Introduction
Brucella species, the cause of brucellosis in
humans and animals, are facultative intracellular bacteria.
Brucella invades phagocytic and non-phagocytic cells and
survives inside the host cells (1,2). The
properties of the intracellular lifestyle of Brucella limit
the number of antibiotics that are effective against these
organisms once they form Brucella-containing vacuoles (BCVs)
(3). Under most conditions,
control of brucellosis in animal reservoirs is achieved via
vaccination. Human brucellosis has also been controlled by
immunization and culling within cattle, goat and sheep herds
(4,5).
Currently there are no vaccines for humans and the
useful vaccines for livestock are (Brucella abortus) B.
abortus S19 and RB51 for cattle and Brucella melitensis
(B. melitensis) Rev1 for small ruminants (6,7).
B. abortus S19 has been widely used to prevent cattle
brucellosis, as it usually has low virulence. However, it is
infectious in humans and always causes abortion when used in
pregnant animals (8,9). Since B. abortus S19 induces
antibodies to the O-polysaccharide, it is difficult to distinguish
from wild-type infection. The relevant diagnostic antigen is the
smooth lipopolysaccharide (LPS) present in field strains, as well
as in B. abortus S19 and B. melitensis Rev 1
(10–12). The development of a safe and
efficacious vaccine that conquers this serological obstacle may
have a broad impact on public health.
LPS provides bacterial resistance to antimicrobial
attacks and modulates the host immune response, which makes it an
important virulence factor for survival and replication in the host
cell (1,13). It provides Brucella with
resistance to innate immunity antibacterial responses by inhibiting
complement and antibacterial peptide attacks, and by preventing the
synthesis of immune mediators (14–16).
The O-chain appears to help Brucella to invade cells in the
early entry stage (17).
Brucellae without O-side chains are termed as
rough or ‘R’ strain. R Brucella species or mutants lack the
antigenic O-side chain and they do not induce anti-O-side chain
antibodies. Currently, vaccinated hosts are difficult to
distinguish from wild-type-infected hosts by common serological
tests. It has been shown that Brucella R mutants are
attenuated; therefore, R mutants have potential for use as vaccines
(18–22). Several genes of B.
melitensis 16M LPS synthesis were analyzed and ABC-type
transporter (integral membrane protein, Wzm) and ABC-type
transporter (ATPase domain, Wzt) were determined to be putative
components of the ABC transporter system. The wzm/wzt
mutant was proven to lead to the absence of the O-side-chain on the
bacterial surface (23,24).
The wzt mutant of B. melitensis 16M
was evaluated and determined to have virulence-reducing potential
in a mouse model (25). In recent
years, LPS has been shown to interfere with major
histocompatibility complex (MHC)-II presentation, which inhibits
peptide presentation in cells (13,16).
In the present study, we constructed wzm and wzt gene
partial deletion mutants with no DNA marker addition, in order to
estimate the survival in vivo and the serological response.
This may provide us with an improved understanding of the effect of
wzm and wzt genes on the induction of immunity caused
by the S19 vaccine strain.
Materials and methods
Bacterial strains and growth
conditions
Escherichia coli DH5α strain was grown on
Luria-Bertani broth (LB) agar at 37°C. Brucella strains B.
abortus S19, Δwzm and Δwzt were grown on tryptic soy broth
(TSB, Sigma, St. Louis, MO, USA) agar at 37°C. Ampicillin (100
μg/ml) and kanamycin (50 μg/ml) were added for plasmid screening if
required. TSB medium (7% sucrose) was prepared for screening of
allelic-exchange mutants.
Construction of wzt and wzm mutants
The allelic exchange plasmids were constructed by
pBKCMV (kanr) with a sacB gene and fragments
upstream and downstream of target genes. The sacB gene along
with its promoter was amplified from pIBP279 (provided by Nanjing
Agricultural University) using PCR methods and ligated into
pBKsacB to construct pBKsacBwzm and pBKsacBwzt
(Table I). Competent cells of
B. abortus S19 were prepared and the constructed plasmid was
electroporated into the cells at 1,500 kV [1-mm gap cuvette; BTX,
Harvard Apparatus, Inc, Holliston, MA, USA]. Subsequently, 1 ml SOC
(2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM
CaCl2, 10 mM MgSO4 and 20 mM glucose) was
added and cells were grown under agitation at 28°C for 24 h and
then plated on TSB agar (kanr) and cultured for 96 h at
28°C. The mutants were confirmed using PCR. The phenotype of the
mutants was then determined by agglutination with acriflavine at a
dilution of 1:100 (26).
 | Table IBacteria and plasmids. |
Table I
Bacteria and plasmids.
| Strain or
plasmid | Phenotype and/or
genotype | Source |
|---|
| Strains |
| B. abortus
S19 | Vaccine strain,
smooth | IVDC |
| B. abortus
Δwzm | B. abortus
S19 Δwzm | This study |
| B. abortus
Δwzt | B. abortus
S19 Δwzt | This study |
| Plasmid |
| pBKCMV | Kanamycin | Stratagene |
| pIBP279 | With sacB
gene | NJAU |
Animals
The 4–6-week-old female specific pathogen-free (SPF)
BALB/c mice were provided by the animal centre of Jilin University
(Changchun, China). Mice were bred in the animal facilities with
filtered air in a restricted-access room and under pathogen-limited
conditions. Mice were acclimatised for a minimum of one week prior
to the experiment and water and food was provided ad
libitum. The animal experiments were approved by the Center of
Laboratory Animals, Jilin University, China.
Serological test and antibody
dynamics
Female BALB/c mice of 6–8 weeks of age were housed
with water and food. Animals were randomly allocated to groups and
acclimatised for 1 week prior to the initiation of experiments
(n=5). To prepare the inoculated samples, bacteria were suspended
in PBS and adjusted to the appropriate 108 CFU/ml in the
same buffer. Blood samples from BALB/c mice were collected and
allowed to clot for 12 h at 4°C and centrifuged. Serum was divided
into Eppendorf tubes (Eppendorf, Hamburg, Germany), and stored at
−80°C. The Rose Bengal plate agglutination test (RBPT, Harbin
Pharmaceutical Group Bio-vaccine Co., Harbin, China) was carried
out by mixing 30 μl serum and 30 μl antigen, and the reaction was
observed after 4 min.
The IgG antibody titer was estimated by indirect
enzyme-linked immunosorbent assay (ELISA). The 96-well plates were
coated by diluted Brucella antigens S19, Δwzm and
Δwzt (pH 9.6, 0.05 M carbonate buffer 100 μl each well,
antigen concentration 108 pfu), at 4°C overnight. The
plate was washed with 200 μl PBST buffer (pH 7.4, 0.01 M PBS:
Tween-20, 1:1,000) three times, for 3 min each time, and then
blocked by 100 μl 1% BSA (pH 7.4) and incubated at 37°C for 1 h
followed by three washes with PBST. The sera samples diluted from
1:100 to 1:3,200 were added and incubated at 37°C for 1 h. After
three washes with PBST, enzyme-labeled goat anti-mouse IgG
(1:5,000) was added and incubated at 37°C for 1 h and then washed
with PBST three times. TMB substrate solution (100 μl) was then
added and incubated at 37°C for 10 min (in light). Then, 50 μl 2 M
sulfate buffer was added to stop the reaction, followed by
detection of optical density (OD) at 490 nm. The antibody titer was
described as the diluted ratio controlled by the S/N ratio. If the
S/N ratio was ≥2.1, the sample of serum was considered positive.
The S/N ratio was calculated as: (sample -
blank)OD490/(negative - blank)OD490.
Lymphocyte proliferation
After injecting antigens for 4 weeks, the spleens of
mice were removed under sterile conditions, put in a sterile Petri
dish with 4 ml lymphocyte separation liquid (Dakewe Biotech Co.,
Shenzhen, China) and then ground with a disposable syringe core in
a 200-mesh nylon sieve (74 μm pore diameter). The spleen cell
suspension was then added to a sterile centrifuge tube and 500 μl
of RPMI-1640 medium was gradually added. The cells were then
centrifuged for 10 min at 300 × g. The lymphocyte layer was placed
into new centrifuge tubes, resuspended in 10 ml of RPMI-1640 and
centrifuged for 2 min at 1,500 rpm. Subsequently, 5 ml of 0.15 M
Tris-NH4Cl solution was added to the cells and the cells
were centrifuged at 200 × g for 5 min after 5 min incubation at
room temperature. The supernatant was removed and the cells were
washed twice with RPMI-1640. The lymphocytes were resuspended by
RPMI-1640 with 5% FBS and the cell concentration was adjusted to
2×106/ml.
The lymphocytes (1×104 per well in
triplicate) were incubated with corresponding antigens
(multiplicity of infection of 200 CFU/cell; PBS in RPMI-1640 medium
as negative control) in 96-well plates at 37°C in an atmosphere
containing 5% (v/v) CO2. After 24 h of culture, 20 μl
MTS solution was added to each well and the cells were incubated
for 4 h at 37°C in an atmosphere containing 5% (v/v)
CO2. The absorbance (optical density, OD) at 490 nm was
recorded. Lymphocyte proliferation ratio (IS) was calculated as:
(OD-OD1640)/(ODPBS-OD1640).
Cytokine induction in vitro and in
vivo
The lymphocyte cells (1×105 per well in
triplicate) were incubated with corresponding antigens
(multiplicity of infection of 200 CFU/cell; PBS in RPMI-1640 as
negative control) for 24 h in 24-well plates at 37°C in an
atmosphere containing 5% (v/v) CO2. The cell culture
supernatants were collected and stored at −80°C. The cytokine
levels were analyzed using R&D Quantikine TNF-α, INF-γ, IL-2,
IL-4 and IL-10 kits according to the manufacturer's instructions
(Minneapolis, MN, USA). The INF-γ levels of serum were estimated
using the same method.
Statistical analysis
The data were analyzed using Original 7.5 software
and presented as the means ± standard deviation (mean ± SD).
Significant differences between the groups were identified by
one-way ANOVA (significant difference, P<0.01 and
P<0.05).
Results
Screening of mutant strains
In order to obtain partial mutants of wzm and
wzt genes, the plasmids pBKsacBwzm and
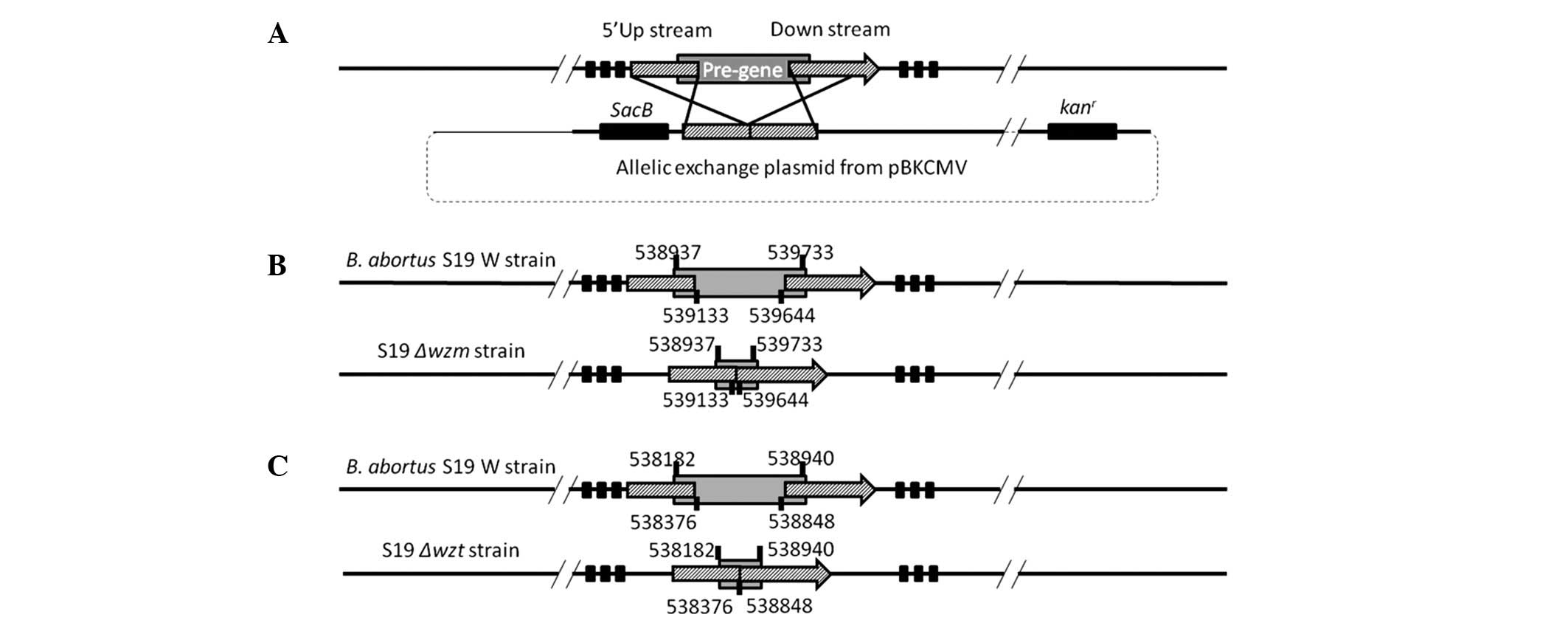
pBKsacBwzt were constructed as shown in Fig. 1A–C. The plasmid was then
electroporated into B. abortus S19 cells. The transformed
samples were plated on TSB agar medium (Kanr) for the
first screen. The colonies were added to TSB medium and detected by
PCR with sacB primers for the second screen. The positive
culture was spread on 7% sucrose TSA medium for the allelic
exchange screen (27). The
colonies from 7% sucrose TSB agar medium were inoculated into TSB
medium and screened by pre-gene primers (wzm or wzt
gene) for the fourth screen. If the cells were mutants, the target
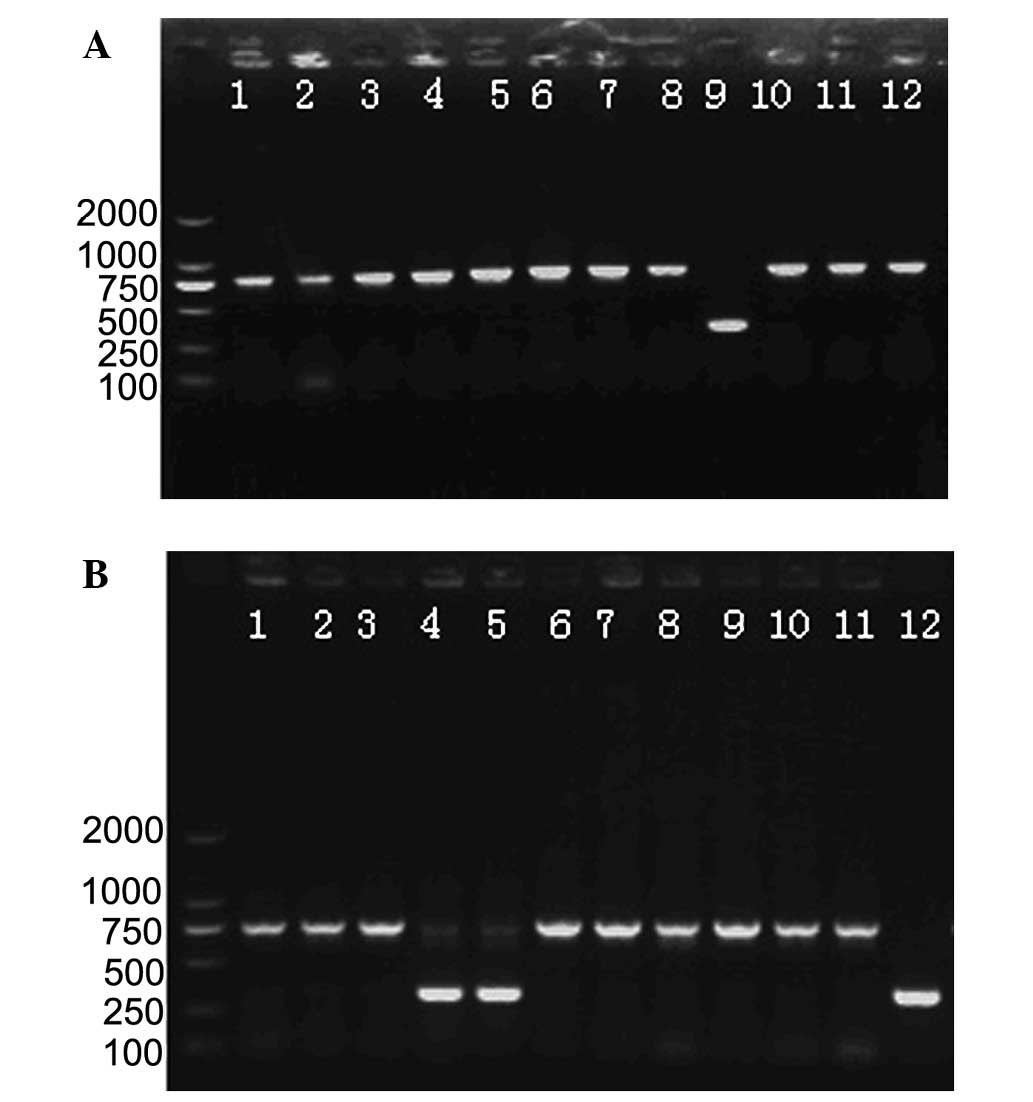
gene was found to be shorter than the pre-gene. The positive
mutants were those with only one band ~300 bp after the screening
process (Fig. 2). The
putative-positive mutants were inoculated into TSB medium
(Kanr) to remove the false-positive mutants.
Mutant strains were rough mutants
After 30 passage cultures for genetic stability, the
mutants were detected by PCR using target gene primers and upstream
and downstream fragment primers, and the sequences were analyzed.
The mutants were prepared for acriflavine agglutination. The
Δwzm and Δwzt mutants were positive and the S19
strain was negative for acriflavine agglutination.
The results of the Rose Bengal plate agglutination
test (RBT) showed positive and negative serum for the S19 group and
the Δwzm and Δwzt groups, respectively. Therefore,
Δwzm and Δwzt mutants did not elicit the antibody
response to O-antigen in the host. These results indicated that the
mutants were rough mutants.
Antibody dynamics
The IgG antibody changes of Δwzm and
Δwzt mutants and S19 are shown from the second to the ninth
week (Fig. 3). The antibody titer
of Δwzm induced in mice was higher than S19 strain before
the fourth week and after the sixth week. Particularly at the
eighth week, the log10 antibody titer of mutant strains (S/N value
of Δwzm and Δwzt mutants was 3.42±0.20 and 3.48±0.26,
respectively) was significantly higher than the S19 strain
(S/N=2.60±0.25, P<0.01). These results indicated that the rough
mutants may induce higher antibody titers than the S19 strain.
Lymphocyte proliferation
Lymphocyte proliferation is an important stage of
the immune response. The results showed (Fig. 4) that the IS of S19 (7.13±1.09) was
significantly higher than that of Δwzm (2.48±0.21) and
Δwzt (1.38±0.07) mutants (P<0.01), which indicated that
S19 induced higher lymphocyte proliferation. The IS of the
Δwzm mutant was approximate to that of the Δwzt
mutant (P>0.05). The disruption of wzm and wzt
genes caused significantly decreased lymphocyte proliferation
ability compared with the wild-type strain S19.
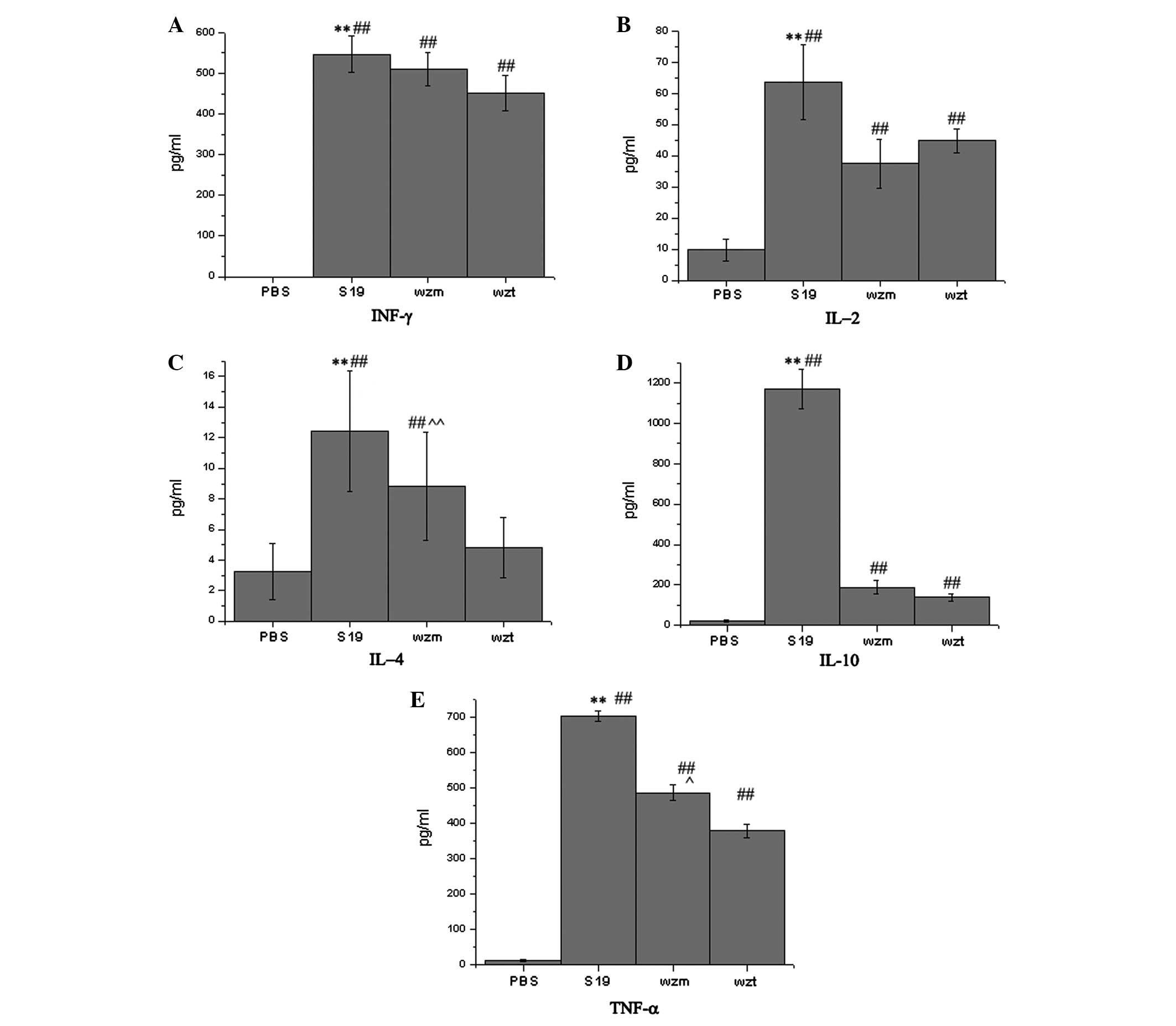
Cytokine secretion
The important immune-related cytokines INF-γ, IL-2,
IL-4, IL-10 and TNF-α were detected in vitro (lymphocytes
cultured in 24-well plates). The TNF-α, INF-γ, IL-2, IL-4 and IL-10
results showed that the cytokines induced by the Δwzm and
Δwzt rough mutants were decreased and significantly lower
than the S19 parent strain (P<0.01, Fig. 5A–E). IL-4 and TNF-α levels induced
by Δwzt mutants were lower than those induced by Δwzm
mutants (P<0.01) and INF-γ, IL-10 and IL-2 levels induced by
Δwzt mutants were approximate to those induced by
Δwzm mutants (P>0.05).
The INF-γ levels in serum were also detected from
weeks 2 to 9. The curve (Fig. 6)
showed that S19 and Δwzm rough mutant induced higher INF-γ
levels (S19, 127.6±1.1 pg/ml at the third week; Δwzm rough
mutant, 67.6±6.8 pg/ml at the fourth week), while the INF-γ levels
induced by Δwzt rough mutants were lower than those of S19
and Δwzm (17.8±14.7 pg/ml on fourth week). The peak time of
the INF-γ induction of Δwzm rough mutants (the fifth week
was the peak of the curve) was delayed compared with S19 (peak of
the curve was evident at the fourth week), and the Δwzt
rough mutants were even more delayed. The concentrations of INF-γ
induced by rough mutants were lower than those of S19, and the
concentrations induced by Δwzt rough mutants were the
lowest.
Discussion
In the present study, knockout of wzm and
wzt genes caused the rough mutant. The wzm and
wzt genes are the membrane-spanning homologs and the
ATP-binding homologs of ABC-transporters involved in transmembrane
export for O-polysaccharide chain biosynthesis (28). Previous studies on B.
melitensis 16M observed that the mutant of wzm or
wzt gene was a rough mutant (23–25).
The acriflavine agglutination results indicated that the
Δwzm and Δwzt mutants were rough mutants (21). Smooth strains did not induce
acriflavine agglutination, suggesting that vaccination with these
mutant strains may allow for differentiation between vaccinated and
wild-type smooth strain-infected animals.
The cytokine-inducing ability of rough mutants was
reduced. INF-γ is one of the most important cytokines in resistance
to Brucella invasion, which enhances the macrophage
bactericidal activity (29). The
INF-γ levels induced by the Δwzm and Δwzt rough
mutants was lower than those of the smooth strain S19 in
vivo although not significantly lower. By contrast, INF-γ
levels induced by the Δwzm and Δwzt mutants were
significantly lower as compared to S19. IL-2 was detected in low
quantities and IL-4 was detected at very low levels. IL-10 levels
induced by S19 were significantly higher than those induced by the
rough mutants, while IL-10 levels induced by the wzm mutant
were higher than those of the wzt mutant.
Secretion of the inflammatory molecule TNF-α was
reduced by rough mutants although not significantly as compared to
S19. LPS is considered to be the most important modulator of TNF-α
that is required for host defense against intracellular pathogens
(30,31). The results showed that the
disruption of LPS caused a reduction in TNF-α levels and spleen
weights, and the spleen kinetics caused by rough mutants were
significantly lower than those of the S19 strain. The TNF-α levels
induced by the Δwzt mutant were lower than those induced by
the Δwzm mutant.
These data indicate that the wzt gene may
affect the host immune response indirectly. As previously reported,
the wzm mutant provides efficient protection against
Brucella invasion compared with mutants (25). Wzt disruption may affect the
expression of more genes associated with the induction of
immunity.
Acknowledgements
This study was funded by a project supported by the
National Science and Technology Ministry (2010BAD04B03) and the Key
Project of Chinese National Programs for Fundamental Research and
Development (No. 2012CB722501).
References
|
1
|
Cardoso PG, Macedo GC, Azevedo V and
Oliveira SC: Brucella spp noncanonical LPS: structure,
biosynthesis and interaction with host immune system. Microb Cell
Fact. 5:132006. View Article : Google Scholar
|
|
2
|
Rambow-Larsen AA, Petersen EM, Gourley CR
and Splitter GA: Brucella regulators: self-control in hostil
environment. Trends Microbiol. 17:371–377. 2009. View Article : Google Scholar
|
|
3
|
Al-Tawfiq JA: Therapeutic options for
human brucellosis. Expert Rev Anti Infect Ther. 6:109–120. 2008.
View Article : Google Scholar
|
|
4
|
Pappas G, Papadimitrious P, Akritidis N,
Christou L and Tsianos E: The new global map of human brucellosis.
Lancet Infect Dis. 6:91–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa
AM and Rice-Ficht AC: Brucellosis: the case for live, attenuated
vaccines. Vaccine. 27:D40–D43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spink WW, Hall JW, Finstad J and Mallet E:
Immunization with viable Brucella organisms. Results of a
safety test in humans. B World Health Organ. 26:409–419. 1962.
|
|
7
|
Perkins SD, Smither SJ and Atkins HS:
Towards a Brucella vaccine for humans. FEMS Microbiol Rev.
34:379–394. 2010.
|
|
8
|
Monreal D, Grilló MJ, González D, Marín
CM, De Miguel MJ, López-Goni I, Blasco JM, Cloeckaert A and Moriyón
I: Characterization of Brucella abortus O-polysaccharide and
core lipopolysaccharide mutant and demonstration that a complete
core is required for rough vaccines to be efficient against
Brucella abortus and Brucella ovis in the mouse
model. Infect Immun. 71:3261–3271. 2003.
|
|
9
|
Fugier E, Pappas G and Gorvel JP:
Virulence factors in brucellosis: implications for
aetiopathogenesis and treatment. Expert Rev Mol Med. 9:1–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bundle DR, Cherwonogrodzky JW, Gidney MA,
Meikle PJ, Perry MB and Peters T: Definition of Brucella A and M
epitopes by monoclonal typing reagents and synthetic
oligosaccharides. Infect Immun. 57:2829–2836. 1989.PubMed/NCBI
|
|
11
|
Weynants V, Gilson D, Cloeckaert A, Tibor
A, Denoel PA, Godfroid F, Limet JN and Letesson JJ:
Characterization of smooth lipopolysaccharides and
O-polysaccharides of Brucella species by competition binding
assays with monoclonal antibodies. Infect Immun. 65:1939–1943.
1997.PubMed/NCBI
|
|
12
|
Ugalde JE, Comerci DJ, Leguizamón MS and
Ugalde RA: Evaluation of Brucella abortus phosphoglucomutase
(pgm) mutant as a new live rough-phenotype vaccine. Infect Immun.
71:6264–6269. 2003.PubMed/NCBI
|
|
13
|
Lapaque N, Moriyon I, Moreno E and Gorvel
JP: Brucella lipopolysaccharide acts as a virulence factor. Curr
Opin Microbiol. 8:60–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenschenk FC, Houle JJ and Hoffmann EM:
Serum sensitivity of field isolates and laboratory strains of
Brucella abortus. Am J Vet Res. 56:1592–1598.
1995.PubMed/NCBI
|
|
15
|
Velasco J, Bengoechae JA, Brandenberg K,
Lindner B, Seydel U, Gonzalez D, Zahringer U, Moreno E and Moriyon
I: Brucella abortus and its closest phylogenetic relative,
ochrobacterum spp, differ in outer membrane permeability and
cationic peptide resistance. Infect Immun. 68:3210–3218. 2000.
View Article : Google Scholar
|
|
16
|
Lapaque N, Forquet F, de Chastellier C,
Mishal Z, Jolly G, Moreno E, Moriyon I, Heuser JE, He HT and Gorvel
JP: Characterization of Brucella abortus lipopolysaccharide
macrodomains as mega rafts. Cell Microbiol. 8:197–206. 2006.
|
|
17
|
Porte F, Naroeni A, Ouahrani-Bettache S
and Liautard JP: Role of the Brucella suis
lipopolysaccharide O antigen in phagosomal genesis and in
inhibition of phagosome-lysosome fusion in murine macrophages.
Infect Immun. 74:1481–1490. 2003.
|
|
18
|
Fernandez-Prada CM, Zelazowska EB,
Nikolich M, Hadfield TL, Roop RM, Robertson GL and Hoover DL:
Interactions between Brucella melitensis and human
phagocytes: bacterial surface O-polysaccharide inhibits
phagocytosis, bacterial killing and subsequent host cell apoptosis.
Infect Immun. 71:2110–2119. 2003.
|
|
19
|
Jiménez de Bagüés MP, Terraza A, Gross A
and Dornand J: Different responses of macrophages to smooth and
rough Brucella spp: relationship to virulence. Infect Immun.
72:2429–2433. 2004.PubMed/NCBI
|
|
20
|
Moriyón I, Grilló MJ, Monreal D, González
D, Marín C, López-Goñi I, Mainar-Jaime RC, Moreno E and Blasco JM:
Rough vaccines in animals brucellosis: structural and genetic basis
and present status. Vet Res. 35:1–38. 2004.PubMed/NCBI
|
|
21
|
Adone R, Ciuchini F, Marianelli C,
Tarantino M, Pistoia C, Marcon G, Petrucci P, Francia M, Riccardi G
and Pasquali P: Protective properties of rifampin-resistant rough
mutants of Brucella melitensis. Infect Immun. 73:4198–4204.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haag AF, Myka KK, Arnold MF,
Caro–Hernández P and Ferguson GP: Importance of lipopolysaccharide
and cyclic β-1,2-glucans in Brucella-mammalian infections.
Int J Microbiol. 2010:1245092010.
|
|
23
|
Cloeckaert A, Grayon M, Verger JM,
Letesson JJ and Godfroid F: Conservation of seven genes involved in
the biosynthesis of the lipopolysaccharide O-side chain in
Brucella spp. Res Microbiol. 151:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godfroid F, Cloeckaert A, Taminiau B,
Danese I, Tibor A, de Bolle X, Mertens P and Letesson JJ: Genetic
organization of the lipopolysaccharide O-antigen biosynthesis
region of Brucella melitensis 16M (wbk). Res Microbiol.
151:655–668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
González D, Grilló MJ, De Miguel MJ, Ali
T, Arce-Gorvel V, Delrue RM, Conde-Alvarez R, Muñoz P, López-Goñi
I, Iriarte M, Marín CM, Weintraub A, Widmalm G, Zygmunt M, Letesson
JJ, Gorvel JP, Blasco JM and Moriyón I: Brucellosis vaccines:
assessment of Brucella melitensis lipopolysaccharide rough
mutants defective in core and O-polysaccharide synthesis and
export. PLoS One. 3:e27602008.
|
|
26
|
Allen CA, Adams LG and Ficht TA:
Transposon-derived Brucella abortus rough mutants are
attenuated and exhibit reduced intracellular survival. Infect
Immun. 66:1008–1016. 1998.PubMed/NCBI
|
|
27
|
Ried JL and Collmer A: An nptI-sacB-sacR
cartridge for constructing directed, unmarked mutations in
gram-negative bacteria by marker exchange-eviction mutagenesis.
Gene. 57:239–246. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christian RHR and Chris W:
Lipopolysaccharide endotoxins. Annu Rev Biochem. 71:635–700. 2002.
View Article : Google Scholar
|
|
29
|
Grilló MJ, Blasco JM, Gorvel JP, Moriyón I
and Moreno E: What have we learned from brucellosis in the mouse
model? Vet Res. 43:292012.PubMed/NCBI
|
|
30
|
Skyberg JA, Thornburg T, Rollins M, Huarte
E, Jutila MA and Pascual DW: Murine and bovine γδT cells enhance
innate immunity against Brucella abortus infections. PLoS
One. 6:e219782012.
|
|
31
|
Li X and He Y: Caspase-2-dependent
dendritic cell death, maturation and priming of t cells in response
to Brucella abortus infection. PLoS One. 7:e435122012.
View Article : Google Scholar : PubMed/NCBI
|