Introduction
Following injury, the central nervous system (CNS)
exhibits limited endogenous repair and poor functional recovery. It
is known that Schwann cells (SCs) are pivotal in the repair of the
peripheral nervous system following injury (1,2).
These cells engulf degenerated axons and myelin and form the bands
of Büngner and cell bridges to direct regenerating axons across the
lesion. They also synthesize and secrete various neurotrophic
factors and the extracellular matrix (1,2).
However, no similar cells are located in the CNS. Findings of
previous studies have demonstrated that the transplantation of
exogenous SCs provides trophic support for spared axons and
participates in remyelination of the injured spinal cord (3,4).
However, invasive surgical biopsies are required to harvest nerves
and the difficulties faced in the purification and expansion of SCs
from adult nerves, have complicated their clinical application
(5). The ideal transplantable cell
should be easily accessible, proliferate rapidly in culture and
successfully integrate into host tissue with immunological
tolerance (6). Adipose-derived
stem cells (ADSCs) are a self-renewing and multipotent precursors
with a high growth rate and represent a highly accessible and
potentially autologous source of adult precursors that are capable
of generating functional SCs (7).
Therefore, whether ADSC-SCs could be used to promote functional
recovery in the brain contusion in rat was investigated. The
results demonstrated that in contrast to transplanted ADSCs or
neural stem cells (NSCs), transplanted ADSCs-SCs significantly
promoted predominant functional locomotor recovery.
Materials and methods
Animals
Sixty-eight adult Sprague-Dawley (SD) rats (weight,
230–260 g) were used in the current study. All the procedures were
approved by the Experimental Animal Center of Xiangya School of
Medicine, Central South University (Changsha, China).
Equipment
All optical equipment was purchased from Leica,
(Buffalo, IL, USA) and all surgical equipment was purchased from
Zhejiang Shangyu Biotechnology (Shangyu, China).
Preparation of ADSCs and NSCs
ADSCs were cultured as described previously
(7,8). Briefly, adipose cells from the groin
of SD rats were digested with 0.15% collagenase type I for 60 min
at 37°C. The tissue was mechanically dissociated, filtered through
a 70 μm filter and centrifuged at 1,200 × g for 10 min to obtain a
pellet. The resulting cell population was maintained at 37°C with
5% CO2 in control medium [low glucose α-minimum
essential medium (MEM) and 10% fetal bovine serum (FBS)], until the
cells had reached 80–90% confluency. To passage, ADSCs were
digested in 0.25% trypsin for 5 min, mechanically detached to
provide single cells and subcultured at a density of 10–25,000
cells/ml. All ADSCs underwent 3–5 passages prior to
transplantation.
NSCs were isolated as previously described (9). Rat brains were obtained on day 2 or 3
following birth. The tissue was incubated in 0.25% trypsin, gently
dissociated to single cells by trituration and grown at a
concentration of 10,000 cells/ml. Dissociated cells were grown as
neurospheres in the neurobasal medium supplemented with 20 ng/ml
basic fibroblast growth factor (FGF-2), 20 ng/ml epidermal growth
factor and 1:50 dilution of B27. The cells were passaged every five
days using trypsin digestion and mechanical dissociation. The
neurospheres underwent 3–5 passages prior to transplantation.
Differentiation of ADSCs to SCs
ADSCs were differentiated as previously described
(7,10,11).
Briefly, ADSCs (at passages 3–5) were dissociated and plated on 10
cm dishes and cultured in mesenchymal stem cell (MSC) growth medium
containing α-MEM, 1 mM β-mercaptoethanol and 10% FBS for 24 h. The
cells were washed and the medium was replaced with MSC growth
medium containing α-MEM, 35 ng/ml all-trans-retinoic acid and 10%
FBS, and then incubated for 72 h. The cells were washed and the
medium was replaced with MSC growth medium containing α-MEM, 5
ng/ml platelet-derived growth factor-AA, 10 ng/ml FGF, 5.7 μg/ml
forskolin, 252 ng/ml glial growth factor-2 and 10% FBS. Cultures
were incubated for 14 days and the medium was replaced every 3
days. Proliferating colonies of ADSC-SCs were isolated using
cloning cylinders, trypsinized from the dish and replated in the
same medium. Cultures of increasing purity were obtained by
sequential passaging when the cultures had reached confluency.
Tissue processing and
immunofluorescence
Immunocytochemical staining was performed on plastic
chamber slides. Cells were washed in Hank’s balanced salt solution
for 5 min, fixed with phosphate-buffered 4% paraformaldehyde for 20
min and washed again. Non-specific binding sites were subsequently
blocked with 10% non-specific serum corresponding to the species in
which secondary antibodies were generated. For characterization of
the cells, the cultures were incubated overnight at 4°C with
primary antibodies, washed and then incubated with secondary
antibodies for 2 h at room temperature. The primary antibodies used
were: Rabbit anti-glial fibrillary acidic protein (GFAP), mouse
anti-neuron specific enolase (NSE), mouse anti-S100b, rabbit
anti-p75 and mouse anti-nestin. Secondary antibodies used were:
Goat polyclonal secondary antibody to rabbit immunoglobulin G
(IgG)-Cy3 and goat polyclonal secondary antibody to mouse IgG-Cy3.
Controls in which primary antibodies were omitted or replaced with
irrelevant IgG resulted in no detectable staining for fluorescent
protocols.
Brain contusion injury and cell
transplantation
Surgery was performed under aseptic conditions. Rats
were anesthetized with phenobarbital sodium (120 mg/kg) via
intraperitoneal injection, and simultaneously atropine (50 μg/kg)
was administered intramuscularly to decrease tracheal secretion.
The rat heads were mounted in stereotactic apparatus. The skin of
the head was disinfected and shaved along the midline and the
anterior fontanel, sagittal suture, and coronal suture were
exposed. A small piece of periosteum was removed and maintained in
sodium chloride. Craniectomy was performed at the left frontal
bone, 2 mm behind the coronal suture to 4 mm in front of it, and 4
mm next to the midline. The dura was cut and the cortex under this
bone window was contused diffusely using a fine micro device. The
depth of contusion was set at 3 mm. A 2×2×2 mm3 area of
the motor cortex was drawn off as a reserved cavity in the center
of the bone window. The wound was stanched thoroughly and covered
by the periosteum. The operation was conducted under a microscope
(DM300, Lecia). Six rats underwent sham operation, receiving only
scalp cutting and osteosomy procedures, with the brain structure
remaining intact.
For transplantation, 7 days following the contusion
injury, rats were randomly divided into 4 groups: no treatment
(n=8) or transplanted with NSCs (n=16), undifferentiated ADSCs
(n=18) or ADSCs-SCs (n=20). Immediately prior to transplantation,
the cells were resuspended at a final concentration of
2×105 cells/μl. A total volume of 10 μl was
stereotaxically injected into the reserved cavity using a 10-μl
Hamilton syringe fitted with a glass micropipette.
Behavioral assessment
Behavioral assessments of locomotor function were
conducted to compare the functional outcomes of various treatments
over time. Locomotor function was assessed using the Longa and
Faden scores, respectively (12,13).
Behavioral tests were conducted 1–2 days prior to injury to
establish the baseline values and again 6 days subsequent to the
injury. The resulting Longa and Faden scores were used to eliminate
animals that demonstrated a score of 2–3 and 9–11, respectively, at
that time point to standardize the injury severity. Behavioral
assessments were performed on day 6, 10, 14, 21 and 30 following
injury.
For motor functional tests, the rats were placed in
the center of an empty, bright square arena surrounded by walls to
prevent the animals from escaping and were observed by two
investigators (blinded to treatment) for 4 min to assess their
performance using the Longa score. Briefly, the Longa score is used
to assess the animals on the following 0–4 scale: 0, no
neurological deficits; 1, the left forepaw is not able to be
extended completely; 2, the rat circles towards the side of
hemiparalysis; 3, the rat is skewed towards the side of
hemiparalysis; 4, the rat is not able to walk spontaneously and
exhibits a disturbance of consciousness.
Motor function was analyzed again using three
separate tests and two different investigators (blinded to
treatment) who assessed the rats’ performance using the Faden
score. Each test was scored via an ordinal scale of 0–5. Tests
measured the ability of maintaining position on an inclined plane
in the vertical and two horizontal positions for 5 sec, forelimb
flexion and forced lateral pulsion. Forelimb flexion measured the
reflex extension of the forelimb to break a fall when suspended by
the tail, while lateral pulsion measured the degree of resistance
to a lateral push. Each of the seven individual scores (vertical
angle, right and left horizontal angle, right and left forelimb
flexion and right and left lateral pulsion) were added to yield a
composite neurological score of 0–35.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows version 10.0 (SPSS Inc., Chicago, IL, USA). The data are
presented as the mean ± SEM. One-way analysis of variance plus post
hoc pairwise comparison was used for multi-group means comparison.
For data that violated the assumptions of normality and/or
homogeneity of variance, Dunnett’s C test was used. The
significance level for all tests was set at two-sided
P<0.05.
Results
Isolation, culture and characterization
of ADSCs and NSCs
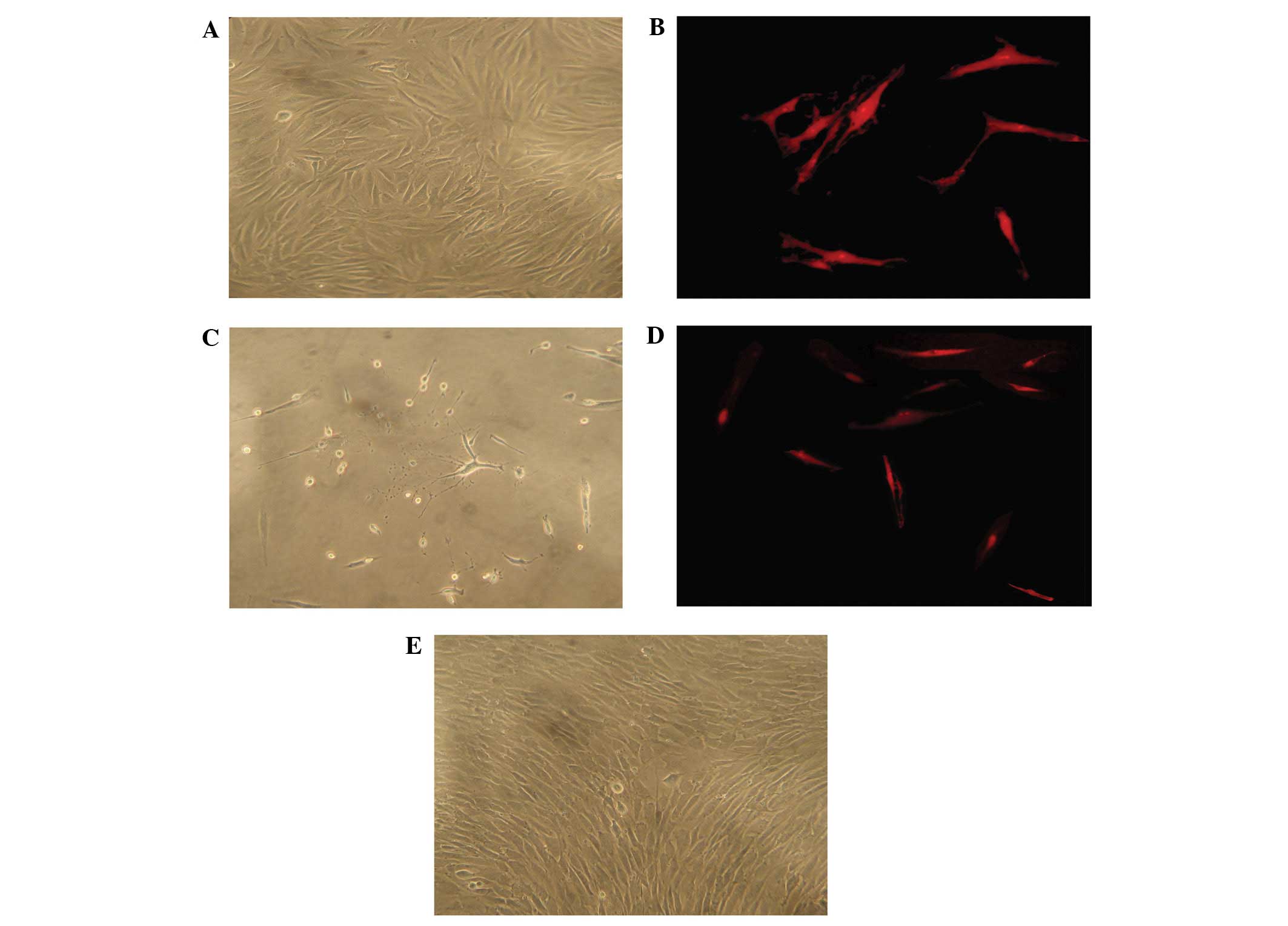
Twenty-four hours following the initial plating of
the primary culture, a few ADSCs were adherent to the plate. After
72 h, the cell number increased significantly and formed
fibroblast-like colonies. One week subsequent to that, ADSCs
appeared as a monolayer of fibroblast-like cells and grew in a
certain direction (Fig. 1A).
Within five passages, ADSCs markedly expanded in a swirl-like
formation (Fig. 1B). To determine
the expression of neural markers in ADSCs following the induction
of neural differentiation, GFAP (gliocyte marker) and NSE (neuron
marker) (14–16) were examined using
immunocytochemical analysis. Prior to neural induction, ADSCs did
not stain for NSE and showed faint staining for GFAP. Following
neural induction, ADSCs showed obvious neuron-like morphology
(Fig. 1C) and began to stain for
NSE (Fig. 1D), without an increase
in the staining of GFAP (Fig.
1E).
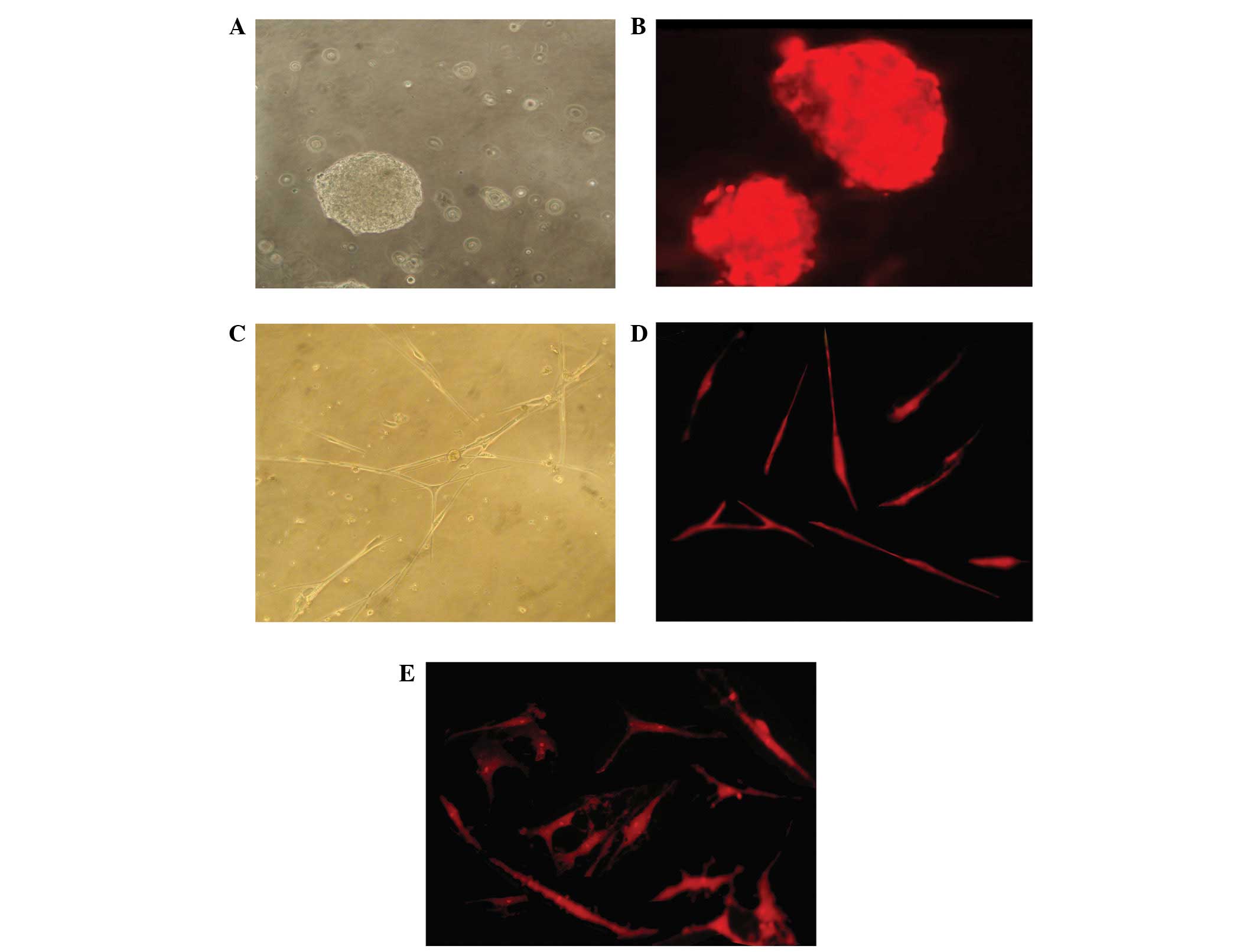
To characterize the cultured NSCs used in the
present study, typical neurospheres (Fig. 2A) were analyzed for nestin
expression. Results of immunofluorescence analysis revealed that
the neurospheres expressed nestin (Fig. 2B). To determine the neural
differentiation potential, immunostaining of NSE and GFAP was
performed in the cells. Prior to neural induction, a relatively low
proportion of NSCs expressed GFAP and no cells stained for NSE.
However, a week following differentiation, NSCs exhibited an
obvious neuron-like morphology (Fig.
2C) and numerous NSE-positive cells (Fig. 2D) and GFAP-positive cells (Fig. 2E) were observed.
Differentiation of ADSCs to SCs
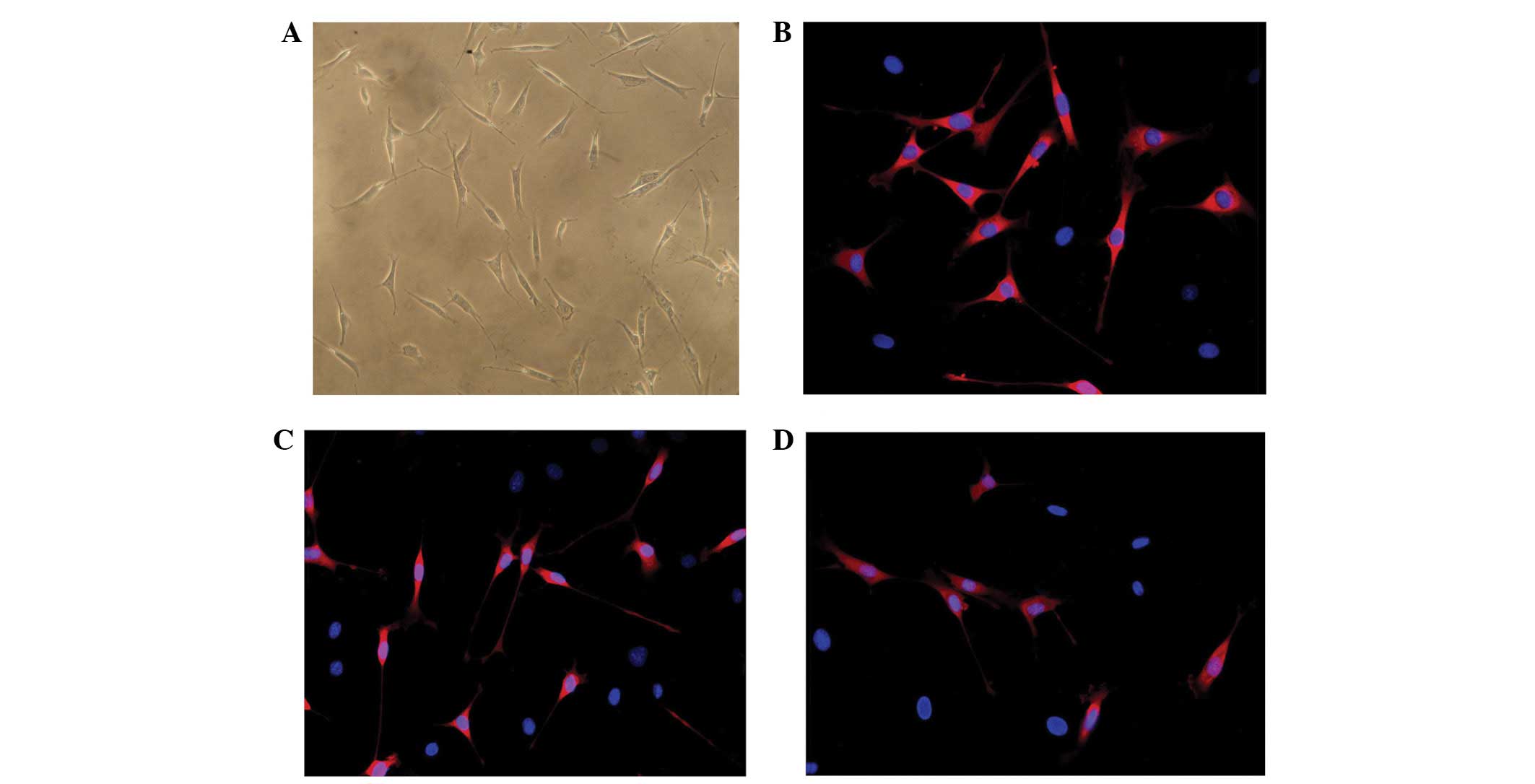
Following induction as described in a previous
section, the size and shape of cultured ADSCs were similar to those
of genuine SCs, which exhibit an elongated spindle shape (Fig. 3A). To characterize the
differentiated ADSCs, immunostaining for SCs markers, such as GFAP,
S-100 and p75 was performed (17,18).
Results demonstrated that ADSCs faintly stained for GFAP and did
not stain for S-100 and p75. By contrast, differentiated ADSCs
showed an increased expression of GFAP (Fig. 3B) and began to stain for S-100
(Fig. 3C) and p75 (Fig. 3D).
ADSC-SCs promote functional improvement
following brain contusion
The target of cell transplantation following CNS
injury was to promote functional improvement. Behavioral
assessments were used to verify the functional locomotor recovery
of the injured rats. ADSCs and NSCs have been demonstrated to
exhibit the ability to promote functional improvement following CNS
injury (16,19,20).
Therefore, the present study aimed to identify whether
transplantation of ADSC-SCs promoted functional recovery following
brain contusion. To investigate, locomotor function was analyzed
prior to the injury and on day 6, 10, 14, 21 and 30 following
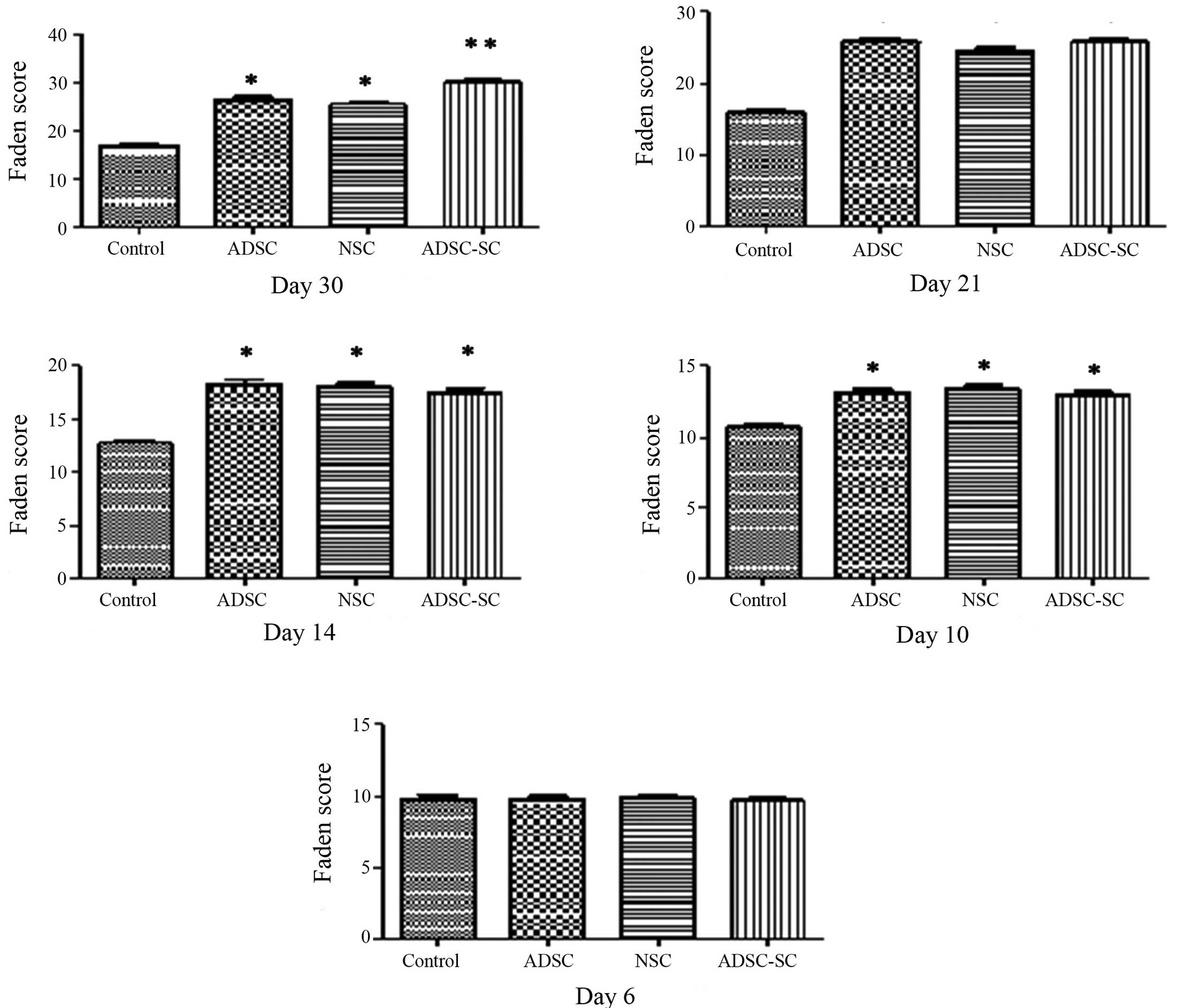
injury. Longa and Faden scores, respectively, were used to assess
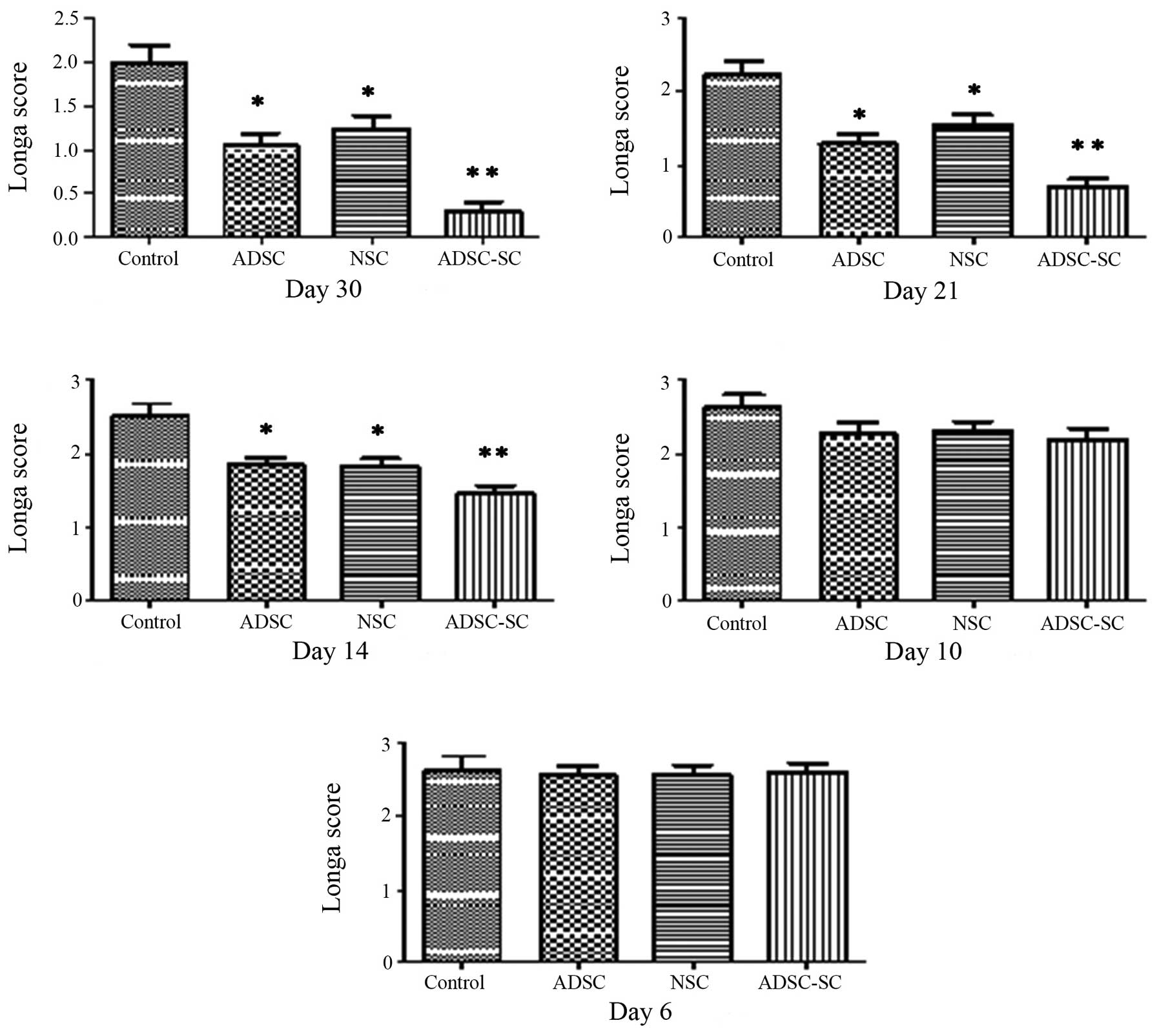
the locomotor function. No deficit of locomotor function was
detected among all experimental rats prior to surgery. Following
brain contusion, the transplanted animals demonstrated gross motor
impairment but no rats exhibited complete paralysis and no
significant difference was identified in the locomotor functional
score among the groups on day 6 (Figs.
4 and 5). On day 10 following
contusion (three days after transplantation), no obvious difference
was identified among the groups (Figs.
4 and 5). However, significant
recovery of motor behavior was observed in the animals transplanted
with ADSCs or NSCs, particularly with ADSC-SCs, compared with the
control animals on day 14, 21 and 30 (Figs. 4 and 5). Moreover, a significant and sustained
recovery was identified in rats transplanted with ADSC-SCs,
compared with those transplanted with ADSCs or NSCs, revealing a
slow pace of recovery until day 21 and no further recovery
following this time point. It was also observed that
transplantation of ADSCs and NSCs showed similar ability in
promoting functional recovery following brain contusion.
Discussion
SCs are differentiated from neural crest cells. All
axons of the peripheral nerve are wrapped by SCs. When the axon is
injured, the distal end is degenerated and the proliferating SCs
engulf the degenerated axons. In addition, the axon also arranges
along the inside of neural tube and forms a solid cell cord (termed
Büngner cord) to connect the two ends. Moreover, SCs were shown to
synthesize and secrete various neurotrophic factors, which are
beneficial to neuronal survival and promote neurite outgrowth
(1,2). David and Lacroix (21) demonstrated that transplanting
exogenous SCs using a matrigel vessel in injured spinal cord
promotes axonal regeneration. Sasaki et al (22) demonstrated that bone marrow MSCs
differentiate into myelinating cells, and repair the demyelinated
axon in spinal cord. Biernaskie et al (23) differentiated skin-derived
precursors (SKP) to myelinating SCs. Twelve weeks following
transplantation in the injured spinal cord, the SKPs-SCs had
survived and provided an environment that was highly conducive to
axonal growth. In the present study, ADSC-SCs were transplanted
into rat brains with cerebral cortex contusion and a direct
comparison of functional outcomes following transplantation of
purified ADSC-SCs, ADSCs and NSCs was performed. The results
demonstrated that ADSC-SCs may provide a highly suitable transplant
candidate for the treatment of brain injury, with higher efficiency
than ADSCs or NSCs. Although SCs differentiated from ADSCs may
promote axonal regeneration, the change in the microenvironment
following the transplantation, such as upregulation of neurotrophic
factors, reactive gliosis and the mobilization of endogenous NSCs
were also important. Further studies are required to determine the
underlying mechanisms of these effects.
The motor cortex of rats is located at the
frontoparietal junction (24).
Cortex contusion results in contralateral hemiplegia and the degree
of paralysis depends on the area and extent of the injury. In the
present study the contusion injury resulted in contralateral
hemiplegia. A 2×2×2 mm3 reserved cavity accommodated the
transplantation. As the operation was performed under a microscope,
rat mortality was significantly reduced.
In conclusion, the results indicated that ADSC-SCs
are a viable alternative with a number of advantages. ADSCs were
derived from adipose tissue and thus represent an accessible,
potentially autologous tissue source. ADSCs are an adult human
precursor population, the use of which circumvents potential
ethical issues. In addition, human ADSCs are markedly expanded
(16,25) and ADSC-SCs behave similar to
immature, developing SCs, in contrast to nerve-derived SCs, which
are mature and thus have limited proliferation potential.
Furthermore, ADSCs were obtained, cultured and expanded easily
in vitro. In the present study, transplantation of ADSC-SCs
following brain contusion was more efficient in promoting locomotor
function recovery than ADSCs and NSCs.
References
|
1
|
Wiberg M and Terenghi G: Will it be
possible to produce peripheral nerves? Surg Technol Int.
11:303–310. 2003.PubMed/NCBI
|
|
2
|
Yamamoto M, Sobue G, Li M, Arakawa Y,
Mitsuma T and Kimata K: Nerve growth factor (NGF), brain-derived
neurotrophic factor (BDNF) and low-affinity nerve growth factor
receptor (LNGFR) mRNA levels in cultured rat Schwann cells;
differential time- and dose-dependent regulation by cAMP. Neurosci
Lett. 152:37–40. 1993. View Article : Google Scholar
|
|
3
|
Oudega M and Xu XM: Schwann cell
transplantation for repair of the adult spinal cord. J Neurotrauma.
23:453–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pearse DD and Barakat DJ: Cellular repair
strategies for spinal cord injury. Expert Opin Biol Ther.
6:639–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiyama Y, Radtke C and Kocsis JD:
Remyelination of the rat spinal cord by transplantation of
identified bone marrow stromal cells. J Neurosci. 22:6623–6630.
2002.PubMed/NCBI
|
|
6
|
Tohill M and Terenghi G: Stem-cell
plasticity and therapy for injuries of the peripheral nervous
system. Biotechnol Appl Biochem. 40:17–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kingham PJ, Kalbermatten DF, Mahay D,
Armstrong SJ, Wiberg M and Terenghi G: Adipose-derived stem cells
differentiate into a Schwann cell phenotype and promote neurite
outgrowth in vitro. Exp Neurol. 207:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang SK, Lee DH and Jung JS: Effect of
brain-derived neurotrophic factor on neural differentiation of
mouse embryonic stem cells and neural precursor cells. Neurosci
Res. 29:183–192. 2002. View Article : Google Scholar
|
|
10
|
Caddick J, Kingham PJ, Gardiner NJ, Wiberg
M and Terenghi G: Phenotypic and functional characteristics of
mesenchymal stem cells differentiated along a Schwann cell lineage.
Glia. 54:840–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani C, Mahay D, Kingham M, Terenghi
G, Shawcross SG and Wiberg M: Bone marrow- and adipose-derived stem
cells show expression of myelin mRNAs and proteins. Regen Med.
5:403–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faden AI, Fox GB, Fan L, Araldi GL, Qiao
L, Wang S and Kozikowski AP: Novel TRH analog improves motor and
cognitive recovery after traumatic brain injury in rodents. Am J
Physiol. 277:R1196–R1204. 1999.PubMed/NCBI
|
|
14
|
Tholpady SS, Katz AJ and Ogle RC:
Mesenchymal stem cells from rat visceral fat exhibit multipotential
differentiation in vitro. Anat Rec A Discov Mol Cell Evol Biol.
272:398–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Safford KM, Safford SD, Gimble JM, Shetty
AK and Rice HE: Characterization of neuronal/glial differentiation
of murine adipose-derived adult stromal cells. Exp Neurol.
187:319–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY
and Jung JS: Improvement of neurological deficits by intracerebral
transplantation of human adipose tissue-derived stromal cells after
cerebral ischemia in rats. Exp Neurol. 183:355–366. 2003.
View Article : Google Scholar
|
|
17
|
Jessen KR and Mirsky R: Developmental
regulation in the Schwann cell lineage. Adv Exp Med Biol. 468:3–12.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murakami T, Fujimoto Y, Yasunaga Y, Ishida
O, Tanaka N, Ikuta Y and Ochi M: Transplanted neuronal progenitor
cells in a peripheral nerve gap promote nerve repair. Brain Res.
974:17–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen CC, Lin CH, Yang YC, Chiao MT, Cheng
WY and Ko JL: Intravenous implanted neural stem cells migrate to
injury site, reduce infarct volume, and improve behavior after
cerebral ischemia. Curr Neurovasc Res. 7:167–179. 2010.PubMed/NCBI
|
|
20
|
Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS,
Lee YW, Kim WH, Kang KS and Kweon OK: Functional recovery and
neural differentiation after transplantation of allogenic
adipose-derived stem cells in a canine model of acute spinal cord
injury. J Vet Sci. 10:273–284. 2009. View Article : Google Scholar
|
|
21
|
David S and Lacroix S: Molecular
approaches to spinal cord repair. Annu Rev Neurosci. 26:411–440.
2003. View Article : Google Scholar
|
|
22
|
Sasaki M, Honmou O, Akiyama Y, Uede T,
Hashi K and Kocsis JD: Transplantation of an acutely isolated bone
marrow fraction repairs demyelinated adult rat spinal cord axons.
Glia. 35:26–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biernaskie J, Sparling JS, Liu J, Shannon
CP, Plemel JR, Xie Y, Miller FD and Tetzlaff W: Skin-derived
precursors generate myelinating Schwann cells that promote
remyelination and functional recovery after contusion spinal cord
injury. J Neurosci. 27:9545–9559. 2007. View Article : Google Scholar
|
|
24
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 6th edition. Academic Press; Waltham, MA:
pp. 152–154. 2007
|
|
25
|
Rodríguez-Serrano F, Alvarez P, Caba O,
Picón M, Marchal JA, Perán M, Prados J, Melguizo C, Rama AR,
Boulaiz H and Aránega A: Promotion of human adipose-derived stem
cell proliferation mediated by exogenous nucleosides. Cell Biol
Int. 34:917–924. 2010.PubMed/NCBI
|