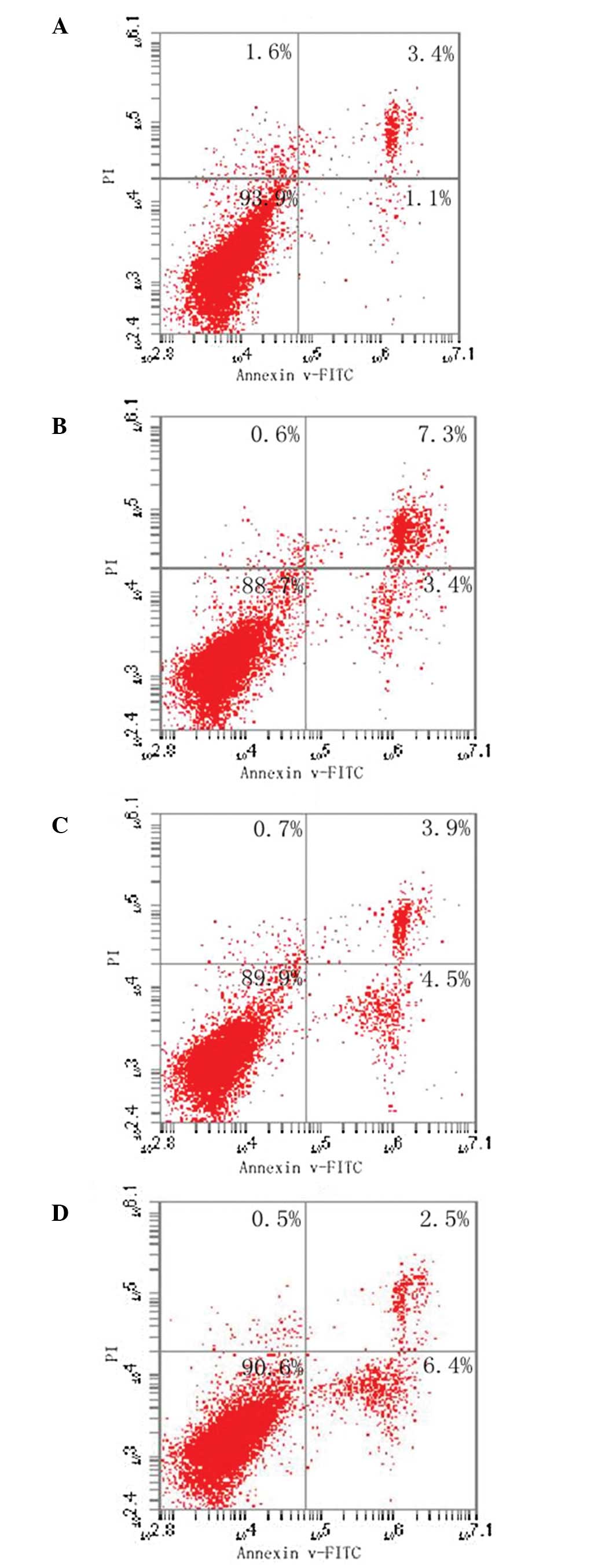
Flow cytometry
The lower right quadrant of each plot indicates the
percentage of early apoptotic cells that are only annexin
V-FITC-positive. The upper right quadrant shows late apoptotic
cells or necrotic cells with positive staining for annexin V-FITC
and PI. In order to avoid interference from necrotic cells, the
apoptotic cells in this portion of the plot were not included in
the calculations. The viable cells (annexin V-FITC- and
PI-negative) are shown in the lower left quadrant. As shown in
Fig. 3, there was a trend of
increased apoptosis in the 1 and 10 μg/ml groups compared with the
control groups (up to 4.5 and 6.4%, respectively; P<0.05).
However, no significant difference was observed between the 0.5
μg/ml LPS group and the control group.
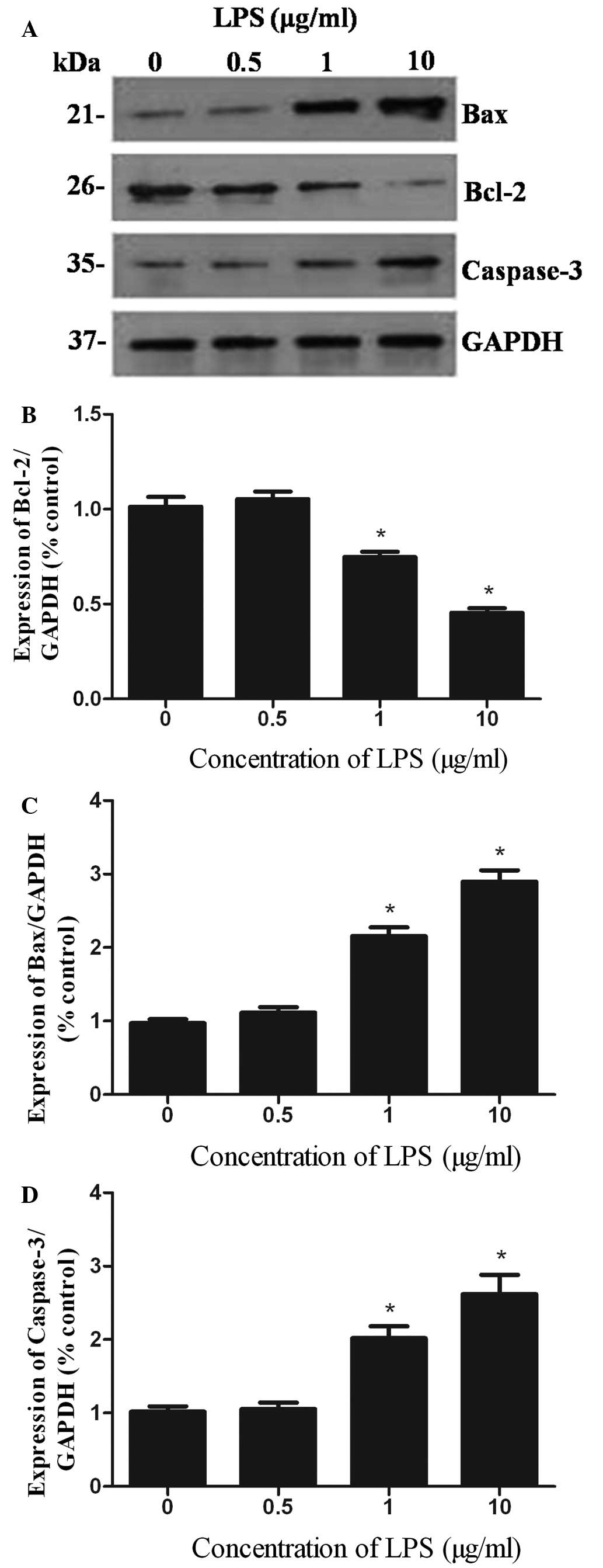
Effect of LPS on the expression of
caspase-3, Bcl-2 and Bax proteins
As measured by western blotting (Fig. 4), incubation with LPS (0.5, 1 and
10 μg/ml) for 24 h markedly reduced the expression of Bcl-2
protein, to 75 and 45% (compared with the controls) in the 1 and 10
μg/ml LPS groups, respectively. The increased expression level of
Bax protein paralleled the decrease in the expression level of
Bcl-2 protein. However, Bax protein expression increased 2.2 and
3.0 times in STC-1 cells following treatment with 1 and 10 μg/ml
LPS, respectively (controls are set as 100%). Furthermore, higher
concentrations of LPS led to the increased cleavage of caspase-3.
The levels of caspase-3 increased 2.0 and 2.6 times following
treatment with 1 and 10 μg/ml of LPS, respectively. There were no
significant changes in the expression of any of the three proteins
in the 0.5 μg/ml LPS group.
Discussion
The growing epidemics of obesity and type 2 diabetes
have led the scientific community to identify and develop new
therapeutic targets. Over the past few years, the modulation of the
secretory functions of enteroendocrine L cells has been the focus
of research due to the powerful anti-diabetic activities of GLP-1.
However, studies on the apoptosis of intestinal endocrine L cells
remain limited. The secretion of GLP-1 has been shown to be
markedly reduced in type 2 diabetes. Therefore, we hypothesized
that this incretin defect may be related to the increased apoptosis
of enteroendocrine L cells. A series of studies have shown that LPS
may induce apoptosis in various cell types, such as fibroblasts,
macrophages and endothelial cells (11–13).
However, whether LPS is capable of inducing the apoptosis of
enteroendocrine L cells has not been confirmed.
The results of this study demonstrated that LPS was
capable of inhibiting the growth of STC-1 cells in a
concentration-dependent manner. Cell viability, as determined by
MTT assay, decreased with an increase in the concentration of LPS.
Typical apoptotic features, such as cytoplasmic shrinkage, nuclear
chromatin condensation and apoptotic bodies, were observed in STC-1
cells treated with 1 or 10 μg/ml LPS using Hoechst 33258.
Phosphatidylserine (PS) is located on the
cytoplasmic surface of normal living cell membranes. Following
apoptosis, PS is translocated from the inner plasma membrane to the
outer plasma membrane and faces the extracellular environment.
Annexin V has a high affinity for PS; therefore,
fluorophore-labeled annexin V may be used to identify apoptotic
cells. Our study revealed that the number of green cells, namely
the apoptotic cells, increased with the increasing concentration of
LPS. Flow cytometry data provide further evidence for this increase
in apoptosis. This is consistent with the findings demonstrated by
Hamada et al(14), in which
LPS induced a dose-dependent increase in the number of
TUNEL-positive hepatocytes.
Apoptosis, or programmed cell death, is an essential
physiological process in which cells that are no longer required or
are injured are eliminated (15).
The best-studied mechanisms of apoptosis regulation involve members
of the Bcl-2 family (16–18). A minimum of 15 Bcl-2 proteins have
been identified, and they may be divided into proapoptotic
proteins, such as Bax, Bak, Bad, Bcl-xs, Bid, Bik, Bim and Hrk, and
antiapoptotic proteins, such as Bcl-2, Bcl-xl, Bcl-w, Bfl-1 and
Mcl-1 (19). The balance between
proapoptotic and antiapoptotic Bcl-2 family proteins determines
whether a cell lives or dies. Therefore, the ratio of Bcl-2/Bax is
of greater importance in determining the apoptosis of cells
following apoptotic stimulation than the quantity of Bcl-2 alone
(18). In this study, we found
that the level of Bcl-2 protein decreased, whereas the levels of
Bax increased in STC-1 cells following exposure to LPS. The low
ratio of Bcl-2/Bax in STC-1 cells may explain the high apoptotic
rate of LPS-treated STC-1 cells. Furthermore, caspase family
members play vital roles in apoptosis. Most notably, caspase-3 is
activated in apoptotic cells by the extrinsic (death ligand) and
intrinsic (mitochondrial) pathways (20,21).
As an executioner caspase, the caspase-3 zymogen has virtually no
activity until it is cleaved by initiator caspases after apoptotic
signaling events have occurred (22). Once caspase-3 is activated,
apoptosis proceeds and cannot be reversed. Our results showed that
the level of cleaved caspase-3 increased after STC-1 cells had been
exposed to LPS for 24 h, which indicated that LPS induced apoptosis
in STC-1 cells. This was consistent with the results of Deaciuc
et al(23), who showed that
liver ECs, derived from mice who had been intravenously
administered LPS, had enhanced caspase-3 activity.
Modulation of the composition of the intestinal
flora may become an effective treatment strategy for type 2
diabetes. New approaches for the therapeutic exploitation of the
microbiota, such as probiotics and prebiotics, may be capable of
changing the balance of gut inhabitants, which might then be used
to treat intestinal inflammatory disorders, metabolic syndromes and
systemic immune conditions (24).
Reducing the concentration of LPS by decreasing the number of
Bacteroidetes in the gut may inhibit the apoptosis of
enteroendocrine L cells and increase GLP-1 excretion in type 2
diabetes.
In conclusion, we have demonstrated that LPS induces
apoptosis in STC-1 cells by decreasing the expression of Bcl-2/Bax
protein and increasing caspase-3 activities. Enteroendocrine L cell
apoptosis may correlate with a decrease in GLP-1 secretion in type
2 diabetes.
References
|
1
|
Larsen N, Vogensen FK, van den Berg FW, et
al: Gut microbiota in human adults with type 2 diabetes differs
from non-diabetic adults. PLoS One. 5:e90852010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drucker DJ and Nauck MA: The incretin
system: glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toft-Nielsen MB, Damholt MB, Madsbad S, et
al: Determinants of the impaired secretion of glucagon-like
peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab.
86:3717–3723. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vilsboll T, Krarup T, Deacon CF, Madsbad S
and Holst JJ: Reduced postprandial concentrations of intact
biologically active glucagon-like peptide 1 in type 2 diabetic
patients. Diabetes. 50:609–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cani PD and Delzenne NM: Gut microflora as
a target for energy and metabolic homeostasis. Curr Opin Clin Nutr
Metab Care. 10:729–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Creely SJ, McTernan PG, Kusminski CM, et
al: Lipopolysaccharide activates an innate immune system response
in human adipose tissue in obesity and type 2 diabetes. Am J
Physiol Endocrinol Metab. 292:E740–E747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lü F, Jin T and Drucker DJ: Proglucagon
gene expression is induced by gastrin-releasing peptide in a mouse
enteroendocrine cell line. Endocrinology. 137:3710–3716.
1996.PubMed/NCBI
|
|
8
|
Jin T and Drucker DJ: The proglucagon gene
upstream enhancer contains positive and negative domains important
for tissue-specific proglucagon gene transcription. Mol Endocrinol.
9:1306–1320. 1995.PubMed/NCBI
|
|
9
|
Abello J, Ye F, Bosshard A, Bernard C,
Cuber JC and Chayvialle JA: Stimulation of glucagon-like peptide-1
secretion by muscarinic agonist in a murine intestinal endocrine
cell line. Endocrinology. 134:2011–2017. 1994.PubMed/NCBI
|
|
10
|
Stoscheck CM: Quantitation of protein.
Methods Enzymol. 182:50–68. 1990. View Article : Google Scholar
|
|
11
|
Alikhani M, Alikhani Z, He H, Liu R, Popek
BI and Graves DT: Lipopolysaccharides indirectly stimulate
apoptosis and global induction of apoptotic genes in fibroblasts. J
Biol Chem. 278:52901–52908. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xaus J, Comalada M, Valledor AF, et al:
LPS induces apoptosis in macrophages mostly through the autocrine
production of TNF-alpha. Blood. 95:3823–3831. 2000.PubMed/NCBI
|
|
13
|
Hull C, McLean G, Wong F, Duriez PJ and
Karsan A: Lipopolysaccharide signals an endothelial apoptosis
pathway through TNF receptor-associated factor 6-mediated
activation of c-Jun NH2-terminal kinase. J Immunol. 169:2611–2618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamada E, Nishida T, Uchiyama Y, et al:
Activation of Kupffer cells and caspase-3 involved in rat
hepatocyte apoptosis induced by endotoxin. J Hepatol. 30:807–818.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peter ME: Programmed cell death: Apoptosis
meets necrosis. Nature. 471:310–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bannerman DD and Goldblum SE: Mechanisms
of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J
Physiol Lung Cell Mol Physiol. 284:L899–L914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang YY, Peng CH, Yang YP, et al:
Desipramine activated Bcl-2 expression and inhibited
lipopolysaccharide-induced apoptosis in hippocampus-derived adult
neural stem cells. J Pharmacol Sci. 104:61–72. 2007. View Article : Google Scholar
|
|
18
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamm I, Schriever F and Dörken B:
Apoptosis: implications of basic research for clinical oncology.
Lancet Oncol. 2:33–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salvesen GS: Caspases: opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ghavami S, Hashemi M, Ande SR, et al:
Apoptosis and cancer: mutations within caspase genes. J Med Genet.
46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walters J, Pop C, Scott FL, et al: A
constitutively active and uninhibitable caspase-3 zymogen
efficiently induces apoptosis. Biochem J. 424:335–345. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deaciuc IV, Fortunato F, D’Souza NB, Hill
DB, Schmidt J, Lee EY and McClain CJ: Modulation of caspase-3
activity and Fas ligand mRNA expression in rat liver cells in vivo
by alcohol and lipopolysaccharide. Alcohol Clin Exp Res.
23:349–356. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hord NG: Eukaryotic-microbiota crosstalk:
potential mechanisms for health benefits of prebiotics and
probiotics. Annu Rev Nutr. 28:215–231. 2008. View Article : Google Scholar : PubMed/NCBI
|